Introduction
Infection with Helicobacter pylori
(Hp) is a substantial public health problem that affects
20–50% of the population in industrialized nations and up to 80% of
the population in less developed countries (1). Furthermore, Hp is associated
with a number of gastro-duodenal disorders (2–4),
including gastric carcinoma, gastric mucosa-associated lymphoid
tissue lymphoma and peptic ulcer disease, particularly duodenal
ulcers, where this bacterium is observed in the stomachs of ∼95% of
patients (5). It is widely
recognized that the eradication of Hp accelerates duodenal
ulcer healing and prevents ulcer relapse. However, recent studies
(6) have indicated that increasing
antibiotic resistance may be responsible for the current low
Hp eradication rate by classical clarithromycin-based triple
therapy.
Alternative approaches have attempted to increase
the Hp eradication rate, including bismuth-containing
quadruple therapy, non-bismuth-containing quadruple therapy,
sequential therapy and levofloxacin-containing regimens (7). However, by changing the
administration strategy of antibiotics to initially improve the
environment in which they exert their effects, we may be able to
enhance their Hp eradication efficacy.
Accumulating evidence suggests that overproduction
of gastric acid is important in the development of ulcer disease.
Although numerous factors are known to affect Hp
eradication, gastric pH levels have attracted considerable
attention. Our study aimed to observe the healing rate and
Hp eradication rate of Hp-infected duodenal ulcers
following clarithromycin-based triple therapy combined with
different gastric juice pH levels. Furthermore, we aimed to explore
new administration strategies which may elevate the Hp
eradication rate by providing a more suitable environment for
anti-Hp drugs.
Materials and methods
Ethics approval and consent
For this human clinical study, we obtained the
approval of The Institutional Human Research Review Committee and
the research carried out on humans was in compliance with the
Helsinki Declaration. Verbal and written informed consent was
obtained from each patient prior to study enrollment. Informed
consent was composed of five parts: patient name, aim, expected
benefits and possible risks of the study and the rights of the
patient during the study.
Case selection
We enrolled 160 cases of Hp-infected duodenal
ulcers from the outpatients and inpatients of the First Hospital of
Hebei Medical University, between February 2010 and October 2011.
The inclusion criteria were as follows: i) Patients who were
diagnosed with duodenal ulcers (active stage or healing stage) by
gastroscopy; ii) patients who were Hp-positive, confirmed by
a 14C-urea breath test and staining microscopy; and iii)
patients with a treatment course of 4 weeks. The exclusion criteria
were as follows: i) Patients who were under 18 years old; ii)
patients whose ulcer was actively bleeding; iii) patients who were
pregnant or lactating; iv) patients who were allergic to proton
pump inhibitors (PPIs) or antibiotics; v) patients who had been
treated with PPIs or relevant antibiotics up to 2 weeks ago; vi)
patients with a history of alcohol or drug abuse; and vii) patients
with heart, liver or kidney dysfunction. The suspension criteria
were as follows: i) Patients exhibiting poor compliance and who
were not able to take medicine according to the arrangement; ii)
patients who experienced adverse reactions; and iii) patients who
did not attend the follow-up or were lost to the study. The study
was approved by the ethics committee of the Fourth Hospital of
Hebei Medical University, Shijiazhuang, Hebei, China.
Therapy methods
The patients (n=160) were randomly assigned into 2
groups (Table I). Eighty patients
(44 males, 36 females) were allocated to the treatment group; the
average age was 48.5±4.6 years (range, 21–68) and 56 cases were in
A2 stage, compared with 24 in H1 stage. The remaining 80 patients
(48 males, 32 females) were allocated to the control group, which
had an average age of 47.6±4.5 years (range, 23–66) and 52 cases
were in A2 stage, compared with 28 cases in H1 stage. Staging was
performed as described previously (8). No statistical difference was
identified between the two groups with regard to age, gender or
ulcer degree. Prior to treatment, the gastric juice was collected
by gastroscopy and stored at −20°C for pH testing and evaluation of
anti-Hp IgA content in the two groups. Patients in the
treatment group were initially administered a 20-mg dose of
omeprazole enteric-coated capsules twice daily. After 1 week,
gastric juice pH and anti-Hp IgA content were reexamined.
