Introduction
Shock is a common intensive clinical event that
leaves patients vulnerable to multiple organ distress syndrome
(MODS), particularly in the case of septic shock (1,2).
Mechanical ventilation (MV) is an important measure used in shock
care (3,4); however, studies concerning the use of
MV in shock resuscitation are very rare. Since the strategy of
using small tidal volumes (Vts) for acute respiratory distress
syndrome (ARDS) patients has been proven and widely accepted in the
Cochrane research (5–7), a judgment of the appropriate Vt for
shock has yet to be established.
When considering protective mechanical strategy,
protection of the body as a whole is necessary. Previous studies
have suggested a possible link between MV and the development of
MODS (8–11). Therefore, the present study aimed
to compare multiple organ injury caused by hemorrhagic shock (HS)
and endotoxic shock (ES) under MV at different Vts. This study also
aimed to provide an approach for determining the appropriate Vt
during early resuscitation from the two types of shock.
Materials and methods
Animal preparation
A total of 64 New Zealand white rabbits weighing
2.5–3.2 kg (provided by the Animal Laboratory of China Medical
University) were randomly divided into eight groups (n=8 each
group; Table I).
 | Table IGrouping of animals. |
Table I
Grouping of animals.
| Group | HS | ES |
|---|
| Control | HS-C | ES-C |
| Low Vt (4–6
ml/kg) | HS-L | ES-L |
| Moderate Vt (8–10
ml/kg) | HS-M | ES-M |
| High Vt (12–15
ml/kg) | HS-H | ES-H |
The rabbits were anesthetized using 300 g/l urethane
(3–4 ml/kg intravenously) via a marginal ear vein and tracheotomy
was then performed. The right carotid arteries were catheterized
for blood pressure monitoring (HP monitor D-1034; HP, Boeblingen,
Germany) and blood withdrawal for HS induction. The right regular
veins were catheterized for re-infusion of the shed blood, isotonic
saline and dopamine for shock resuscitation. After 15 min of
stabilization breathing without MV, the artery blood gas was
measured (using an AVL Omni 3 blood gas analyzer; AVL, Graz,
Austria). The selected index was pH 7.30–7.40,
PaO2>80 mmHg and PaCO2=35–45 mmHg. During
the entire animal experiment, the central body temperature was kept
between 38 and 39°C with the use of an electric blanket. All animal
work was conducted in accordance with NIH guidelines (NIH Pub. No.
85-23, revised 1996) and was approved by Animal Care and Use
Committee of China Medical University (Shenyang, China).
HS, ES and resuscitation model
For the HS groups, HS was induced by withdrawing
blood from the right carotid in aliquots of 2 ml/min to reduce the
mean arterial pressure (MAP) to 40 mmHg over 20 min. The MAP was
maintained at 38–42 mmHg over the next 40 min by withdrawing or
re-injecting blood as required. Afterwards, the shed blood and
isotonic saline were infused intravenously at an infusion speed of
2 ml/min to maintain the MAP at 65–80 mmHg and the heart rate (HR)
at 160–240 bpm for 120 min. The blood was anti-coagulated with 400
U/kg heparin and the syringes containing the shed blood were placed
on a horizontal rotator at 37°C at 170 × g (12). Dopamine infusions were used to
reach the required MAP and HR when the saline infusion speed was
>20 ml/kg/h or HR <160 bpm, for fast saline infusion.
For the ES groups, ES was induced via the
intravenous infusion of 1 mg/kg endotoxin (L-2880 from E.
coli sero-type 055:B5; Sigma, St. Louis, MO, USA) into the
rabbits at a speed of 0.1 mg/min. MAP was reduced to 38–42 mmHg
over ∼20 min and maintained at 65–80 mmHg for the next 40 min.
Afterwards, 400 U/kg heparin was infused at a speed of 20 U/min/kg
for 20 min. Isotonic saline and dopamine were also infused to
maintain the MAP at 65–80 mmHg and HR at 160–240 bpm over 60
min.
Dopamine was administered to zero, five, one and two
animals in groups HS-C, HS-L, HS-M and HS-H, respectively. All
animals in the ES groups were administered dopamine. There were no
significant differences in the amounts of dopamine administered
among the four ES groups (data not shown).
MV
The 60-min shock period without MV was followed by a
120-min resuscitation period during which the animals of the
ventilated groups were myo-relaxed via peritoneal injection of
vecuronium bromide (0.2 mg/kg/h). The animals were also ventilated
(Bear 1000/es respirator; Viasys Healthcare, Palm Springs, CA, USA)
with a specific Vt, FiO2 40%, HR 40 bpm, and positive
end-expiratory pressure (PEEP) 0 cmH2O. The animals of
the control groups were breathing oxygen independently (via
catheter, 5 l/min) without the administration of myo-relaxin.
Hemodynamic data and arterial blood
gas
After the 120-min resuscitation period, MAP, HR and
the amounts of resuscitation fluid and dopamine were measured.
Arterial blood gas was measured simultaneously, immediately after
sampling.
