Introduction
Fluvoxamine, a common and widely used antidepressant
agent, is intended to exert its therapeutic effects in patients
with depression by inhibiting the reuptake of serotonin in synaptic
clefts (1). Certain studies have
supported the theory that fluvoxamine exerts therapeutic effects
not only on depression but also on schizophrenia and bipolar
depression (2,3). Consequently, the conventional
serotonin hypothesis is not able to not fully elucidate the
pharmacological mechanisms of action of fluvoxamine.
σ receptors are recognized as non-opioid,
intracellular receptors that modulate a variety of types of signal
transduction in cells (4). A
number of studies have demonstrated the existence of at least two
subtypes of the σ receptor, σ1 and σ2, and
σ1 receptors are expressed in numerous organs such as
the brain, liver, pancreas, spleen and adrenal glands (5–7). The
wide distribution of σ1 receptors in a variety of
tissues suggests a critical role in living systems (6). A previous study by Niitsu et
al(8) suggested that a
σ1 receptor agonist caused a significant therapeutic
effect in the treatment of schizophrenia. Additionally, SA4503, a
σ1 receptor agonist, alleviated schizophrenia symptoms
in an animal model (9). A study
using fluvoxamine for the treatment of a patient with schizophrenia
suggested that σ1 receptors are probably associated with
the mechanism of action of fluvoxamine (10).
Mammalian target of rapamycin (mTOR),
Ca2+/calmodulin-dependent protein kinase 2γ (Camk2γ) and
glycogen synthase kinase-3β (GSK-3β) are three fundamental
biomarkers implicated in the underlying mechanisms of depression,
schizophrenia, mania and certain neuropsychiatric diseases
(11–13). The present study aimed to
investigate the effects of fluvoxamine on the expression of these
biomarkers by studying fluvoxamine-treated N2a cells and attempted
to elucidate whether σ1 receptors mediate the
pharmacological effects of fluvoxamine. Thus, BD1047, a
σ1 receptor antagonist, was applied to
fluvoxamine-treated N2a cells in order to observe its effects on
the fluvoxamine-elicited pharmacological action.
Materials and methods
Reagents
Fluvoxamine and BD1047 were purchased from Tocris
Bioscience (Minneapolis, MN, USA). Primary antibodies against mTOR,
Camk2γ and GSK-3β were purchased from Cell Signalling Technology,
Inc. (Danvers, MA, USA). The mouse N2a neuroblastoma cells were
obtained from the Medical College, Soochow University (Suzhou,
China).
Cell culture
N2a cell culture was performed as described
previously (14,15). The N2a cells were cultured in DMEM
(Gibco, Grand Island, NY, USA) solution supplemented with 10% fetal
bovine serum (FBS; Gibco), 0.3 mM L-glutamine and 50 U/ml
penicillin/streptomycin. The N2a cells were randomly divided into
three groups (six duplicates per group): DMEM group (D group), 0.5
μmol/l fluvoxamine group (F group) and 0.2 μmol/l BD1047
(σ1 receptor antagonist) + 0.5 μmol/l fluvoxamine group
(BF group). Each culture well contained 2×105 N2a cells.
The N2a cells were prepared for analysis 48 h after the initiation
of incubation,.
Western blotting
The N2a cells were washed with phosphate-buffered
saline (PBS). Protein levels were determined using the
bicinchoninic acid method, according to the manufacturer’s
instructions (Nanjing Kaiji Biochemistry Company, Nanjing, China).
Briefly, bovine serum albumin (BSA) was applied as a standard
protein. Prior to electrophoresis, a mixture of bromophenol blue
and dithiothreitol (DTT; final concentration, 10 mM) was added to
the samples. For western blotting, 50 μg of the total protein from
each sample was separated by SDS-PAGE under reducing conditions.
