Introduction
Essential hypertension is a complex disease
associated with an increased risk of cardiovascular disorders.
Twenty-four-hour ambulatory blood pressure monitoring (ABPM) is
easily able to detect the circadian blood pressure (BP) pattern of
an individual: systolic blood pressure (SBP) and diastolic blood
pressure (DBP) show a nocturnal reduction of ≥10% in healthy
subjects. Patients whose BP does not decrease during sleep compared
with daytime levels are defined as nondippers. Nondipper
hypertensive patients have been reported to be at high risk for
target organ damage, including stroke, left ventricular
hypertrophy, carotid artery disease, microalbuminuria, nephropathy
and myocardial infarction (1).
Currently, the pathogenesis of nondipper hypertension remains
largely unclear in patients without any renal or endocrine
pathology. Previous studies have identified differences in several
types of serum biochemical concentrations between dipper and
nondipper hypertensive patients, including adiponectin (2), serum calcium and phosphate (3) and cystatin C (4). Further clinical investigations are
vital for the exploration of the complicated network involved in
nondipper hypertension.
Sphingomyelin (SM) is a ubiquitous substance present
in cell membranes where cellular processes including signal
transduction, membrane trafficking and protein sorting occur
(5). Cross-sectional studies have
shown an association of high plasma SM levels with subclinical
atherosclerosis (6) and clinical
coronary artery disease (7). The
majority of studies have focused on the association among SM
levels, lipid metabolism and atherosclerosis. To the best of our
knowledge, the correlation of SM with dipper or nondipper status in
hypertension has not yet been studied. The present study was
designed to investigate plasma SM levels in patients with dipper
and nondipper hypertension.
Subjects and methods
Subjects
The study was conducted on patients who attended the
outpatient department of Tongji Hospital Affiliated to Tongji
University (Shanghai, China). The study participants consisted of
200 consecutive hypertensive patients. BP was measured from the
right arm using a standard mercury sphygmomanometer after 10 min of
rest with the patient in the sitting position. SBP was measured at
Korotkoff phase I and DBP at Korotkoff phase V, following
recommendations of the American Heart Association (8). BP was measured three times with an
interval of ≥30 sec and the mean values were used for analysis.
Hypertension was defined as an SBP of >140 mmHg and/or a DBP of
>90 mmHg on repeated measurements and/or receipt of
antihypertensive treatment. Exclusion criteria included the
presence of the following: Known coronary artery disease (angina
and/or electrocardiogram signs of ischemia on treadmill-exercise
test), chronic renal failure, chronic liver disorder, moderate or
severe valvular disease, diabetes mellitus, congenital heart
disease, left ventricular systolic dysfunction on echocardiography
(ejection fraction <50%), anemia, thyroid disorder, pregnancy,
obstructive sleep apnea, Alzheimer's Disease (it has been revealed
that high plasma SM level is associated with Alzheimer's Disease)
(9), hyperuricemia and secondary
hypertension. All participants provided their informed consent.
This study was approved by the Ethics Committee of Tongji Hospital
Affiliated to Tongji University.
Laboratory tests
A venous blood sample was collected from each
participant under fasting conditions. Fasting blood glucose, total
cholesterol, high-density lipoprotein cholesterol, low-density
lipoprotein cholesterol, triglyceride, urea nitrogen, creatinine
and uric acid were measured by standard laboratory methods.
Plasma SM levels were measured with an enzymatic
method using a four-step procedure according to a previous study
(7). In the first step, bacterial
sphingomyelinase hydrolyzed SM to phosphorylcholine and
N-acylsphingosine. Thereafter, the addition of alkaline phosphatase
generated choline from phosphorylcholine. The newly formed choline
was used to generate hydrogen peroxide in a reaction catalyzed by
choline oxidase. Finally, with peroxidase as a catalyst, hydrogen
peroxide was used together with phenol and 4-aminoantipyrine to
generate a red quinone pigment with an optimal absorption at 505
nm. The plasma SM levels were measured in a blinded fashion and the
interassay coefficient of variation ranged from 2.2 to 4.5%.