Tissue, plasma and tissue
preparation
At the end of the experiment, the animals were
sacrificed using an intravenous overdose of sodium pentobarbital.
The abdomen and chest of the animals were opened. Surgical silk was
tied around the left pulmonary artery and vein.
Small tissue fragments (5×5×5 mm3) were
removed from the left dorsal lobe and stored in 2.5% glutaraldehyde
for transmission electron microscopy (EM) analysis. The right lobes
from the right pulmonary artery were fixed by instilling 0–4°C
saline for 30 min and 4% buffered paraformaldehyde for 15 min, at a
constant pressure of 15 cmH2O (10). The small fragments of the instilled
right dorsal were then removed and stored in 10% formalin for light
microscopy (LM) histology and terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) staining analysis.
Small fragments of the cortex and outer medulla of
the right kidney (5×5×5 mm3) and small intestine (10×10
mm2) (10 cm below the Treitz ligament) were harvested
and stored in 2.5% glutaraldehyde for EM analysis, and in 10%
formalin for LM and cell death (TUNEL) analysis.
Histology, EM and in situ cell death
detection
The histological sections (5 μm) of the lung,
right kidney and small intestine were stained with hematoxylin and
eosin (H&E). Histological assessment was performed by a
professional histopathologist, blinded for the treatment, using the
assessment methods of Brégeon et al (13), Paller et al (14) and Chiu et al (15). TUNEL staining was performed using