The proteins were then transferred onto polyvinylidene fluoride
membranes (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China). The membranes were blocked for 2 h at room temperature
using non-fat dried milk blotting-grade blocker and incubated
overnight with primary antibodies. The primary antibodies used were
goat anti-mTOR (1:1,000), rabbit anti-Camk2γ (1:1,000) and rabbit
anti-GSK-3β (1:1,000). The primary antibodies were diluted in
Tris-buffered saline (Thermo Fisher Scientific Inc., Rockford, IL,
USA) containing 0.1% Tween-20 (TBS-T) and 2% BSA. Following
extensive washing (three times for 15 min each in TBS-T), the mTOR,
Camk2γ and GSK-3β protein levels were measured with horseradish
peroxidase-conjugated rabbit anti-goat IgG (1:100,000 dilution)
using enhanced chemiluminescence reagents (Beyotime, Nantong,
China). Equal protein loading and transfer were assessed by
subjecting each sample to western blotting for GAPDH with rabbit
anti-GAPDH IgG (1:2,000 dilution).
Statistical analysis
Data are expressed as the mean ± standard deviation
and were analyzed using one-way analysis of variance, and post hoc
analyses were performed using the least significant difference
test. Statistical analysis was conducted using SPSS Software,
version 17.0 (IBM, Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference for all the data
analyzed.
Results
Effects of fluvoxamine on the expression
of mTOR, Camk2γ and GSK-3β in cultured N2a cells
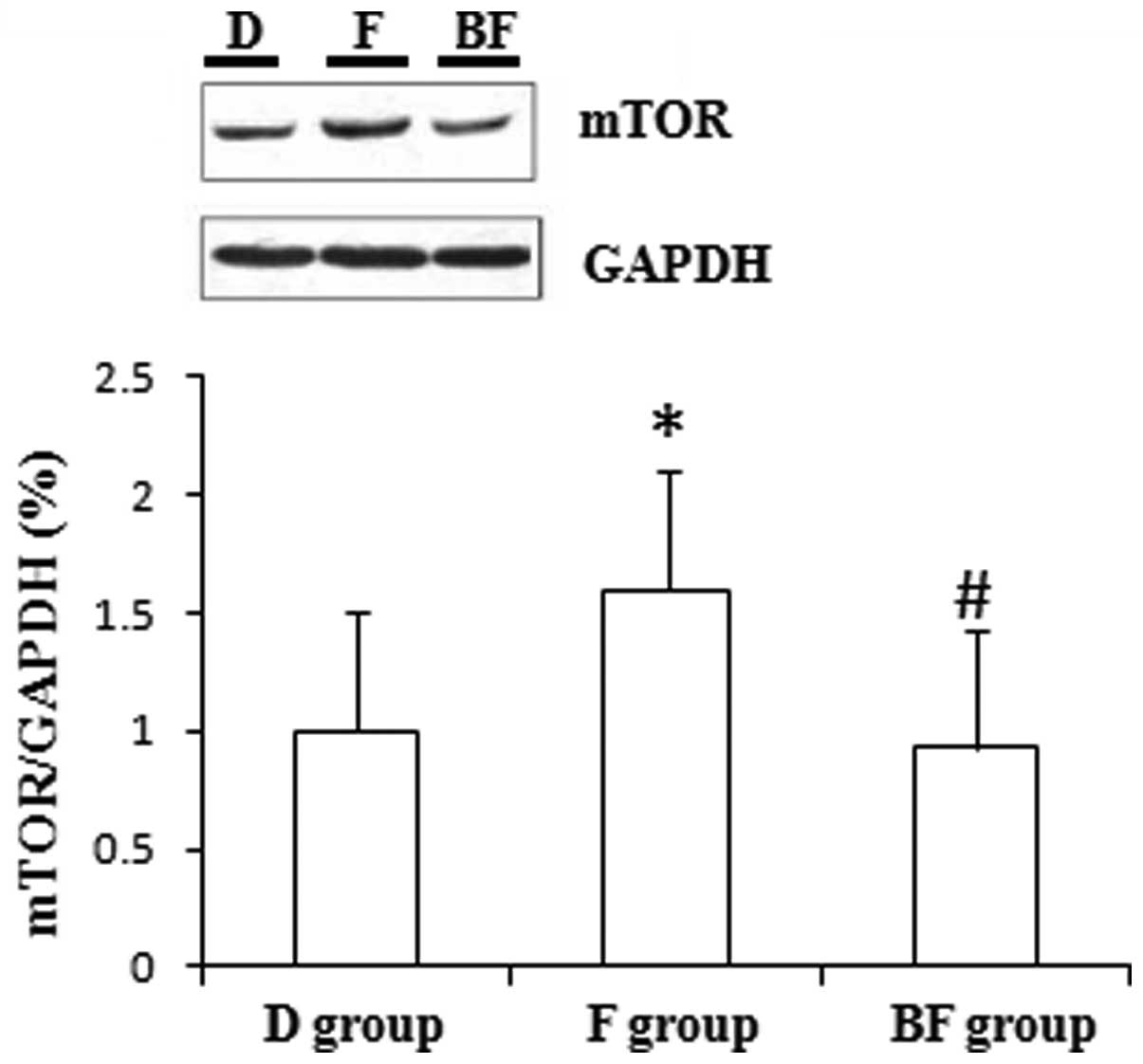
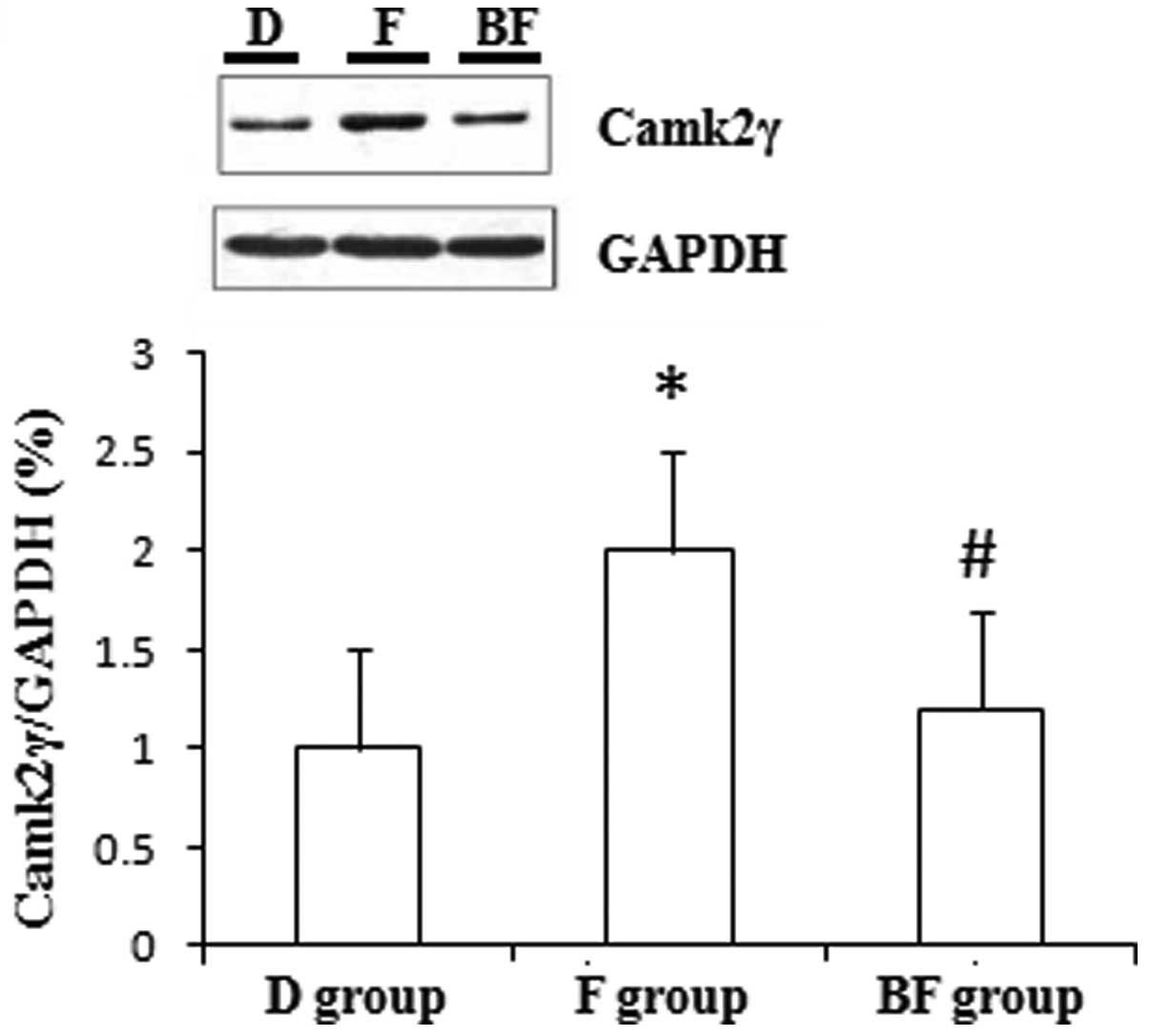
The administration of fluvoxamine significantly
increased the levels of mTOR and Camk2γ expression compared with
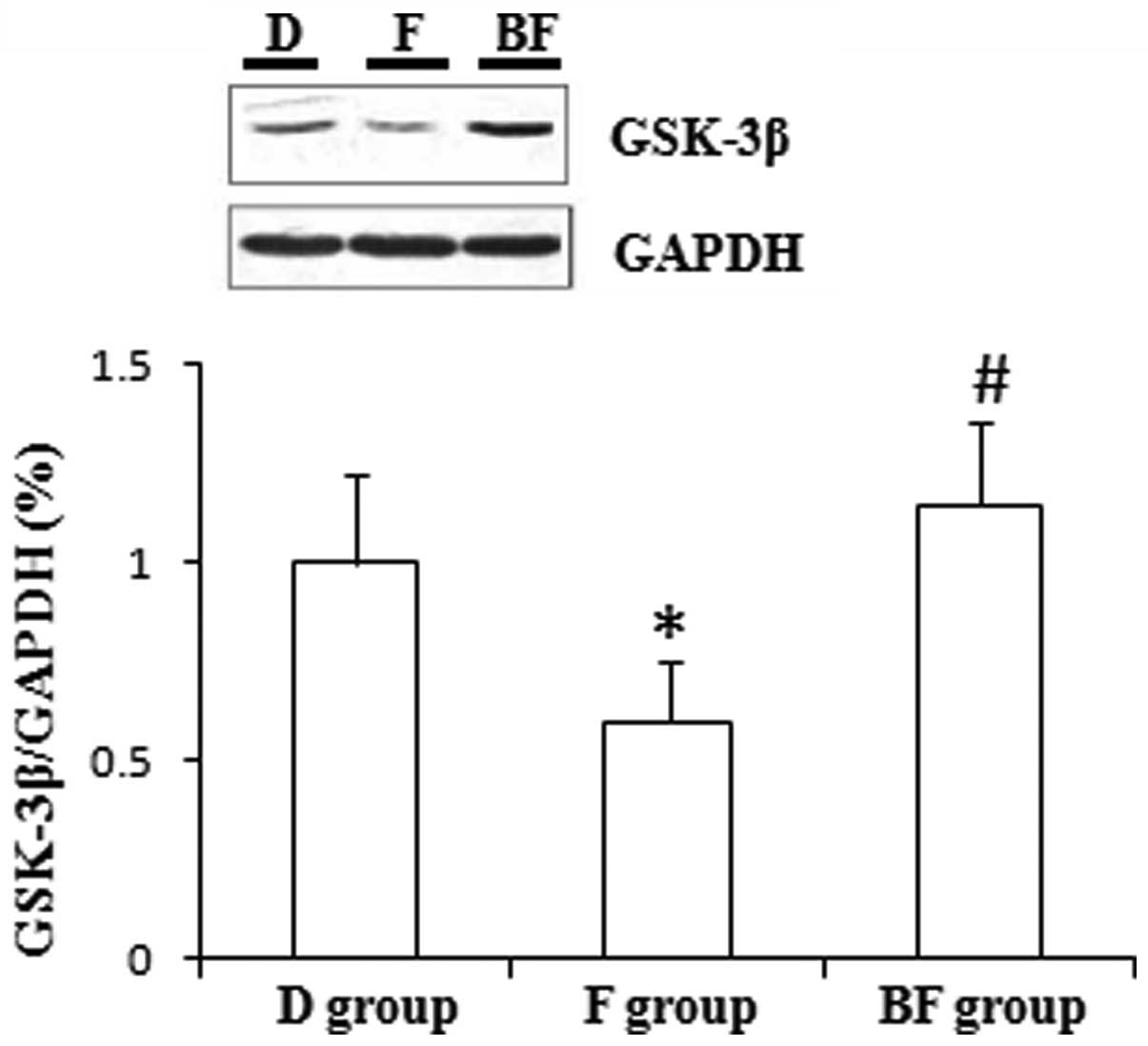
those of the D group in the cultured N2a cells (P<0.01; Figs. 1 and 2). Fluvoxamine significantly decreased
the levels of GSK-3β expression compared with those of the D group
in the cultured N2a cells (P<0.01; Fig. 3).