Ambulatory BP recordings
Ambulatory 24-h BP monitoring was performed using a
SunTech Oscar2 ABPM recorder (Suntech Medical Inc., Morrisville,
NC, USA). Automatic BP recordings were obtained every 30 min during
the 24-h period. The cuff was placed around the non-dominant arm of
the subjects. Daytime was defined as 7:00 a.m. to 11:00 p.m. and
nighttime was defined as 11:00 p.m. to 7:00 a.m. The percentage of
nocturnal BP dipping was calculated using the following formula:
100 × [1-(nighttime SBP/daytime SBP)]. A nocturnal BP dip was
defined as a >10% reduction in both nocturnal SBP and DBP
compared with the average day-time BP. Detection of a <10%
reduction in either SBP or DBP was regarded as nondipper
hypertension.
Transthoracic echocardiography
examination
All participants underwent complete transthoracic
echocardiographic studies (Vivid 7 system; General Motors Co.,
Detroit, MI, USA), including two-dimensional, color flow and
spectral Doppler imaging, using a 2.5–4.0 MHz transducer. An
electrocardiograph was recorded simultaneously for every subject.
Echocardiographic measurements were obtained with participants in
the left lateral decubitus position. Three consecutive cycles were
averaged for each parameter. The examinations were performed by an
experienced cardiologist who had no knowledge of the participant's
clinical information.
Standard views, including the parasternal long-axis,
short-axis at the papillary muscle level, apical four-chamber and
two-chamber views were recorded. Left atrial volume index (LAVI;
left atrial volume divided by body surface area), left ventricular
end-diastolic diameter (LVDd), left ventricular end-systolic
diameter (LVSd), interventricular septum thickness in diastole
(IVST), posterior ventricular septum thickness in diastole (PVST)
and left ventricular mass index (LVMI; left ventricular mass
divided by body surface area) were collected. The left ventricular
mass (LVM) and body surface area (BSA) were calculated using the
formula (10): LVM (g) =
1.04[(IVST + LVDd + PVST)3 − (LVDd)3]− 13.6
and BSA (kg/m2) = 0.06 × height + 0.0128 × weight −
0.1529. M-mode tracing of LV was obtained in the parasternal
long-axis view with the cursor placed at the tip of the mitral
valve leaflets. Left ventricular ejection fraction (LVEF) was
estimated using a modified Simpson's biplane method (11).
Pulsed Doppler recordings of mitral flow velocities
were obtained from the apical 4-chamber view by placing the sample
volume between the tips of the mitral leaflets and LV outflow
velocities were obtained by placing the sample volume in the
outflow tract below the aortic valve leaflets. Peak early (E) and
late diastolic (A) transmitral filling flow velocities, the E/A
ratio and the deceleration time (DT) of the E wave were measured.
Isovolumic relaxation time (IRT), defined as the time from aortic
valve closure to mitral valve opening, was assessed by
simultaneously measuring the flow into the LV outflow tract and
mitral inflow by Doppler echocardiography.
Statistical analysis
Statistical analyses were made using SPSS
(Statistical Package for the Social Sciences version 15, SPSS Inc.,
Chicago, IL, USA) software. Numerical variables are presented as
mean ± standard deviation and categorical variables are presented
as percentage values. The Student's t-test was used for group
comparisons. The Mann-Whitney U test was used for comparison of
abnormally distributed data. Categorical data were compared with
the χ2 test. Pearson correlation was used to evaluate
the association between SM levels and demographics or laboratory
parameters. P<0.05 was considered to indicate a statistically
significant result.
Results
According to 24-h ABPM monitoring, dipper and
nondipper hypertension was noted in 84 patients (42%) and 116
patients (56%), respectively. Comparisons of clinical and
biochemical variables in the dipper and nondipper groups are shown
in Table I. There was no
statistically significant difference between the two groups in
terms of age, gender distribution, body mass index (BMI), smoking
status or antihypertensive medications. The concentrations of total
cholesterol, high density lipoprotein, low density lipoprotein,
triglyceride, fasting glucose, urea nitrogen and creatinine were
similar in the two groups. The SM levels were significantly lower
in the dipper group than in the nondipper group (41.9±17.5 vs.
