Introduction
The pathogenesis of myocardial ischemia-reperfusion
injury (MIRI) is complex and the mechanisms involved have not yet
been fully elucidated. In previous years, basic and clinical
research of molecular cardiology have demonstrated that apoptosis
occurs in a number of physiological and pathological processes of
the cardiovascular system (1).
Studies have shown that apoptosis may be one of the pathogenetic
processes of reperfusion injury. The possibility of reducing
apoptosis and the severity of reperfusion injury through
interfering with the expression of apoptosis-related genes has
received considerable attention. Therefore, determining how to
reduce myocardial apoptosis during the MIRI process has become one
of the hot topics in the field of myocardial protection (2,3).
Apoptosis is an inevitable phenomenon and plays an
important role in biological development and the maintenance of
biological balance. It is capable of clearing unnecessary cells,
thus promoting normal body development. When an individual matures,
apoptosis is a mechanism of physiological regulation and
self-protection (4), through which
injured cells are removed to maintain the regeneration of normal
cells. Under physiological conditions, apoptosis is the main cause
of cell death and under pathological conditions, abnormal apoptosis
may lead to a variety of diseases (5). Myocardial apoptosis involves a
variety of genes and proteins, which are controlled by the
anti-apoptosis promoting signal transduction pathway. A deficiency
in the myocardial cell survival factor means the signal
transduction pathway may not be able to be activated or myocardial
cells undergo apoptosis under the stimulation of apoptotic factors
(6).
This study aimed to observe the effects of limb
ischemic preconditioning (LIPC) on myocardial apoptosis-related
protein in MIRI with the specific phosphoinositide 3-kinase (PI3k)
inhibitor LY294002 in vivo.
Materials and methods
Materials
A terminal deoxynucleotidyl transferase deoxyuridine
triphosphate (dUTP) nick end labeling (TUNEL) kit and reverse
transcription-polymerase chain reaction (RT-PCR) kit were purchased
from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.
(Beijing, China). Primers for amplifying β-actin, caspase-3 and B
cell lymphoma 2 (Bcl-2) were synthesized by Beijing Sanbo Yuanzhi
Biotechnology Co., Ltd. (Beijing, China). DNA markers and TRIzol
reagent were also used.
Experimental groups and treatment
A total of 50 Sprague-Dawley (SD) male rats,
weighing 220–280 g, were provided by the Experimental Animal Center
of Henan Province. The rats were divided into five groups (n=10 in
each group): i) sham surgery group: the surgical procedure was
similar to that of the MIRI group, with the exception that the left
anterior descending coronary artery was strung without ligation;
ii) MIRI group: the left anterior descending coronary artery was
ligated for 30 min, followed by perfusion for 120 min; iii) LIPC
group: the femoral artery was continuously blocked at the upper 1/3
section for 5 min, followed by continuous reperfusion for 5 min and
this process was repeated 3 times. The ischemia-reperfusion of the
femoral artery was implemented for 3 days and MIRI was performed on
the 4th day with the same surgical procedure as in the MIRI group.
iv) LY294002 pretreatment group: the rats were pretreated with
LY294002 following myocardial ischemia and 15 min before
reperfusion (injection dose into the femoral vein, 0.3 μg/g
body weight) and v) LY294002+LIPC group: rats were pretreated with
LY294002 following LIPC and 15 min before reperfusion. Following
the treatment of each group, myocardial tissue was immediately
removed from the ischemic center of the hearts of the rats. One
portion of the tissue was placed in sterile pyrogen-free vials and
stored at −78°C and the other portion was fixed with 4%
paraformaldehyde. This study was approved by the ethics committee
of Xinxiang Medical University.