an in situ cell death detection kit (Roche, Mannheim,
Germany) according to the manufacturer’s instructions. The
apoptotic index (AI) was the mean number of apoptotic cells per 100
cells in five fields of view (×400). The ultrastructure changes
were determined by transmission EM (×8,000). Professional
pathologists, who were blinded for the treatment, measured the AI
and observed the ultrastructural changes.
Statistical analysis
SPSS software for Windows (version 11.5) was used
for statistical analysis. The data are presented as the mean ± SEM
and tested for normal distribution using the Kolmogorov-Smirnov
test. The data of the four groups per shock model were compared
using one-way analysis of variance (ANOVA). The
Student-Newman-Keuls post hoc test was used to compare significant
ANOVA results among the groups. Comparisons among the data of the
two shock groups using the same Vt (or control groups) were
performed using a Student’s t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
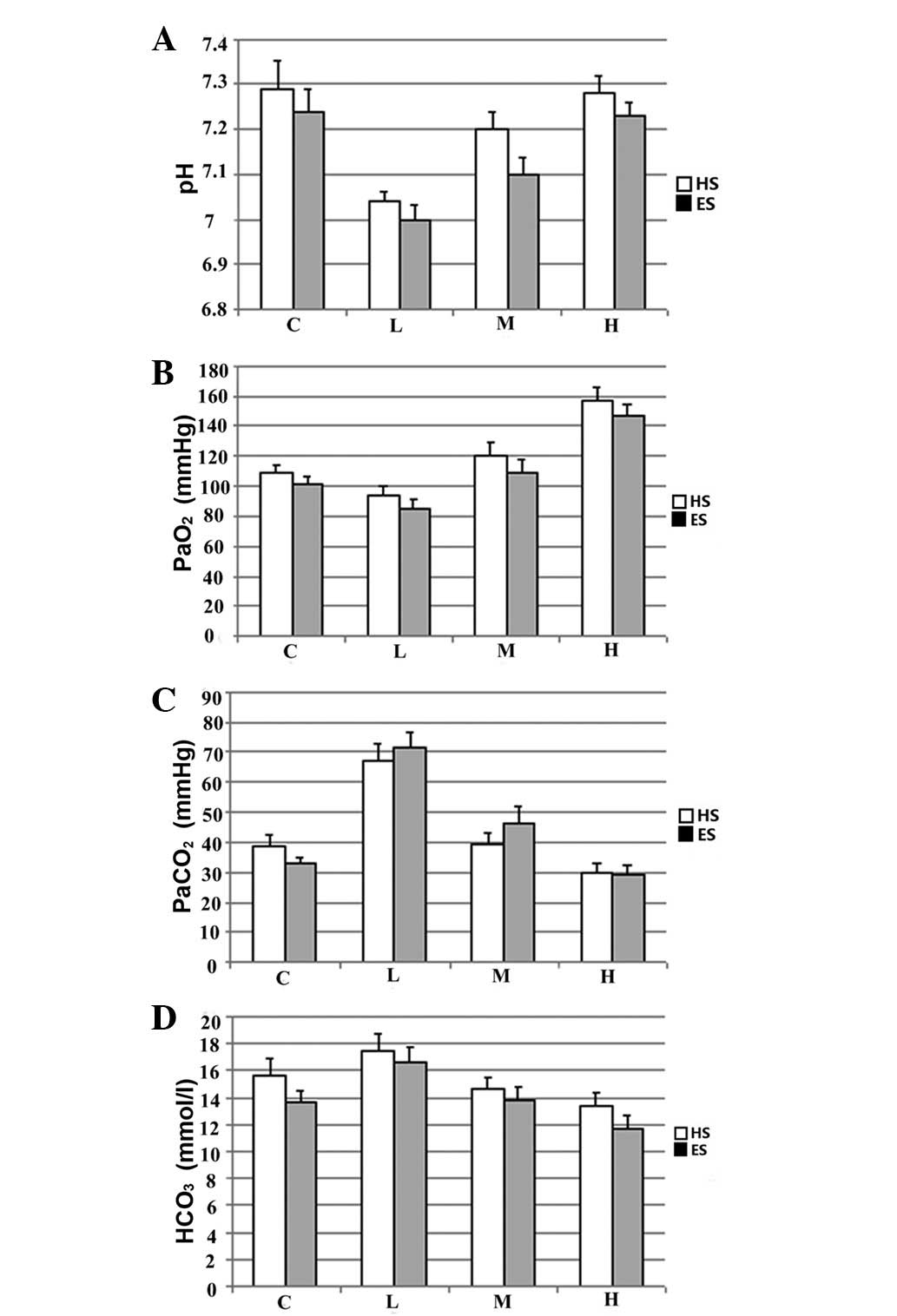
Blood gas
Following the 120-min resuscitation with ventilation
at a specific Vt, arterial PaO2 and pH were
significantly increased. By contrast, PaCO2
significantly decreased with increasing Vt. When ventilated with
the same Vt, no significant differences were identified in the
blood gas data between the rabbits in the HS and ES groups
(Fig. 1).
Pathological changes in the lung, kidney
and small intestine
For HS and ES, the high-Vt groups showed more severe