96.4±14.3 mg/dl, P=0.003).
 | Table IComparisons of the clinical and
biochemical variables between dipper and nondipper hypertensive
patients. |
Table I
Comparisons of the clinical and
biochemical variables between dipper and nondipper hypertensive
patients.
| Variable | Dipper hypertensive
patients (n=84) | Nondipper
hypertensive patients (n=116) | P-value |
|---|
| Age (years) | 57.3±8.4 | 58.6±9.1 | 0.740 |
| Gender, M/F (n) | 40/44 | 49/67 | 0.260 |
| BMI
(kg/m2) | 27.8±3.3 | 26.9±4.2 | 0.360 |
| Smoking history (n,
%) | 30 (35.7) | 37 (31.9) | 0.400 |
| ACEI-ARB (n, %) | 47 (60.0) | 66 (56.9) | 0.560 |
| CCB (n, %) | 34 (40.5) | 54 (46.6) | 0.530 |
| β-blocker (n, %) | 9 (10.7) | 10 (8.6) | 0.280 |
| Diuretics (n, %) | 7 (8.3) | 9 (7.8) | 0.610 |
| Total cholesterol
(mg/dl) | 208.1±22.6 | 199.7±25.4 | 0.370 |
| HDL (mg/dl) | 40.6±12.1 | 41.2±11.8 | 0.460 |
| LDL (mg/dl) | 122.6±27.8 | 115.9±30.4 | 0.130 |
| Triglyceride
(mg/dl) | 186.5±31.0 | 192.6±28.2 | 0.620 |
| Fasting glucose
(mg/dl) | 98.7±10.4 | 101.3±12.5 | 0.270 |
| Urea nitrogen
(mg/dl) | 26.4±8.2 | 28.7±7.1 | 0.800 |
| Creatinine
(mg/dl) | 0.82±0.13 | 0.86±0.17 | 0.780 |
| SM (mg/dl) | 41.9±17.5 | 96.4±14.3 | 0.003 |
The ABPM parameters of the patients are summarized
in Table II. No significant
differences were identified in 24-h mean SBP, between the two
groups. However, mean nighttime SBP and mean nighttime DBP were
lower in the dipper group than in the nondipper group (118.2±13.3
vs. 129.4±12.7 mmHg and 71.6±7.0 vs. 79.1±7.2 mmHg, respectively;
both P<0.001). Additionally, the rates of SBP and DBP fall in
the nighttime were clearly different between the two groups
(12.8±6.4 vs. 1.1±5.8% and 11.4±5.2 vs. 0.8±6.1%, respectively;
both P<0.001).
 | Table IIComparison of ambulatory blood
pressure monitoring results between dipper and nondipper
hypertensive patients. |
Table II
Comparison of ambulatory blood
pressure monitoring results between dipper and nondipper
hypertensive patients.
| Variable | Dipper hypertensive
patients (n=84) | Nondipper
hypertensive patients (n=116) | P-value |
|---|
| 24-h mean SBP
(mmHg) | 132.6±12.8 | 133.7±11.9 | 0.460 |
| 24-h mean DBP
(mmHg) | 78.3±11.6 | 81.5±10.1 | 0.370 |
| Mean daytime SBP
(mmHg) | 137.9±10.4 | 136.8±10.2 | 0.510 |
| Mean daytime DBP
(mmHg) | 84.8±8.3 | 83.0±9.5 | 0.250 |
| Mean nighttime SBP
(mmHg) | 118.2±13.3 | 129.4±12.7 | <0.001 |
| Mean nighttime DBP
(mmHg) | 71.6±7.0 | 79.1±7.2 | <0.001 |
| Rate of SBP fall at
nighttime (%) | 12.8±6.4 | 1.1±5.8 | <0.001 |
| Rate of DBP fall at
nighttime (%) | 11.4±5.2 | 0.8±6.1 | <0.001 |
The results of the echocardiographic examinations
are shown in Table III. No
significant differences in LVSd, LVDd, IVST, PVST or LVEF were
identified between the two groups, but LAVI, LVMI, DT and IRT were
higher in the nondipper group than in the dipper group (26.5±4.6
vs. 23.2±3.6 ml/m2, P=0.02; 122.8±12.1 vs. 108.9±14.6
g/m2, P=0.007; 234.9±19.5 vs. 211.3±25.4 msec, P=0.03;
and 100.1±7.3 vs. 85.7±8.2 msec, P=0.02, respectively), while the
E/A ratio was lower in the nondipper group than in the dipper group
(0.74±0.21 vs. 0.91±0.13, P=0.009).