RT-PCR detection
TRIzol reagent was used to extract total RNA from
the myocardial tissue. RT-PCR was performed by two steps: i) a
total of 12 μg total RNA from the myocardial tissue was used
to prepare 20 μl for the reverse transcription system and
the RNA was reverse transcribed to cDNA using oligo (dT) 15 primer
and AMV reverse transcriptase at 72°C for 10 min, 42°C for 1 h and
70°C for 10 min. ii) The cDNA was used as a template to amplify
adenosine diphosphate (ADP) and ADP receptor 1 (ADPR1) with PCR
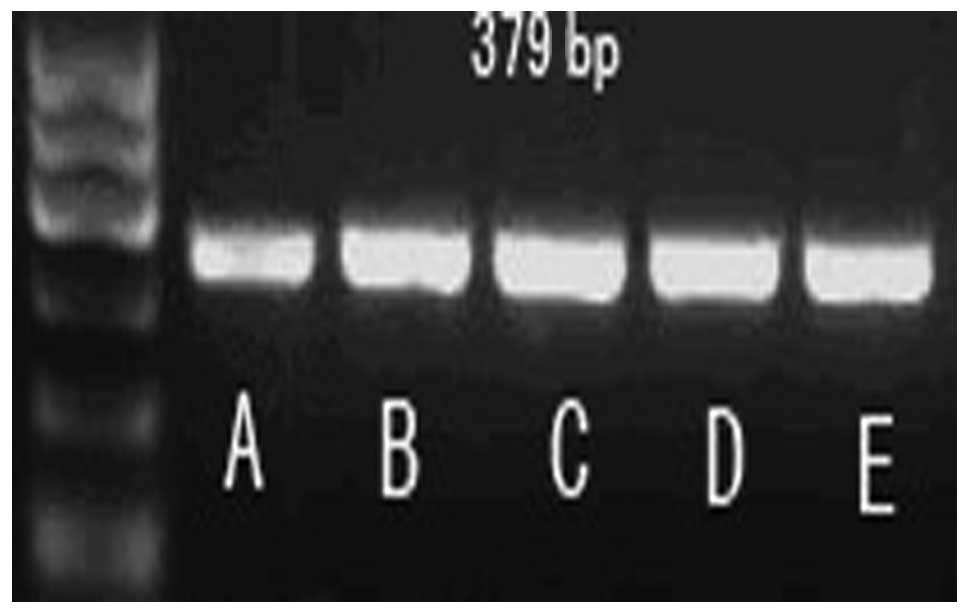
amplification of β-actin as an internal control. β-actin: 379 bp,
forward primer: 5′-CAGTAACAGTCCGCCTAGAA-3′, reverse primer:
5′-GATTACTGCTCTGGCTCCTA-3′ (94°C for 45 sec, 58°C for 45 sec and
72°C for 45 sec, 35 cycles). Bcl-2, 315 bp, forward primer:
5′-CCGCTACCGCCGCGACTTC-3′, reverse primer:
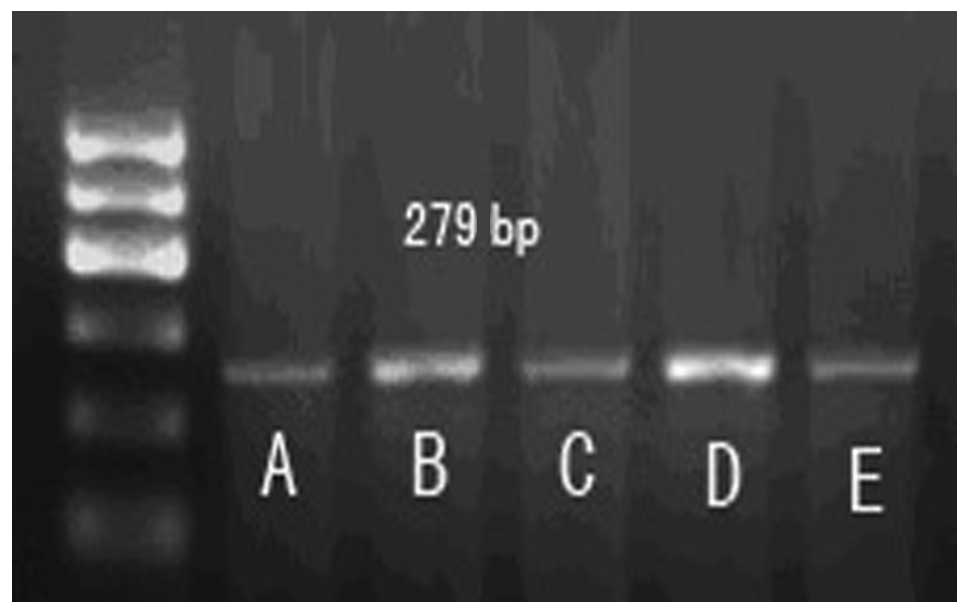
5′-AAACAGAGGCCGCATGCTG-3′. Caspase-3, 279 bp, forward primer:
5′-TGTCATCTCGCTCTGGTACG-3′, reverse primer:
5′-AAATGACCCCTTCATCACCA-3′. PCR products were observed by
ionization on a 1.5% agarose gel and imaged.