For the following 1 week, a 0.5-g dose of clarithromycin disperse
tablets twice daily and a 0.1-g dose of furazolidone twice daily
were added. Omeprazole enteric-coated capsules were continued for a
further 2 weeks. Two weeks after the course of treatment had ended,
14C-urea breath tests, gastroscopy and staining
microscopy were carried out again and gastric juice pH and content
of anti-Hp IgA were reexamined. The treatment course
differed for the control group; a 20-mg dose of omeprazole
enteric-coated capsules twice daily, a 0.5-g dose of clarithromycin
disperse tablets twice daily and a 0.1-g dose of furazolidone twice
daily were administrated for the first week. Antibiotics were
subsequently stopped, while omeprazole continued for a further 3
weeks. Two weeks after this course of treatment had ended,
14C-urea breath tests, gastroscopy and staining
microscopy were carried out again and gastric juice pH and content
of anti-Hp IgA were reexamined.
 | Table I.Patient data. |
Table I.
Patient data.
| | Age (years)
| | |
|---|
| Group | Number | Mean ± SD | Range | Gender
(female/male) | Stage (A2/H1) |
|---|
| Treatment | 80 | 48.5±4.6 | 21–68 | 36/44 | 56/24 |
| Control | 80 | 47.6±4.5 | 23–66 | 32/48 | 52/28 |
Treatment of biopsy specimens
The biopsy specimens were treated according to the
method described previously (9).
Biopsy specimens were collected from the duodenal bulb by endoscopy
and placed into a transport medium [phosphate-buffered saline (PBS)
at pH 7.2]. Within 3 h of the completion of endoscopy, the samples
were placed onto Columbia Agar enriched with 5% hemolyzed horse
blood. They were subsequently incubated at 37°C in a
microaerophilic atmosphere (5% O2, 10% CO2
and 85% N2) for 5–6 days.
Testing gastric juice pH
When the gastroscope reached the mucus lake in the
fundus of the stomach, a thin plastic straw was used to collect
gastric juice through the biopsy hole and gastric juice pH was
tested with pH indicator paper.
Preparation of antigen
The ultracentrifuged cell sonicate was prepared from
a strain of Hp, Hp NCTC 11637, in accordance with the method
proposed by Hirschl et al(10).
Preparation of gastric juice for the
enzyme-linked immunosorbent assay (ELISA) test
Gastric juice samples were prepared according to the
method described by Rathbone et al(11). Samples were centrifuged at 2000 x g
for 10 min, neutralized to pH 6.5–7 by 0.67 M Tris-HCI (pH 7.4) in
0.15 M saline and diluted to 1:100 for IgA detection.
Evaluation of anti-Hp IgA levels in
gastric juice
Hp-specific IgA antibodies were measured by
ELISA, as described previously (12). The antigen preparation was diluted
in a sodium carbonate-bicarbonate buffer (pH 9.6). Antigen (1
μg/ml) was added to each well of the 12-well plates and
these were incubated at 4°C for 18 h. The plates were washed three
times with PBS solution containing 0.05% Tween-20, following which
2x100 μl of diluted gastric juice was added to each well and
incubated at 37°C for 1 h. After washing, 100 μl
peroxidase-labeled goat anti-human IgA antibody (Sigma, St. Louis,
MO, USA) was added to each well and incubated at 37°C for 1 h. The
plates were washed again and 100 μl ortho-phenylenediamine
dihydrochloride was added to each well as a substrate. The plates
were incubated in the dark at room temperature for 30 min. The
reaction was inhibited by adding 1M H2S04 and
the plates were read for optical density (OD) at 450 nm using a
Dynatech MR5000 reader (Yokohama, Japan). Results were expressed as
the mean OD ± SD. The results were interpreted as positive when the
OD ratio was ≥1.0 for IgA antibodies.