injuries of the lung, kidney and small intestine than the other Vt
groups.
Lung
In the rabbits with HS, changes to the lungs
included widening of the alveolar septum, as observed by LM
(H&E, ×400) and the diminution of the electron density of
luminal bodies, as observed by EM (×8,000). In the rabbits with ES,
LM and EM showed intra-alveolar effusion and widening of the
intercellular junction, respectively (Fig. 2).
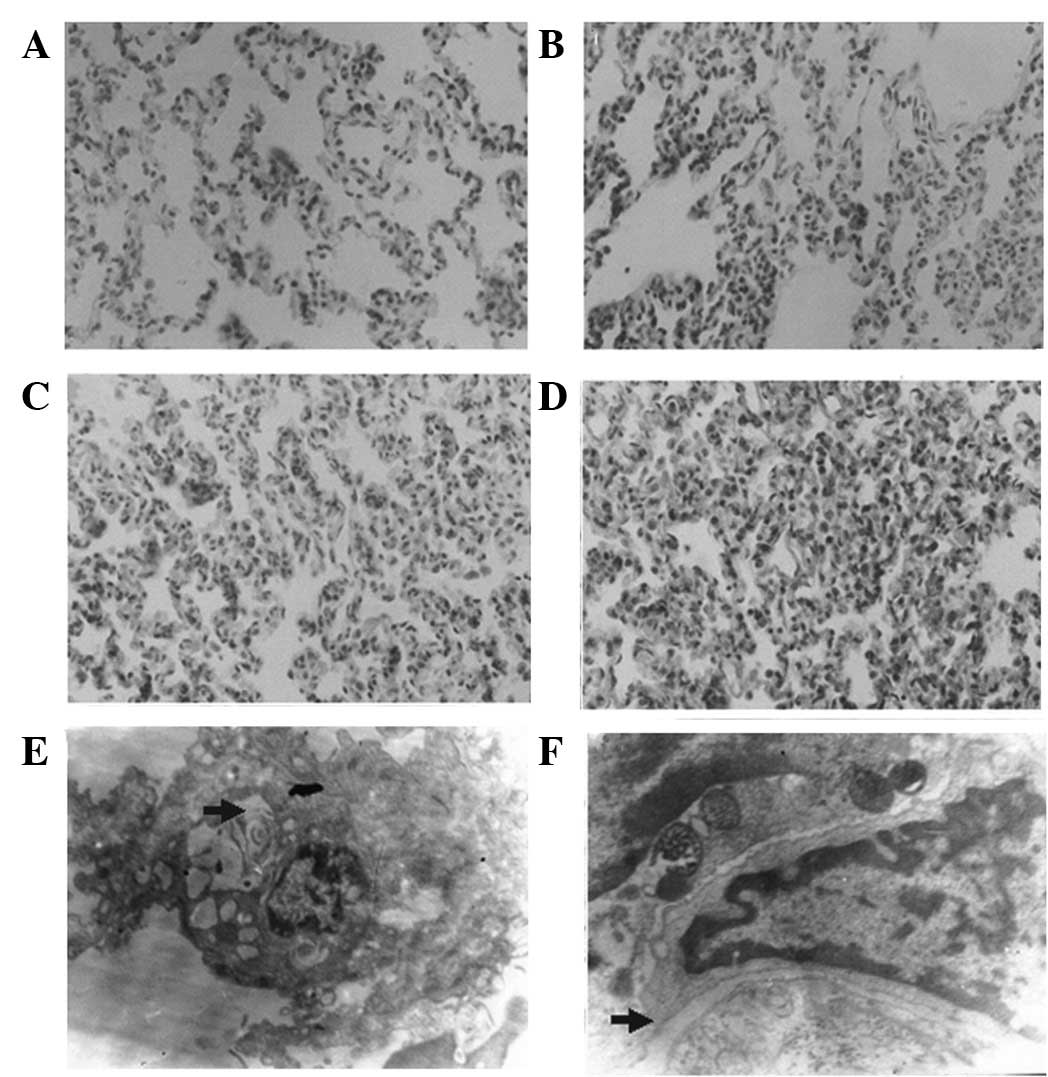 | Figure 2Pathological changes of lung. (A) Lung
histology of group HS-M by LM (H&E, ×400): alveolar septum
widened; (B) lung histology of group HS-H by LM (H&E, ×400):
the injury was more severe than that in group HS-M: alveolar septum
widened and alveoli collapsed; (C) lung histology of group ES-M by
LM (H&E, ×400): the injury was more severe than that in group
HS-M: alveolar septum widened, alveoli collapsed and intra-alveolar
space was effusive; (D) lung histology of group ES-H by LM
(H&E, ×400): the injury was more severe than that in groups
ES-M and HS-H: alveoli collapse and intra-alveolar space effusion
were more severe; (E) diminished electron density of luminal bodies
in HS (EM, ×8,000). (F) Widened intercellular junction in ES (EM,
×8,000). EM, electron microscopy; H&E, hematoxylin and eosin;
LM, light microscopy; HS, hemorrhagic shock; ES, endotoxic shock;
M, moderate Vt; H, high Vt; Vt, tidal volume. |
Kidney
In the kidneys of the rabbits with HS, the
interstitial edema was more marked, as observed by LM, and the