 | Table IIIComparison of echocardiographic
parameters between dipper and nondipper hypertensive patients. |
Table III
Comparison of echocardiographic
parameters between dipper and nondipper hypertensive patients.
| Variable | Dipper hypertensive
patients (n=84) | Nondipper
hypertensive patients (n=116) | P-value |
|---|
| LAVI
(ml/m2) | 23.2±3.6 | 26.5±4.6 | 0.020 |
| LVDd (mm) | 46.3±4.9 | 45.9±5.5 | 0.260 |
| LVSd (mm) | 28.9±3.1 | 29.1±4.0 | 0.370 |
| IVST (mm) | 11.2±1.6 | 11.3±1.5 | 0.590 |
| PVST (mm) | 10.9±1.3 | 10.6±1.4 | 0.470 |
| LVMI
(g/m2) | 108.9±14.6 | 122.8±12.1 | 0.007 |
| LVEF (%) | 64.3±6.8 | 62.4±7.1 | 0.160 |
| E/A | 0.91±0.13 | 0.74±0.21 | 0.009 |
| DT (msec) | 211.3±25.4 | 234.9±19.5 | 0.030 |
| IRT (msec) | 85.7±8.2 | 100.1±7.3 | 0.020 |
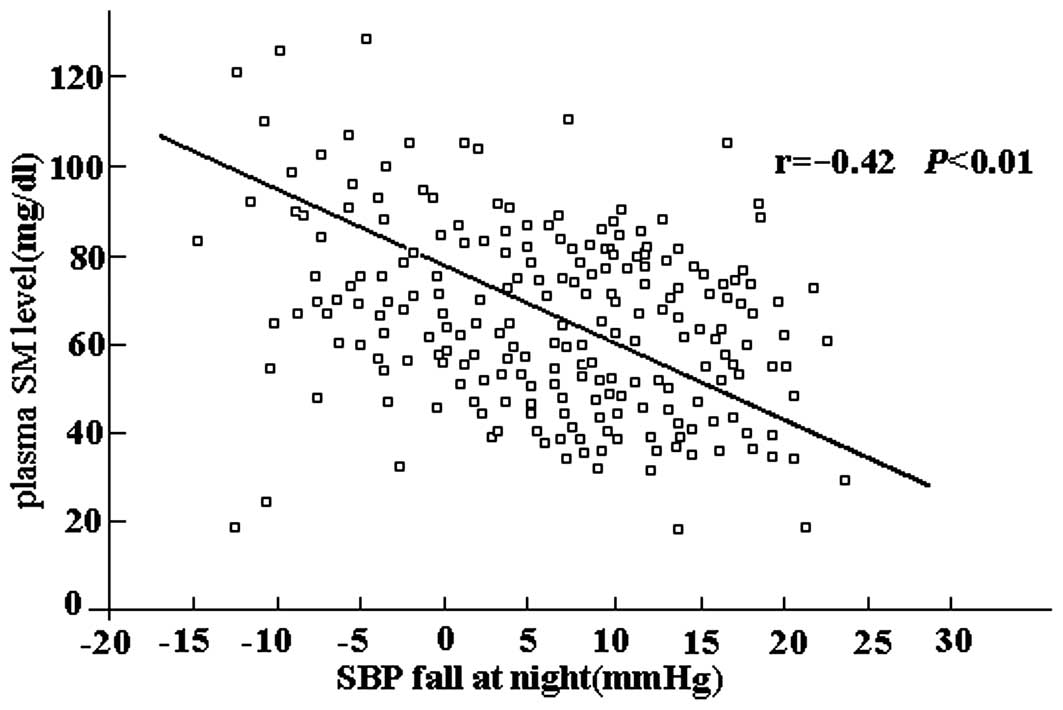
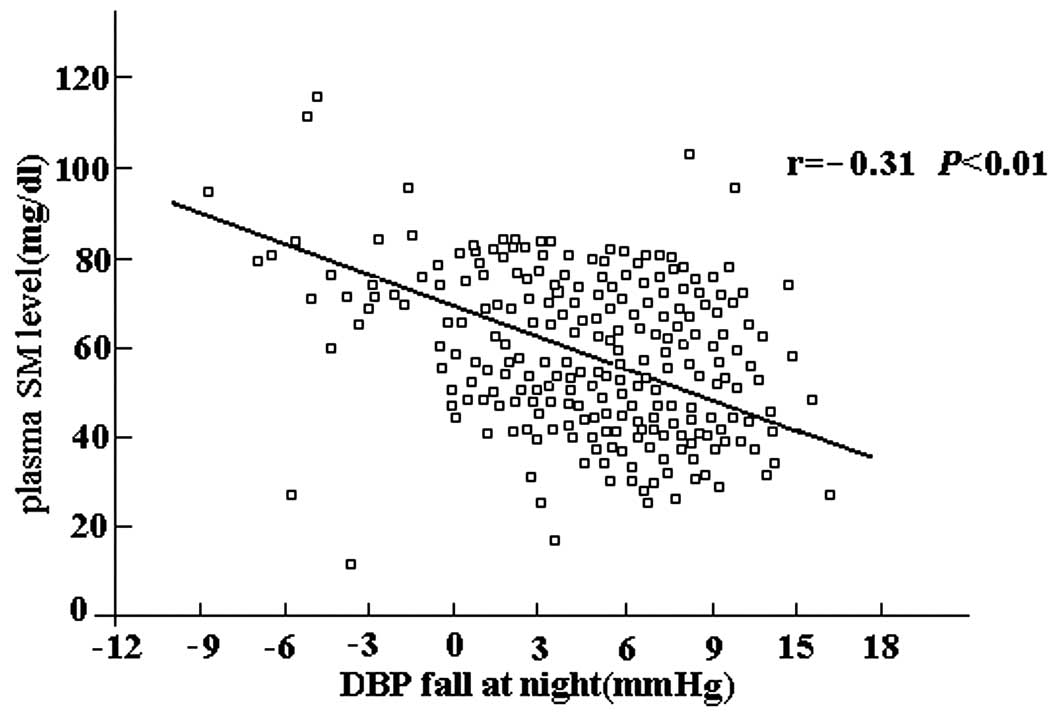
In correlation analyses, the plasma SM level was
identified to be negatively correlated with the magnitude of SBP
fall at night (r=−0.42, P<0.01; Fig. 1) and DBP fall at night (r=−0.31,
P<0.01; Fig. 2). In addition,
the SM level was correlated with age (r=0.39, P=0.02), BMI (r=0.25,
P=0.01) and low density lipoprotein (r=0.43, P<0.01).
Discussion
In this study, it was demonstrated that the LVMI and
left ventricular diastolic parameters were less favorable in the
nondipper hypertensive group compared with the dipper group. In
addition, plasma SM levels were significantly increased in
nondipper hypertensive patients compared with those in dipper
hypertensive patients. Furthermore, plasma SM levels were
negatively correlated with the fall in SBP and DBP at night. To the
best of our knowledge, this is the first study to investigate the
association between plasma SM levels and the BP nondipper
pattern.
A 24-h ABPM is an effective, reliable, noninvasive
and inexpensive method for BP ambulatory measurement and circadian