Diagnostic standard of Hp infection and
criteria for Hp eradication
Patients who had a positive 14C-urea
breath test and histological identification of Hp by biopsy
(where Hp had been cultured from the biopsies or
Helicobacter-like organisms were detected in microscopic
slide biopsies, stained using the Gram method) concurrently were
diagnosed as having a Hp infection. Two weeks after the end
of the therapy, patients who had a negative 14C-urea
breath test and tested negative for histological identification of
Hp by biopsy were classed as having successful eradication
of Hp. For the 14C-urea breath test, patients
were administered a 14C urea capsule to swallow
following an overnight fast. After 10 to 15 min, patients provided
a single breath sample by blowing through a straw into a bottle of
liquid; this was tested for radioactive CO2.
Evaluation of ulcer healing
Ulcer healing refers to ulcer disappearance and is
classified into 2 groups. S1 stage indicates that the red
regenerating epithelium has completely covered the floor of the
ulcer and the white coating has disappeared, thus it is also known
as the ‘red scar’ stage. In S2 stage, the redness has returned to
the color of the surrounding mucosa, thus it may also be referred
to as the ‘white scar’ stage.
Statistical analysis
SPSS 13.0 was used for statistical analysis and data
referring to measurements are expressed in the form of average ±
standard deviation. The Chi-square test was used to compare the two
groups, where P<0.05 was considered to indicate a statistically
significant difference.
Results
Gastric juice pH before and after Hp
eradication
The gastric juice pH of the treatment group was
significantly higher than that of the control group before
Hp eradication (P<0.05), however there was no significant
difference between the two groups after Hp eradication
(P>0.05; Table II).
 | Table II.Gastric juice pH before and after
eradication of Hp in the two groups. |
Table II.
Gastric juice pH before and after
eradication of Hp in the two groups.
| Gastric juice pH,
mean ± SD
|
|---|
| Variable | Before Hp
eradication | After Hp
eradication |
|---|
| Treatment
group | 5.32±0.023 | 2.546±0.692 |
| Control group | 2.35±0.026 | 2.436±0.597 |
| P-value | <0.05 | >0.05 |
Association of gastric pH with content of
anti-Hp IgA in gastric juice before and after Hp eradication
The content of anti-Hp IgA in gastric juice