Effects of BD1047 on the
fluvoxamine-elicited changes in the expression levels of mTOR,
Camk2γ and GSK-3β in cultured N2a cells
The administration of BD1047 significantly decreased
the levels of mTOR and Camk2γ expression compared with those of the
F group in the cultured N2a cells (P<0.01; Figs. 1 and 2). Moreover, BD1047 significantly
increased the levels of GSK-3β expression compared with those of
the F group in the cultured N2a cells (P<0.01; Fig. 3).
Discussion
Fluvoxamine is a widely used clinical antidepressant
agent. Its primary pharmacological action is inhibition of the
reuptake of serotonin, which ultimately increases the levels of
serotonin in synaptic clefts and exerts therapeutic effects in
patients with depression (1). N2a
cells are a semi-adherent, fast growing, mouse neuroblastoma cell
line. In the present study, N2a cells were used to investigate the
pharmacological properties of fluvoxamine, and the results
demonstrated that fluvoxamine significantly increased the mTOR and
Camk2γ expression levels and decreased the GSK-3β expression
levels.
It is generally acknowledged that fluvoxamine acts
as an antidepressant agent with therapeutic effects that alleviate
the symptoms of schizophrenia, obsession, bipolar depression and
certain neuropsychiatric diseases (16–18).
Increasing evidence has suggested that σ1 receptors may
be pivotal in the mechanism of action of fluvoxamine in the
treatment of schizophrenia and other psychiatric diseases (2). Notably, the results of the present
study also demonstrated that BD1047, a σ1 receptor
agonist, abolished the fluvoxamine-elicited pharmacological
effects. This result was consistent with the expectations of the
study.
mTOR is a type of protein that promotes the activity
of neurons (11). The results of
present study indicate that fluvoxamine increased the expression
levels of mTOR in cultured N2a cells. mTOR stimulates the growth of
neurons via increasing the expression levels of neurotropic factors
and supplying nutrients (17). It
has been demonstrated that upregulated mTOR expression levels in
the prefrontal cortex are likely to be associated with the
mechanisms of antidepressant effects, which facilitate the return
of the depression-induced atrophic neurons to normal morphology and
function (18). Additionally, a
postmortem study has demonstrated downregulated mTOR expression
levels in the brain tissues of depressed patients (19). Therefore, in the present study it
was suggested that increased mTOR expression levels are probably
involved in the mechanisms by which fluvoxamine exerts
antidepressant effects. Furthermore, it was observed that BD1047
attenuated the Camk2γ-elicited increased in mTOR expression levels,
which indicates that σ1 receptors are likely to be
involved in the mechanism of action of fluvoxamine.
Camk2γ is a Ca2+-dependent protein kinase
(13). However, there is little
literature reporting whether its expression is associated with the
mechanisms of psychiatric diseases. In the present study, it was
observed that Camk2γ expression levels were significantly increased
following treatment with fluvoxamine in cultured N2a cells. It is
widely accepted that this antidepressant agent has neuroprotective
effects. The results of the present study indicate that increased
Camk2γ expression levels are probably associated with the
neuroprotective and antidepressant effect of fluvoxamine. In
addition, the results suggest that σ1 receptors are
probably involved in the pharmacological effect of fluvoxamine on
the expression of Camk2γ.
GSK-3β is serine/threonine kinase, which has been
acknowledged as a pivotal target for the treatment of depression
and mania (12). In the present
study, it was observed that that fluvoxamine has the potential to
inhibit GSK-3β and that σ1 receptors probably mediate
this process, which suggests that fluvoxamine exerts its
pharmacological effects via the serotonin pathway and also by
stimulating σ1 receptors.
In conclusion, the results of this study demonstrate
that fluvoxamine has an effect on the expression levels of mTOR,
Camk2γ and GSK-3β, and that this process is likely to be associated
with the activation of σ1 receptors. However, an in
vivo study was not conducted to investigate whether
σ1 receptor antagonists are able to attenuate the
therapeutic effects of fluvoxamine. Future large-scale studies are
required to elucidate the pharmacological properties of
fluvoxamine.