In situ TUNEL assay
Experiments were performed according to the
manufacturer’s instructions. Cells were stained with
diaminobenzidine (DAB), counterstained with hematoxylin, dehydrated
with gradient alcohol, cleared with xylene and mounted with neutral
resin. The reaction mixture was replaced with phosphate-buffered
saline (PBS) in the negative control. The nucleus was blue and the
apoptotic nucleus was brownish-black or brown. Four slices from
each rat were observed and five fields (magnification, ×400) on
each slice were counted. The percentage of positive apoptotic
nuclei in the total number of cells per field was calculated and
the mean value was the apoptotic index of the myocardial cells.
Transmission electron microscopy
(TEM)
Myocardial tissues were fixed in 2.5% glutaraldehyde
in PBS and the ultra-microstructure and morphology of the
myocardial tissue were observed and imaged using TEM.
Statistical analysis
SPSS v13.0 (SPSS Inc., Chicago, IL, USA) was used to
perform statistical analysis. Quantitative data were expressed as
mean ± standard deviation. Single factor analysis of variance was
performed to analyze the differences between the groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
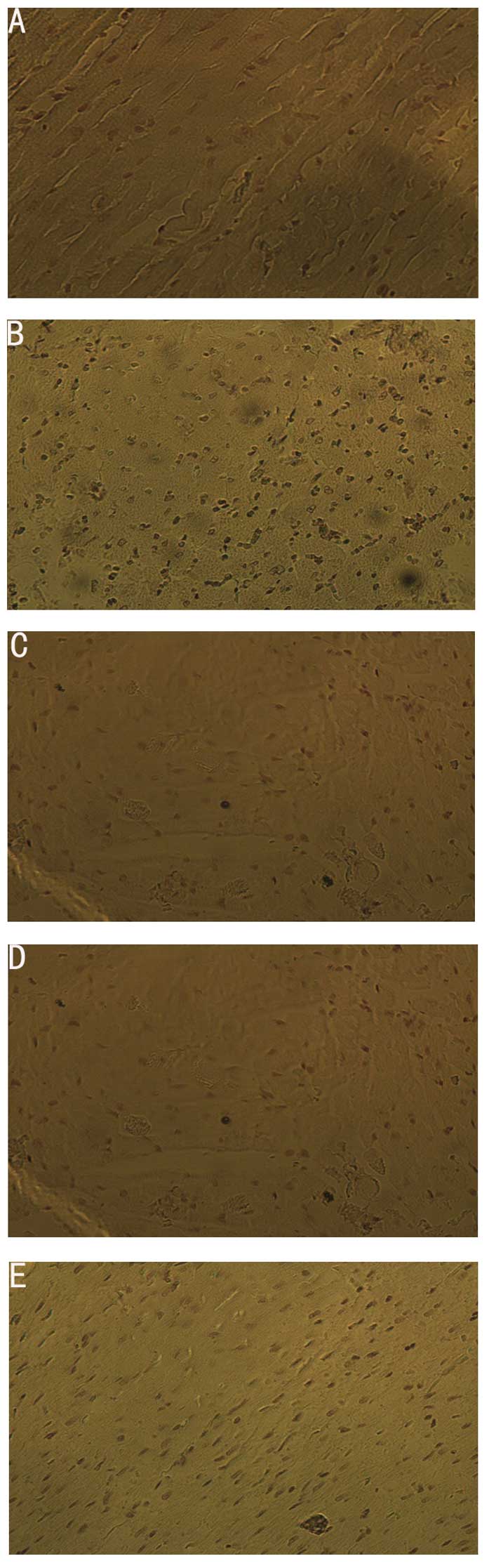
Detection of apoptosis by TUNEL
assay
A brown nucleus represented the occurrence of
apoptosis and the apoptotic index (AI) was calculated. The results
were as follows: apoptosis of myocardial cells in the sham group
was rare; the number of positive apoptotic cells in the MIRI group
increased significantly compared to the sham group (P<0.05); the
number of positive apoptotic cells in the LIPC group decreased
significantly compared to that of the MIRI group (P<0.05); the
number of apoptotic cells in the LY294002 group increased
significantly and the number of apoptotic cells in the
LY290024+LIPC group increased significantly compared to that of the
LIPC group (P<0.05), but was not significantly different from
that of the MIRI group (Table I).