before Hp eradication was significantly higher in the
treatment group than the control group (P<0.05). However, the
content of anti-Hp IgA in gastric juice after Hp
eradication was significantly lower in the treatment group than in
the control group (P<0.05; Table
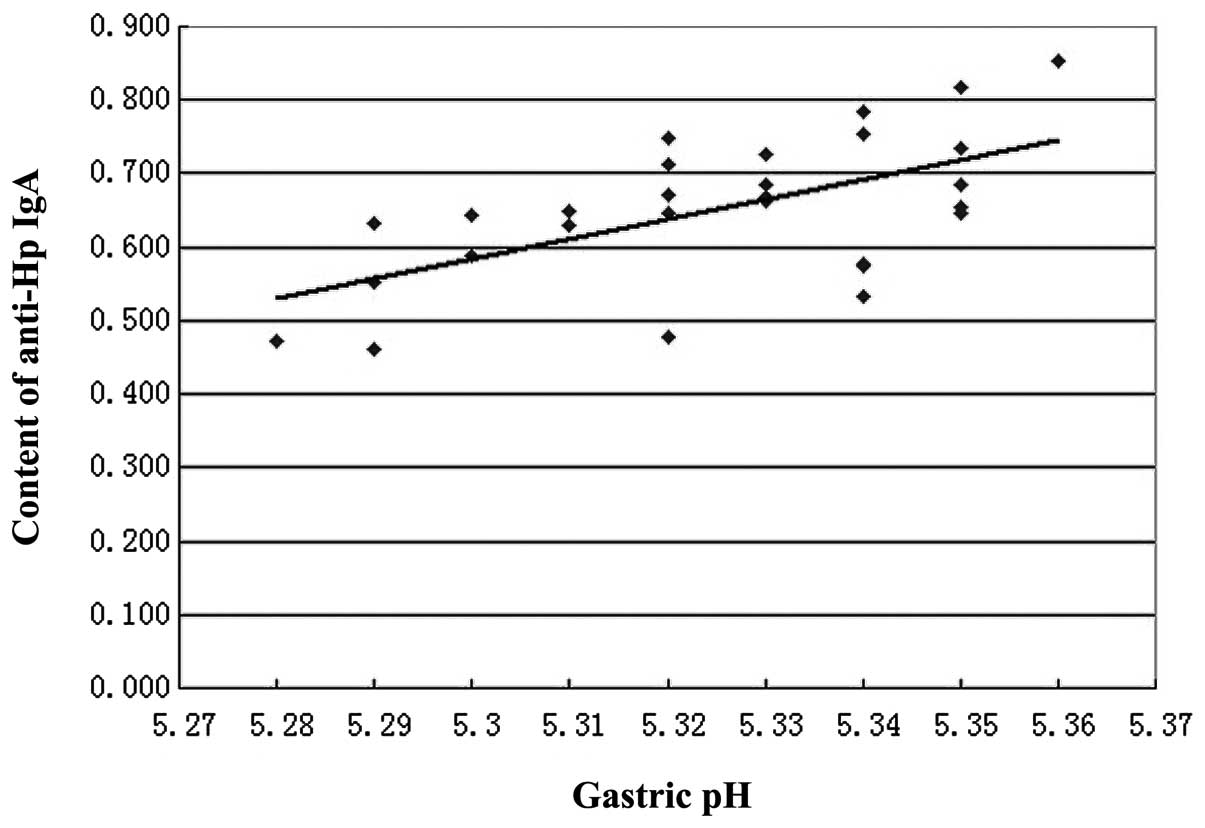
III). In addition, a positive linear correlation was observed
between gastric pH (x) and the content of anti-Hp IgA (y;
r=0.5997, P<0.05, y=−13.661+2.6878x; Fig. 1).
 | Table III.Content of anti-Hp IgA in
gastric juice before and after Hp eradication in the two
groups. |
Table III.
Content of anti-Hp IgA in
gastric juice before and after Hp eradication in the two
groups.
| Content of
anti-Hp IgA in gastric juice (mean OD ± SD)
|
|---|
| Variable | Before Hp
eradication | After Hp
eradication |
|---|
| Treatment
group | 0.658±0.175 | 0.173±0.013 |
| Control group | 0.456±0.198 | 0.202±0.039 |
| P-value | <0.05 | <0.05 |
Efficacy of Hp eradication
All 80 patients in the treatment group completed
their treatment, however 3 patients in the control group did not
finish their treatment due to experiencing adverse reactions. The
efficacy of Hp eradication was improved in the treatment
group compared with the control group and this difference was
statistically significant (P<0.05; Table IV).
 | Table IV.Comparison of Hp eradication
rates between the two groups. |
Table IV.
Comparison of Hp eradication
rates between the two groups.
| Variable | Effective
cases | Ineffective
cases | Hp
eradication rate (%) |
|---|
| Treatment
group | 74 | 6 | 93 |
| Control
groupa | 62 | 15 | 81 |
| P-value | - | - | <0.05 |
Ulcer healing rate
S2 stage achievement rate in the treatment group was
significantly higher than in the control group (P<0.05; Table V).
 | Table V.Comparison of S2 stage achievement
rates between the two groups. |
Table V.
Comparison of S2 stage achievement
rates between the two groups.
| Variable | Effective
casesa | Ineffective
cases | S2 achievement rate
(%) |
|---|
| Treatment
group | 74 | 6 | 93 |
| Control
groupb | 54 | 23 | 70 |
| P-value | - | - | <0.05 |
Adverse reaction
All 80 patients in the treatment group completed
their treatment. Three patients in the control group did not finish
their treatment due to experiencing nausea following ingestion of
the anti-Hp drugs, however the remaining patients in this
group did complete their treatment.