rhythm determination. Based on the ABPM results, hypertension may
be easily classified as dipper or nondipper pattern. The nondipper
pattern has been demonstrated to be associated with an increased
risk of cardiovascular, cerebrovascular and renal complications
(1,12,13).
It is generally acknowledged that transthoracic echocardiographic
examination is also an effective and reliable method for
hypertensive heart follow-up. A tissue Doppler study reported that
the nondipper pattern had an influence on increased LVM, impaired
left ventricular systolic and diastolic dysfunction and higher left
ventricular filling pressures in hypertensive patients (14). In addition, in a recent study, left
atrial appendage filling and ejection flow rates were observed to
be decreased in nondipper hypertensive patients compared with those
in dipper hypertensive patients and control subjects, which is
likely to cause left atrial appendage dysfunction (15). In the present study, the
echocardiographic findings for dipper and nondipper hypertensive
groups were consistent with certain previous results. LVMI in the
nondipper hypertensive group was higher than that in the dipper
group, suggesting that nondipper status had contributory effects to
hypertensive left ventricular concentric hypertrophy. In addition,
the left ventricular diastolic parameters in the nondipper group
were less favorable than those in the dipper group. Left
ventricular diastolic dysfunction may develop much earlier than
left ventricular hypertrophy and systolic dysfunction in
hypertensive patients. The duration of diastole is an important
determinant of myocardial perfusion. Researchers who have obtained
similar results concerning nondipper hypertensive patients having a
less favorable cardiac performance have proposed the adoption of
more aggressive antihypertension treatment for those patients
(14–16).