mitochondria of the tubular epithelium cells showed more severe
damage when examined by EM (Fig.
3).
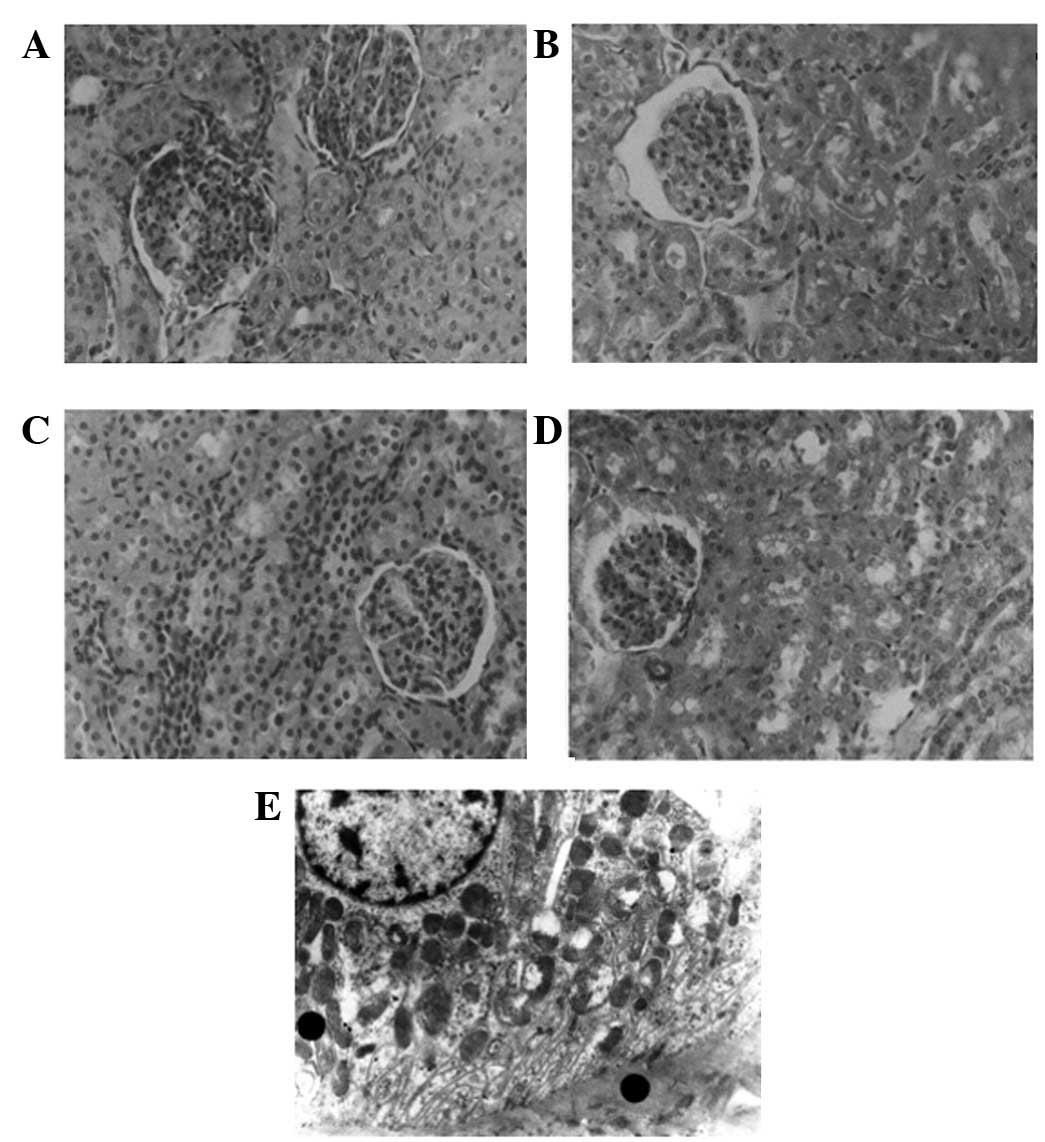 | Figure 3Pathological changes of kidney. (A)
Renal histology of group HS-L by LM (H&E, ×400): renal
interstitial edema; (B) renal histology of group HS-H by LM
(H&E, ×400): renal tubular cell injury was more severe than
that of groups HS-L and ES-H, the interstitial edema was
significant; (C) renal histology of group ES-L by LM (H&E,
×400): renal tubular cell injury was slighter than that of group
HS-L; (D) renal histology of group ES-H by LM (H&E, ×400):
renal tubular cell injury was more severe than that of group ES-L,
numerous cell fragments formed and blocked the tubular lumen; (E)
renal histology of group ES-M under EM (×8,000): the mitochondria
of the tubular epithelial cells were swollen and certain crista
were ruptured. EM, electron microscopy; H&E, hematoxylin and
eosin; LM, light microscopy; HS, hemorrhagic shock; ES, endotoxic
shock; L, low Vt; M, moderate Vt; H, high Vt; Vt, tidal volume. |
Small intestine
We have previously reported the pathological changes
in the small intestine (16). The
intercellular junction of the epithelia in the rabbits with ES was
more severe, as observed by EM.
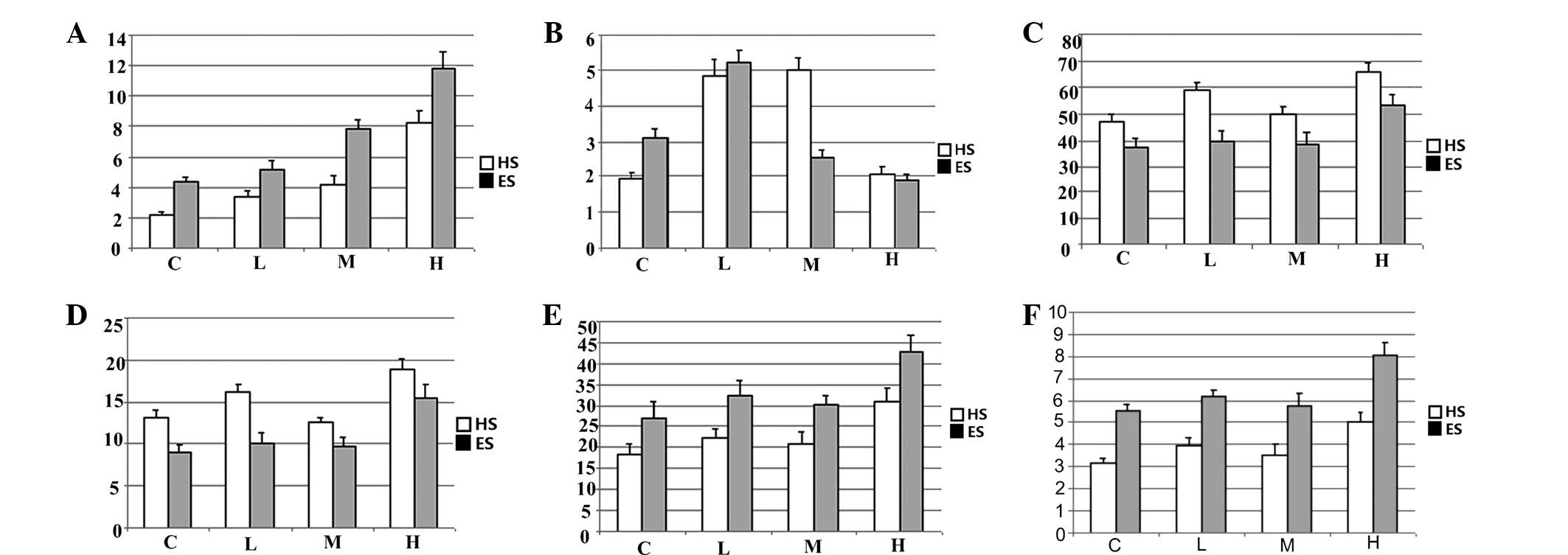
Pathological scores (PSs) and AI
For HS and ES, the PSs and AIs of the organs in the
high-Vt groups (HS-H and ES-H) were higher than those of the organs
from other groups, with the exception of the lung, which had a
lower AI (Fig. 4).