Discussion
Since the discovery of Hp ∼30 years ago, our
understanding of the pathophysiology of peptic ulcer disease has
increased enormously. The presence of Hp leads to a sequence
of pathophysiological events, including mucosal inflammation,
impairment of the mucus-bicarbonate barrier, superficial epithelial
cell damage, elevated serum gastrin levels with defective feedback
control, a possible increase in parietal cell mass and gastric
metaplasia in the duodenal cap. It has been well established that
Hp is a critical contributor to the development of duodenal
ulcers and that eradication of Hp accelerates duodenal ulcer
healing and prevents ulcer relapse. Since the 1940s, it has been
recognized that pH levels in the duodenal bulbs of patients with
ulcer disease was lower than those without ulcers and that antacids
or antisecretory therapy, which reduced the duodenal acid load,
accelerated ulcer healing. Gradually, increasing numbers of studies
started to explore the association between pH and Hp and
this led to the conclusion that the duodenal acid load may be a
critical factor in explaining the ability of Hp to colonize
the duodenal bulb, via the precipitation of glycineconjugated bile
salts. Furthermore, the combination of an elevated duodenal acid
level and Hp infection is suggested to be a critical event
in the pathogenesis of Hp-infected duodenal ulcers. It has
also been reported that the minimum inhibitory concentration (MIC)
of clarithromycin against Hp is an order of magnitude lower
when pH is high (13). Therefore,
among the numerous factors which are known to affect Hp
eradication, gastric pH, which affects the activity of Hp
and anti-Hp drugs, is one of the most crucial. Thus
eliminating Hp in an environment with a suitable gastric pH
may enhance Hp eradication, whilst also improving the
healing quality of mucosa (4,14).
In this study, the treatment group was provided with
acid-suppressing drugs for the first week, followed by
anti-Hp therapy the week after. The control group received
no treatment in the first week and the acid suppression and
anti-Hp therapy were administered in the second week.
Subsequently, the two groups were administered PPIs until the end
of the therapy course. The main difference between the two groups
was that gastric pH in the treatment group was changed by
administering acid-suppressing therapy, thus the gastric juice pH
levels were significantly higher than that of the control group
before Hp eradication (P<0.05; Table II). This study observed the healing
stage, the content of anti-Hp IgA in gastric juice and the
Hp eradication rate in Hp-infected duodenal ulcers,
after clarithromycin-based triple therapy in environments with
different gastric pH levels.
Hp colonizes the microaerophilic environment
in the protective layer of mucosal secretions, which are adjacent
to the epithelium of the human stomach, particularly in the antrum.
Numerous studies have shown that Hp is able to provoke a
systemic and local immune response. Anti-whole Hp component
specific IgA antibodies exist in the gastric juice, gastric tissue
and saliva. Due to their ability to prevent the entrance of
external foreign antigenic molecules, secretory IgA antibodies have
generally been considered an immune barrier. However, secretory IgA
(SIgA) antibodies have numerous functions, including the
intracellular and serosal neutralization of antigens, activation of
non-inflammatory pathways and homeostatic control of endogenous
microbiota (15). Furthermore,
studies have suggested that there is an association between the
antibody titer of anti-Hp IgA and the intensity of
inflammation and degree of Hp colonization in different
parts of the stomach (16). This
study has shown that Hp-specific IgA is correlated with
gastric pH (Fig. 1). As indicated
in Tables II and III and Fig.
1, higher gastric pH levels correspond with an increased
content of anti-Hp IgA in gastric juice and vice versa. It
is known that the optimum pH value of pepsin is 1.9, thus when the
gastric pH is increased, its activity decreases and therefore the
decomposition of anti-Hp IgA by pepsin is reduced. This
leads to the increased content and enhanced immunocompetence of
IgA, which causes an improvement in Hp eradication, as
depicted in Table IV. As shown in
Table III, the content of
anti-Hp IgA in gastric juice after Hp eradication was
significantly lower in the treatment group than in the control
group (P<0.05). This may be explained by the higher Hp
eradication rate in the treatment group and the short half-life of
IgA. Additionally, the extent of the decrease in IgA content in the
treatment group was larger than in the control group, which may
reflect the difference in Hp eradication rate between the
two groups.