These results indicate that LIPC is capable of reducing
MIRI-induced myocardial apoptosis through the ADP/PI3k/Akt
signaling pathway (Fig. 1).
 | Table IComparison of the index of
cardiomyocyte apoptosis of each group (mean ± SD, n=4). |
Table I
Comparison of the index of
cardiomyocyte apoptosis of each group (mean ± SD, n=4).
| Group | Apoptotic index
(%) |
|---|
| Sham |
2.01±0.44a,b,c |
| MIRI |
18.68±2.81b,c,d |
| LIPC |
8.17±1.93a,c,d,e |
| LY294002 |
23.76±3.77a,b,d,e |
| LY294002+LIPC |
16.10±2.06b,c,d |
Changes of myocardial ultra-micro
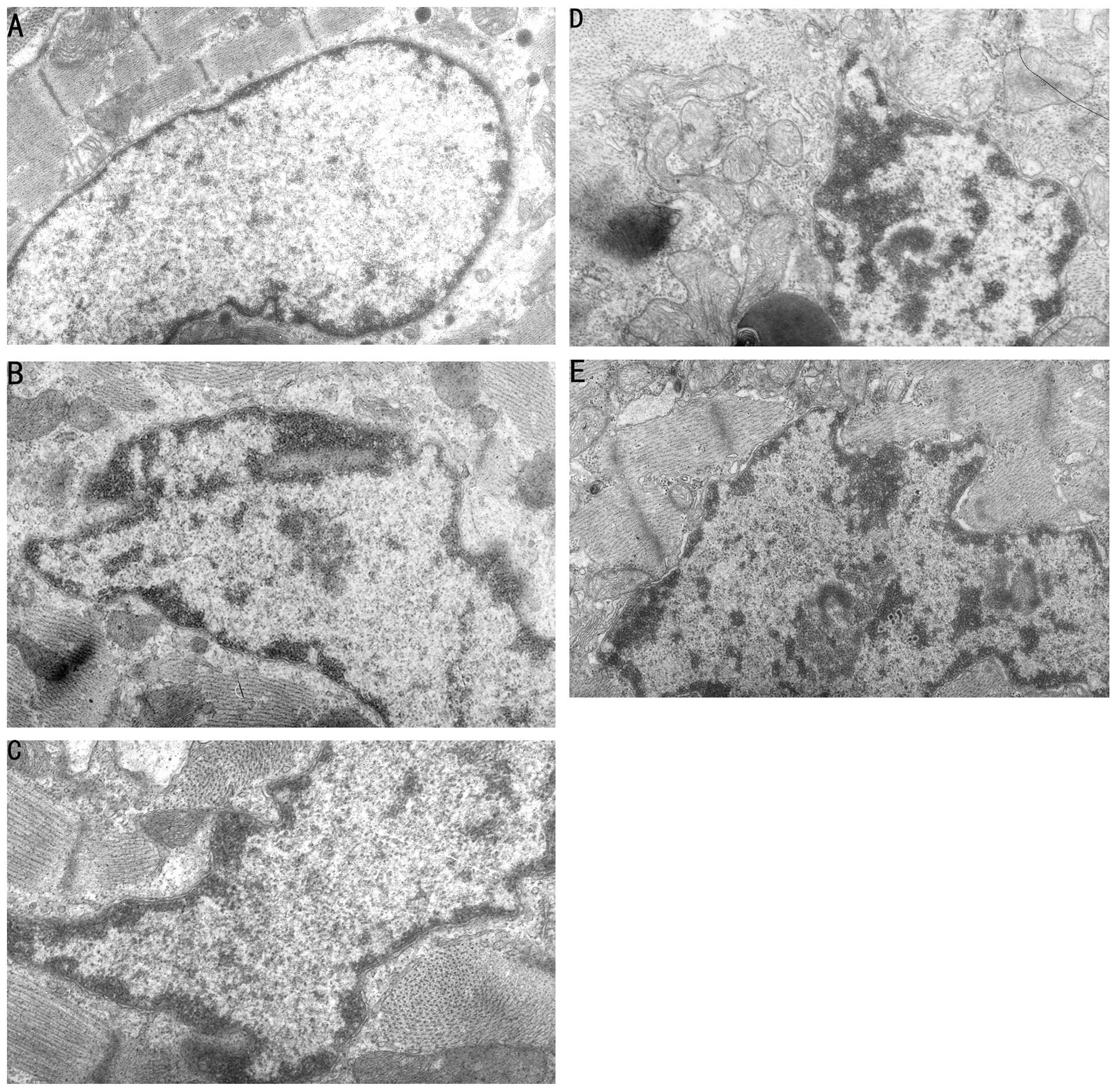
structure under TEM
TEM revealed normal cell structure, a dense
arrangement of myocardial perinuclear myofilaments and developed
intracytoplasmic mitochondria in the sham group. In the MIRI,
LY294002 and LY294002+LIPC groups, TEM revealed damaged myocardial
cell structure, expanded rough endoplasmic reticulum, swollen
mitochondria, faded matrix and unclear crest and increased
heterochromatin with margination, with an appearance of degenerated
and necrotized myocardial cells. In the LIPC group, TEM revealed
mild edema of the myocardial cells, mild disordered arrangement of
perinuclear myofilaments, no evident swelling of mitochondria and
increased heterochromatin without clear margination. The MIRI group
treated with LY294002 and LIPC had significantly reduced
heterochromatin and margination compared to the group treated with