For HS, a comparison between the HS-L and HS-M
groups showed that the renal PS and AI of the HS-M group were
lower, whereas no statistically significant differences were
observed between the groups with regard to the PS and AI of the
lung and intestine. A comparison between the HS-C and HS-M groups
demonstrated that lung PS and AI of the HS-C group were lower,
whereas no statistically significant differences were noted with
regard to the PS and AI of the kidney and intestine (Fig. 4).
For ES, a comparison between the ES-L and ES-M
groups revealed that lung PS of the ES-L group was lower. The PS
and AI of the kidney and intestine showed no statistically
significant differences between the two groups. A comparison
between the ES-C and ES-L groups indicated that the lung AI of the
ES-C group was lower.
In rabbits ventilated with the same Vt, the organs
in the HS groups showed higher PSs and the small intestine and
kidney in the HS groups showed higher AIs. Lung AI was affected
differently: the control groups had lower lung AIs and the high-Vt
groups also had lower lung AIs than the other Vt groups.
Discussion
The correlation between MV and distal organ injury
has been addressed in a series of studies. Imai et al noted
that MV is associated with distal organ damage in rabbits with lung
injury caused by hydrochloric acid during intra-tracheal
administration (8). The injurious
effects of MV on the extra-pulmonary organs of mice with
experimental S. aureus pneumonia were also demonstrated in
the study by Dhanireddy et al (9). O’Mahony et al demonstrated
non-injurious MV strategies using a conventional Vt of 10 ml/kg.
Interaction with endotoxemia induced via the intra-peritoneal
injection of E. coli LPS in mice enhanced pro-inflammatory
mechanisms in the lungs and promoted extra-pulmonary end-organ
injury (10). A study by Wolthuis
et al further indicated that ventilator-associated lung
injury (VALI) occurs despite the absence of a priming pulmonary
insult and despite the use of non-injurious ventilator settings
(11).
Occasionally, MV is required for hemorrhagic or
septic shock patients in intensive care units, who are susceptible
to acute lung injury (ALI), ARDS and MODS, but do not meet the
criteria for ALI or ARDS. We proposed that despite the lack of
significant evidence of lung injury in such cases, protective MV
should be administered to avoid the development of lung and distal
organ injury. Thus, in the present study, the ventilated rabbit
models of HS and ES were developed.
The results of the current study showed that high Vt
(12–15 ml/kg) may increase the pathological injury of the lung and
distal organs when the lung injury does not meet the criteria for
ALI/ARDS. High Vt is harmful during early resuscitation from HS and
ES.
The initial purpose of the present study was to
determine a suitable ventilation Vt. Our data indicated that MV was
injurious and should be avoided if possible during early
resuscitation from shock. Compared with the ventilated groups, the
control groups for the two types of shock showed slightly less
pulmonary injury and extra-pulmonary organ injury. Data from
Wolthuis relating to non-injurious ventilation (11) and commentary from Ng et al
concerning gene expression changes following ventilation (17) also indicated that ‘non-injurious’
ventilation does not necessarily mean ‘protective’. Our data
indicated that MV was injurious and should be avoided if possible
during early resuscitation from shock.
Reduction of PEEP is the most important ventilation
strategy component affecting hemodynamic stability. Zero or even
negative end-expiratory pressure is safe for HS (18–20),
which is why we used a PEEP of 0 cmH2O during the
resuscitation period. Herff et al reported that reducing the
mean airway pressure by decreasing Vt had less effect than 0
cmH2O PEEP on cardiopulmonary function and survival
(20). The effects of varying Vts
on HS observed in our study were as follows. Given constant blood
pressure and fluid infusion, the HS-C, HS-L, HS-M and HS-H groups
contained zero, five, one and two animals, respectively, that
required treatment with dopamine. The greater need for dopamine in
the HS-L group may be attributed to mixed acidemia (respiratory and
metabolic acidosis). Ventilating with 4–6 ml/kg Vt in HS may cause
circulation instability. The HS-M and HS-L groups had similar lung
and small intestine PSs, but the latter group had a higher renal PS
and AI. Increased dopamine use may aggravate renal injury (21). These results indicated that should
MV be required during early resuscitation from HS, a moderate Vt of
8–10 ml/kg may be relatively safe.
By contrast, differences in Vt seemed to have less
effect on hemodynamic stability during ES, given that the
sub-groups had similar blood pressures and used similar amounts of
dopamine and fluid infusion. The ES-L and ES-M groups showed
similar renal and intestinal PSs and AIs, and the former showed a
lower lung PS. If it is not possible to avoid MV and the noxious
stimuli that lead to shock which are also important triggers for
ARDS, a low Vt of 4–6 ml/kg is encouraged during early
resuscitation from ES.