Due to variation between Hp strains, their
increasing resistance to antibiotics and numerous host and
environmental factors, the eradiation of Hp is decreasing
year by year (17,18). Hp colonizes the gastric
epithelial surface and/or deeper into the mucus layer, where the
median pH is 1.4. Due to the reduced activity of antibiotics in low
pH levels and their decreased ability to penetrate the mucus layer,
Hp elimination is unsuccessful. A reduction in pH negatively
affects the activity of antibiotics, as demonstrated by a study
which revealed a marked increase in the MIC of clarithromycin for
clarithromycin-susceptible strains when pH was reduced; at pH 6.5,
the MIC50 and MIC90 increased two- and
eight-fold, respectively; at pH 5.9, eight- and 16-fold increases,
respectively, were observed (13).
Therefore, the negative effect of low gastric pH levels on the
activity of anti-Hp drugs may be one of the most significant
factors in the failure of Hp eradication. In the present
study, we used the commonly administered clarithromycin-based
triple therapy, which is a combination of omeprazole,
clarithromycin and furazolidone. As has been illustrated in
Tables IV and V, the Hp eradication rates and S2
stage achievement rates in the treatment group were significantly
higher than those in the control group (P<0.05). The initial
basic therapy with omeprazole in the treatment group relieved
symptoms which were causing discomfort in patients, thus increasing
their compliance for further treatment. More critically, it
increased gastric pH, which had a positive effect on the efficacy
of the antibiotics. Numerous studies have demonstrated that low pH
conditions in gastric juice results in the rapid decomposition and
decreased antibacterial efficacy of clarithromycin. Scholars have
also investigated the impact of pH on the decomposition rate of
clarithromycin, by calculating the decomposition rate constants of
clarithromycin molecules in solutions and in human gastric juice.
Such studies have demonstrated that the decomposition of
clarithromycin in solutions and gastric juice proceeded in a
pseudo-first order manner and the respective half-lives of
clarithromycin were 0.1 and 1.3 h when the pH levels of the
solutions were 1.0 and 2.0. Clarithromycin scarcely decomposed when
the pH was above 5.0 (19). It has
been suggested that the prolonged elevation of intragastric pH may
increase the concentration of acid-labile antibiotics in gastric
juice, prolong their effectiveness and improve the environment to
allow the defense mechanisms of the host to exert their optimal
effect. Thus the elevation of gastric pH may be a critical factor
in Hp elimination and ulcer healing. Hp is also
susceptible to furazolidone, which has a similar reaction to a
change in gastric pH. In addition, PPIs have the direct ability to
improve the content of anti-Hp IgA and partly eliminate
Hp, which contributes to Hp eradication and ulcer
healing. In summary, this synergistic effect may be due to the
direct effect of omeprazole on the organism, the protection of
antibiotics from acid suppression or a reinforcement in host
defense mechanisms which accompany acid degradation.
Studies have revealed that the gastric pH levels of
patients with duodenal ulcers were too low to allow the
anti-Hp drugs to optimally exert their effects. Therefore,
administering acid-suppressing therapy ahead of anti-Hp
treatment may improve the gastric pH and compliance of patients,
whilst also elevating the content of Hp-specific IgA and
enhancing the sustainability, activity and efficacy of
anti-Hp drugs, which is beneficial to the eradication of
Hp and the healing of duodenal ulcers.
Abbreviations:
|
Hp
|
Helicobacter pylori;
|
|
PPI
|
proton pump inhibitor;
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
Acknowledgements
The authors thank all the members of
the Central Laboratory of the 1st Hospital of Hebei Medical
University. This study was supported by the Natural Science
Foundation of Hebei (C2010000627).
References
|
1.
|
Malfertheiner P, Bazzoli F, Delchier JC,
et al Pylera Study Group: Helicobacter pylori eradication
with a capsule containing bismuth subcitrate potassium,
metronidazole, and tetracycline with omeprazole versus
clarithromycin-based triple therapy: a randomised, open-label,
non-inferiority, phase 3 trial. Lancet. 377:905–913. 2011.