Although the detrimental impacts of the nondipper BP
pattern have been studied extensively among hypertensive patients,
its exact mechanisms of action have not yet been elucidated. It has
been suggested that nondippers display impaired autonomic
dysfunction (17), higher
sympathetic activity (18), higher
inflammatory activity (19,20),
prominent insulin resistance (2)
and increased oxidative stress leading to endothelium-dependent
vasodilation dysfunction (21).
Clinical observations have shown the association of several plasma
biomarkers with nondipper hypertension, including plasma atrial and
brain natriuretic peptides (22)
and vitamin D deficiency (23).
However, there are few data concerning lipoprotein metabolism and
nondipper hypertension. SMs, once considered mainly to be
structural components of cell membranes, have emerged as key
signaling molecules involved in a range of cellular functions,
including cell growth and differentiation, proliferation and
apoptotic cell death (24). An
increasing amount of evidence shows that plasma SM level is an
independent risk factor for atherosclerosis that shares common
features with hypertension, including enhanced oxidative stress,
chronic inflammatory responses and altered endothelial and vascular
muscle smooth cell functions. In an epidemiological case-control
study, Jiang et al observed that the plasma SM level was
positively and independently correlated with age, BMI and SBP
(8). Nelson et al then
investigated whether plasma SM was an early atherogenic risk factor
and examined the associations between plasma SM level and carotid
intimal-medial wall thickness, ankle-arm BP index and the Agatston
coronary artery calcium score in asymptomatic adults. Nelson et
al concluded that plasma SM was associated with subclinical
atherosclerotic disease (6). In
the present study, it was observed that plasma SM levels were
higher in the nondipper hypertensive group than in the dipper
group, and were negatively correlated with the fall in SBP and DBP
at night. The present study is a clinical observational study and
did not investigate the mechanism by which SM levels were increased
in nondipper hypertensive patients. The identification of
mechanistic pathways is extremely important for improving the
understanding of nondipper hypertension and its treatment. As a
previous study has identified that SM biosynthesis is tightly
linked to development of insulin resistance, obesity and
atherosclerosis (25), we suggest
that SM acts as a link between several key chains, including
insulin resistance, mediation of cell proliferation and oxidative
stress, involved in a complicated mechanistic network in nondipper
hypertension.
In conclusion, the present study indicated that the
nondipper pattern had contributory effects on hypertensive
concentric hypertrophy and diastolic functional impairment. In
addition, the plasma SM level was associated with the nondipper
pattern in hypertensive patients. The measurement of SM may be used
to indicate an increased risk of nondipper hypertension-associated
adverse cardiovascular events. The major limitation of this study
is its relatively small sample size and lack of a control group.
Further large-scale studies are required to assess the effects of
SM on the development of nondipper hypertension.
References
|
1
|
Izzedine H, Launay-Vacher V and Deray G:
Abnormal blood pressure circadian rhythm: a target organ damage?
Int J Cardiol. 107:343–349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Della Mea P, Lupia M, Bandolin V, et al:
Adiponectin, insulin resistance, and left ventricular structure in
dipper and nondipper essential hypertensive patients. Am J
Hypertens. 18:30–35. 2005.PubMed/NCBI
|
|
3
|
Kanbay M, Isik B, Akcay A, et al: Relation
between serum calcium, phosphate, parathyroid hormone and
‘nondipper’ circadian blood pressure variability profile in
patients with normal renal function. Am J Nephrol. 27:516–521.