The different recommended Vts should be based on the
physiological differences between the two types of shock. In HS,
the blood capacity is absolutely deficient whereas in ES, the blood
capacity is relatively deficient. Reperfusion injury during
resuscitation may be more severe in HS (22). Endotoxins may cause more damage in
ES. Studies have shown that endotoxins damage the intercellular
junctions in the lung (23) and
intestine (24,25) and may increase the susceptibility
of lung tissue to VALI (10,26).
The present study reports additional differences between ES and HS,
which hitherto had not been directly compared. In HS, pathological
injury to, and apoptosis in, the lung and small intestine were
slighter, but the renal pathological injury and apoptosis were more
severe than in ES. The required dopamine dosage during ES
resuscitation was also higher than in HS resuscitation. Certain
studies have shown that the use of dopamine may improve alveolar
fluid re-absorption (27,28), intestinal microcirculation and
renal β-2-microglobulin excretion (21), as well as reduce intestinal
inflammation (29). The results of
the current study indicate that the use of dopamine may alleviate
lung and intestinal injury and aggravate renal injury.
The decreased lung apoptosis and increased lung PS
observed in the high-Vt groups of rabbits were in stark contrast
with the effects observed in the kidney and small intestine. The
current data were in agreement with the study by Imai et al,
in which rabbits were exposed to two bouts of acid aspiration and
VALI (8) with the hypothesis that
milder injury may result in a greater degree of apoptosis in the
lungs.
The blood gas data in the current study showed no
significant difference between HS and ES resuscitation using the
same Vt. This result, which may be due to the time of observation,
also indicated that ventilation was an important factor affecting
blood gas during early resuscitation. Further comparative studies
are necessary to better understand and improve the treatment of the
two types of shock.
The blood gas data of the high-Vt groups indicated
excessive ventilation. During intensive care, efforts are made to
improve blood gas, oxygen saturation point and artery oxygen
pressure. However, our study identified no association between
higher artery oxygen pressure and reduced organ injury, reinforcing
the standpoint of certain scholars regarding permissive hypoxia
(30). It may be better to balance
the oxygen supply to the organs against the protection of the
lungs. Extracorporeal CO2 removal and extracorporeal
membrane oxygenation may provide improved rest for the injured
lung. The concept of complete rest is not a new approach, as it has
long been applied in renal and liver replacement therapy, although
the hemodynamic problems in the treatment of shock may prove more
difficult.
The clinical Cochrane study of the effects of
different Vts for the MV of shock patients may be more
conclusive.
High Vt (12–15 ml/kg) was injurious to the lung and
distal organs during HS and ES. MV was not recommended for either
shock type, but when it was necessary for MV to be applied, a low
Vt (4–6 ml/kg) protected the lung during ES. Moderate Vt (8–10
ml/kg) may be relatively safe for use during HS.
Acknowledgements
The authors thank Professor Shi Jingpu
for assistance with statistical analysis, Mr Ma Tingxian for
assistance with light microscopy and apoptotic sample preparation
and measurements and Mrs Guo Yukun for assistance with electronic
microscopy sample preparation and measurements.
References
|
1
|
Giuliani D, Mioni C, Bazzani C, et al:
Selective melanocortin MC4 receptor agonists reverse haemorrhagic
shock and prevent multiple organ damage. Br J Pharmacol.
150:595–603. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Madách K, Aladzsity I, Szilágyi A, et al:
4G/5G polymorphism of PAI-1 gene is associated with multiple organ
dysfunction and septic shock in pneumonia induced severe sepsis:
prospective, observational, genetic study. Crit Care.
14:R792010.PubMed/NCBI
|
|
3
|
Dellinger RP, Levy MM, Carlet JM, et al:
Surviving Sepsis Campaign: international guidelines for management
of severe sepsis and septic shock: 2008. Intensive Care Med.
34:17–60. 2008. View Article : Google Scholar
|
|
4
|
Wheeler AP: Recent developments in the
diagnosis and management of severe sepsis. Chest. 132:1967–1976.
2007. View Article : Google Scholar
|
|
5
|
No authors listed. Ventilation with lower
tidal volumes as compared with traditional tidal volumes for acute
lung injury and the acute respiratory distress syndrome. The Acute
Respiratory Distress Syndrome Network. N Engl J Med. 342:1301–1308.
2000.
|
|
6
|
Parsons PE, Eisner MD, Thompson BT, et al:
Lower tidal volume ventilation and plasma cytokine markers of
inflammation in patients with acute lung injury. Crit Care Med.
33:1–6. 2005.
|
|
7
|
Ragaller M and Richter T: Acute lung
injury and acute respiratory distress syndrome. J Emerg Trauma
Shock. 3:43–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Imai Y, Parodo J, Kajikawa O, et al:
Injurious mechanical ventilation and end-organ epithelial cell
apoptosis and organ dysfunction in an experimental model of acute
respiratory distress syndrome. JAMA. 289:2104–2112. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dhanireddy S, Altemeier WA, Matute-Bello
G, et al: Mechanical ventilation induces inflammation, lung injury,
and extra-pulmonary organ dysfunction in experimental pneumonia.