View Article : Google Scholar
|
|
2.
|
Suerbaum S and Michetti P: Helicobacter
pylori infection. N Engl J Med. 347:1175–1186. 2002. View Article : Google Scholar
|
|
3.
|
Malfertheiner P, Chan FK and McColl KE:
Peptic ulcer disease. Lancet. 374:1449–1461. 2009. View Article : Google Scholar
|
|
4.
|
Malfertheiner P, Megraud F, O’Morain C, et
al: Current concepts in the management of Helicobacter
pylori infection: the Maastricht III Consensus Report. Gut.
56:772–781. 2007.PubMed/NCBI
|
|
5.
|
Olbe L, Fändriks L, Hamlet A and
Svennerholm AM: Conceivable mechanisms by which Helicobacter
pylori provokes duodenal ulcer disease. Baillières Best Prac
Res Clin Gastroenterol. 14:1–12. 2000.
|
|
6.
|
Bago J, Majstorovic K, Belosic-Halle Z, et
al: Antimicrobial resistance of H. pylori to the outcome of 10-days
vs. 7-days Moxifloxacin based therapy for the eradication: a
randomized controlled trial. Ann Clin Microbiol Antimicrob.
9:132010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Gasparetto M, Pescarin M and Guariso G:
Helicobacter pylori Eradication Therapy: Current
Availabilities. ISRN Gastroenterol. 2012:1867342012.PubMed/NCBI
|
|
8.
|
Kaneko E, Hoshihara Y, Sakaki N, et al:
Peptic ulcer recurrence during maintenance therapy with H2-receptor
antagonist following first-line therapy with proton pump inhibitor.
J Gastroenterol. 35:824–831. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Gościniak G: IgG and IgA antibodies in
Helicobacter pylori infections. Zentralbl Bakteriol.
286:494–502. 1997.
|
|
10.
|
Hirschl AM, Rathbone BJ, Wyatt JI, et al:
Comparison of ELISA antigen preparations alone or in combination
for serodiagnosing Helicobacter pylori infections. J Clin
Pathol. 43:511–513. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Rathbone BJ, Wyatt JI, Worsley BW, et al:
Systemic and local antibody responses to gastric Campylobacter
pyloridis in non-ulcer dyspepsia. Gut. 27:642–647. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Gościniak G, Klakockar J,
Przondo-Mordarska A and Mauff G: Helicobacter pylori
antibodies in sera of children suffering from chronic abdominal
pain. Zentralbl Bakteriol. 280:214–220. 1993.
|
|
13.
|
Lascols C, Bryskier A, Soussy CJ and
Tanković J: Effect of pH on the susceptibility of Helicobacter
pylori to the ketolide telithromycin (HMR 3647) and
clarithromycin. J Antimicrob Chemother. 48:738–740. 2001.PubMed/NCBI
|
|
14.
|
Malfertheiner P, Mégraud F, O’Morain C, et
al: Current concepts in the management of Helicobacter
pylori infection--the Maastricht 2-2000 Consensus Report.
Aliment Pharmacol Ther. 16:167–180. 2002.PubMed/NCBI
|
|
15.
|
Corthésy B: Role of secretory
immunoglobulin A and secretory component in the protection of
mucosal surfaces. Future Microbiol. 5:817–829. 2010.PubMed/NCBI
|
|
16.
|
Manojlovic N, Babic D, Filipovic-Ljeshovic
I and Pilcevic D: Anti Helicobacter pylori IgG and IgA
response in patients with gastric cancer and chronic gastritis.
Hepatogastroenterology. 55:807–813. 2008.
|
|
17.
|
Heep M, Kist M, Strobel S, et al:
Secondary resistance among 554 isolates of Helicobacter
pylori after failure of therapy. Eur J Clin Microbiol Infect
Dis. 19:538–541. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Wang WH, Wong BC, Mukhopadhyay AK, et al:
High prevalence of Helicobacter pylori infection with dual
resistance to metronidazole and clarithromycin in Hong Kong.
Aliment Pharmacol Ther. 14:901–910. 2000.
|
|
19.
|
Fujiki S, Iwao Y, Kobayashi M, et al:
Stabilization mechanism of clarithromycin tablets under gastric pH
conditions. Chem Pharm Bull (Tokyo). 59:553–558. 2011. View Article : Google Scholar : PubMed/NCBI
|