2007.
|
|
4
|
Ordu S, Ozhan H, Alemdar R, et al:
Cystatin C levels in patients with dipper and nondipper
hypertension. J Investig Med. 60:676–679. 2012.PubMed/NCBI
|
|
5
|
Brown DA and London E: Structure and
function of sphingolipid- and cholesterol-rich membrane rafts. J
Biol Chem. 275:17221–17224. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nelson JC, Jiang XC, Tabas I, Tall A and
Shea S: Plasma sphingomyelin and subclinical atherosclerosis:
findings from the multi-ethnic study of atherosclerosis. Am J
Epidemiol. 163:903–912. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang XC, Paultre F, Pearson TA, et al:
Plasma sphingomyelin level as a risk factor for coronary artery
disease. Arterioscler Thromb Vasc Biol. 20:2614–2618. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kirkendall WM, Feinleib M, Freis ED and
Mark AL: Recommendations for human blood pressure determination by
sphygmomanometers. Subcommittee of the AHA Postgraduate Education
Committee. Circulation. 62:1146A–1155A. 1980.PubMed/NCBI
|
|
9
|
Mielke MM, Haughey NJ, Bandaru VV, et al:
Plasma sphingomyelins are associated with cognitive progression in
Alzheimer's Disease. J Alzheimers Dis. 27:259–269. 2011.PubMed/NCBI
|
|
10
|
Devereux RB, Alonso DR, Lutas EM, et al:
Echocardiographic assessment of left ventricular hypertrophy:
comparison to necropsy findings. Am J Cardiol. 57:450–458. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Otterstad JE, Froeland G, St John Sutton M
and Holme I: Accuracy and reproducibility of biplane
two-dimensional echocardiographic measurements of left
ventriculardimensions and function. Eur Heart J. 18:507–513. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kario K, Pickering TG, Matsuo T, Hoshide
S, Schwartz JE and Shimada K: Stroke prognosis and abnormal
nocturnal blood pressure falls in older hypertensives.
Hypertension. 38:852–857. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oliveras A, Armario P, Martell-Clarós N,
Ruilope LM and de la Sierra A; Spanish Society of
Hypertension-Resistant Hypertension Registry. Urinary albumin
excretion is associated with nocturnal systolic blood pressure in
resistant hypertensives. Hypertension. 57:556–560. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tigen K, Karaahmet T, Fotbolcu H, et al:
The influence of dipper and nondipper blood pressure patterns on
left ventricular functions in hypertensive patients: a tissue
Doppler study. Turk Kardiyol Dern Ars. 37:101–106. 2009.PubMed/NCBI
|
|
15
|
Kumak F and Gungor H: Comparison of the
left atrial appendage flow velocities between patients with dipper
versus nondipper hypertension. Echocardiography. 29:391–396. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rizzo V, Maio FD, Campbell SV, et al: Left
ventricular function, cardiac dysrhythmias, atrial activation, and
volumes in nondipper hypertensive individuals with left ventricular
hypertrophy. Am Heart J. 139:529–536. 2000.PubMed/NCBI
|
|
17
|
Mann S, Altman DG, Raftery EB and
Bannister R: Circadian variation of blood pressure in autonomic
failure. Circulation. 68:477–483. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sherwood A, Routledge FS, Wohlgemuth WK,
Hinderliter AL, Kuhn CM and Blumenthal JA: Blood pressure dipping:
ethnicity, sleep quality, and sympathetic nervous system activity.
Am J Hypertens. 24:982–988. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kaya MG, Yarlioglues M, Gunebakmaz O, et
al: Platelet activation and inflammatory response in patients with
non-dipper hypertension. Atherosclerosis. 209:278–282. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ermis N, Yagmur J, Acikgoz N, et al: Serum
gamma-glutamyl transferase (GGT) levels and inflammatory activity
in patients with non-dipper hypertension. Clin Exp Hypertens.
34:311–315. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maio R, Perticone M, Sciacqua A, et al:
Oxidative stress impairs endothelial function in nondipper
hypertensive patients. Cardiovasc Ther. 30:85–92. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Anan F, Takahashi N, Ooie T, Yufu K,
Saikawa T and Yoshimatsu H: Role of insulin resistance in nondipper
essential hypertensive patients. Hypertens Res. 26:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Demir M, Günay T, Özmen G and Melek M:
Relationship between vitamin D deficiency and nondipper
hypertension. Clin Exp Hypertens. 35:45–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hla T and Dannenberg AJ: Sphingolipid
signaling in metabolic disorders. Cell Metab. 16:420–434. 2012.
View Article : Google Scholar
|
|
25
|
Jiang XC, Goldberg IJ and Park TS:
Sphingolipids and cardiovascular diseases: lipoprotein metabolism,
atherosclerosis and cardiomyopathy. Adv Exp Med Biol. 721:19–39.
2011. View Article : Google Scholar : PubMed/NCBI
|