References
|
1
|
Sugimoto Y, Tagawa N, Kobayashi Y,
Mitsui-Saito K, Hotta Y and Yamada J: Involvement of the sigma1
receptor in the antidepressant-like effects of fluvoxamine in the
forced swimming test in comparison with the effects elicited by
paroxetine. Eur J Pharmacol. 696:96–100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Niitsu T, Iyo M and Hashimoto K: Sigma-1
receptor agonists as therapeutic drugs for cognitive impairment in
neuropsychiatric diseases. Curr Pharm Des. 18:875–883. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hindmarch I and Hashimoto K: Cognition and
depression: the effects of fluvoxamine, a sigma-1 receptor agonist,
reconsidered. Hum Psychopharmacol. 25:193–200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hayashi T and Su TP: An update on the
development of drugs for neuropsychiatric disorders: focusing on
the sigma 1 receptor ligand. Expert Opin Ther Targets. 12:45–58.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Romieu P, Martin-Fardon R, Bowen WD and
Maurice T: Sigma 1 receptor-related neuroactive steroids modulate
cocaine-induced reward. J Neurosci. 23:3572–3576. 2003.PubMed/NCBI
|
|
6
|
Matsumoto RR, McCracken KA, Pouw B, Zhang
Y and Bowen WD: Involvement of sigma receptors in the behavioral
effects of cocaine: evidence from novel ligands and antisense
oligodeoxynucleotides. Neuropharmacology. 42:1043–1055. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gebreselassie D and Bowen WD: Sigma-2
receptors are specifically localized to lipid rafts in rat liver
membranes. Eur J Pharmacol. 493:19–28. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Niitsu T, Fujisaki M, Shiina A, et al: A
randomized, double-blind, placebo-controlled trial of fluvoxamine
in patients with schizophrenia: a preliminary study. J Clin
Psychopharmacol. 32:593–601. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Collina S, Gaggeri R, Marra A, et al:
Sigma receptor modulators: a patent review. Expert Opin Ther Pat.
23:597–613. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iyo M, Shirayama Y, Watanabe H, et al:
Fluvoxamine as a sigma-1 receptor agonist improved cognitive
impairments in a patient with schizophrenia. Prog
Neuropsychopharmacol Biol Psychiatry. 32:1072–1073. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kitagishi Y, Kobayashi M, Kikuta K and
Matsuda S: Roles of PI3K/AKT/GSK3/mTOR pathway in cell signaling of
mental illnesses. Depress Res Treat. 2012:7525632012.PubMed/NCBI
|
|
12
|
Li X and Jope RS: Is glycogen synthase
kinase-3 a central modulator in mood regulation?
Neuropsychopharmacology. 35:2143–2154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tombes RM, Faison MO and Turbeville JM:
Organization and evolution of multifunctional
Ca2+/CaM-dependent protein kinase genes. Gene.
322:17–31. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Strenge K, Schauer R and Kelm S: Binding
partners for the myelin-associated glycoprotein of N2A
neuroblastoma cells. FEBS Lett. 444:59–64. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Björkdahl C, Sjögren MJ, Winblad B and Pei
JJ: Zinc induces neurofilament phosphorylation independent of p70
S6 kinase in N2a cells. Neuroreport. 16:591–595. 2005.PubMed/NCBI
|
|
16
|
Apter A, Ratzoni G, King RA, et al:
Fluvoxamine open-label treatment of adolescent inpatients with
obsessive-compulsive disorder or depression. J Am Acad Child
Adolesc Psychiatry. 33:342–348. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen A, Xiong LJ, Tong Y and Mao M:
Neuroprotective effect of brain-derived neurotrophic factor
mediated by autophagy through the PI3K/Akt/mTOR pathway. Mol Med
Rep. 8:1011–1016. 2013.PubMed/NCBI
|
|
18
|
Li N, Lee B, Liu RJ, et al: mTOR-dependent
synapse formation underlies the rapid antidepressant effects of
NMDA antagonists. Science. 329:959–964. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jernigan CS, Goswami DB, Austin MC, et al:
The mTOR signaling pathway in the prefrontal cortex is compromised
in major depressive disorder. Prog Neuropsychopharmacol Biol
Psychiatry. 35:1774–1779. 2011. View Article : Google Scholar : PubMed/NCBI
|