Lab Invest. 86:790–799. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
O’Mahony DS, Liles WC, Altemeier WA, et
al: Mechanical ventilation interacts with endotoxemia to induce
extrapulmonary organ dysfunction. Crit Care. 10:R1362006.PubMed/NCBI
|
|
11
|
Wolthuis EK, Vlaar AP, Choi G, Roelofs JJ,
Juffermans NP and Schultz MJ: Mechanical ventilation using
non-injurious ventilation settings causes lung injury in the
absence of pre-existing lung injury in healthy mice. Crit Care.
13:R12009. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Douzinas EE, Andrianakis I, Livaditi O, et
al: The level of hypotension during hemorrhagic shock is a major
determinant of the post-resuscitation systemic inflammatory
response: an experimental study. BMC Physiol. 8:152008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brégeon F, Delpierre S, Chetaille B, et
al: Mechanical ventilation affects lung function and cytokine
production in an experimental model of endotoxemia. Anesthesiology.
102:331–339. 2005.PubMed/NCBI
|
|
14
|
Paller MS, Hoidal JR and Ferris TF: Oxygen
free radicals in ischemic acute renal failure in the rat. J Clin
Invest. 74:1156–1164. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chiu CJ, McArdle AH, Brown R, Scott HJ and
Gurd FN: Intestinal mucosal lesions in low flow states. A
morphological, hemodynamic, and metabolic reappraisal. Arch Surg.
101:478–483. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu F, Zhang HY and Liu Z: Effects of
mechanical ventilation with different tidal volumes on small
intestine injury of early resuscitated hemorrhagic and endotoxic
shock rabbits. World Chinese Journal of Digestology. 16:833–838.
2008.(In Chinese).
|
|
17
|
Ng CS, Wan S, Ho AM and Underwood MJ: Gene
expression changes with a ‘non-injurious’ ventilation strategy.
Crit Care. 13:4032009.
|
|
18
|
Krismer AC, Wenzel V, Lindner KH, et al:
Influence of positive end-expiratory pressure ventilation on
survival during severe hemorrhagic shock. Ann Emerg Med.
46:337–342. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Krismer AC, Wenzel V, Lindner KH, et al:
Influence of negative expiratory pressure ventilation on
hemodynamic variables during severe hemorrhagic shock. Crit Care
Med. 34:2175–2181. 2006. View Article : Google Scholar
|
|
20
|
Herff H, Paal P, von Goedecke A, Lindner
KH, Severing AC and Wenzel V: Influence of ventilation strategies
on survival in severe controlled hemorrhagic shock. Crit Care Med.
36:2613–2620. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carcoana OV, Mathew JP, Davis E, et al:
Mannitol and dopamine in patients undergoing cardiopulmonary
bypass: a randomized clinical trial. Anesth Analg. 97:1222–1229.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Legrand M, Mik EG, Johannes T, Payen D and
Ince C: Renal hypoxia and dysoxia after reperfusion of the ischemic
kidney. Mol Med. 14:502–516. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han X, Fink MP, Uchiyama T, Yang R and
Delude RL: Increased iNOS activity is essential for pulmonary
epithelial tight junction dysfunction in endotoxemic mice. Am J
Physiol Lung Cell Mol Physiol. 286:L259–L267. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moriez R, Salvador-Cartier C, Theodorou V,
Fioramonti J, Eutamene H and Bueno L: Myosin light chain kinase is
involved in lipopolysaccharide-induced disruption of colonic
epithelial barrier and bacterial translocation in rats. Am J
Pathol. 167:1071–1079. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han X, Fink MP, Yang R and Delude RL:
Increased iNOS activity is essential for intestinal epithelial
tight junction dysfunction in endotoxemic mice. Shock. 21:261–270.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schreiber T, Hueter L, Schwarzkopf K,
Hohlstein S, Schmidt B and Karzai W: Increased susceptibility to
ventilator-associated lung injury persists after clinical recovery
from experimental endotoxemia. Anesthesiology. 104:133–141. 2006.
View Article : Google Scholar
|
|
27
|
Saldias FJ, Comellas AP, Pesce L, Lecuona
E and Sznajder JI: Dopamine increases lung liquid clearance during
mechanical ventilation. Am J Physiol Lung Cell Mol Physiol.
283:L136–L143. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chamorro-Marín V, García-Delgado M,
Touma-Fernández A, Aguilar-Alonso E and Fernández-Mondejar E:
Intratracheal dopamine attenuates pulmonary edema and improves
survival after ventilator-induced lung injury in rats. Crit Care.
12:R392008.PubMed/NCBI
|
|
29
|
Birnbaum J, Klotz E, Spies CD, et al:
Effects of dopamine on the intestinal microvascular blood flow and
leukocyte activation in a sepsis model in rats. Crit Care.
10:R1172006. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abdelsalam M: Permissive hypoxemia: is it
time to change our approach? Chest. 129:210–211. 2006. View Article : Google Scholar : PubMed/NCBI
|