only LY294002 (Fig. 2).
Expression of apoptotic factors,
caspase-3 and Bcl-2, in each group
Compared to the sham group, the expression level of
caspase-3 mRNA in the MIRI group significantly increased
(P<0.05); however, the expression of Bcl-2 mRNA reduced
significantly. Compared to the MIRI group, LIPC decreased the
caspase-3 mRNA expression level (P<0.05) and increased the Bcl-2
mRNA expression level (P<0.05), while LY294002 increased the
caspase-3 mRNA expression level (P>0.05) and reduced the Bcl-2
mRNA expression level (P>0.05). There was no significant
difference in mRNA expression levels of caspase-3 and Bcl-2 between
the LY294002+LIPC and MIRI groups. These results indicate that
LY294002 is capable of reducing the protection effects of LIPC on
the MIRI cardiac muscles of rats by changing the mRNA expression
levels of caspase-3 and Bcl-2 (Table
II, Figs. 3–5).
 | Table IIRelative levels of caspase-3 and bcl-2
mRNA/β-actin in the myocardial tissue of each group (mean ± SD,
n=6). |
Table II
Relative levels of caspase-3 and bcl-2
mRNA/β-actin in the myocardial tissue of each group (mean ± SD,
n=6).
| Group |
Caspase-3/β-actin | bcl-2/β-actin |
|---|
| Sham |
0.31±0.05a,b,c |
0.51±0.04a,b,c,d |
| MIRI |
0.62±0.09d,e |
0.31±0.05d,e |
| LIPC |
0.35±0.07a,c,d |
0.74±0.12a,b,c,e |
| LY294002 |
0.68±0.13c,d,e |
0.24±0.03c,d,e |
| LY294002+LIPC |
0.56±0.11b,d,e |
0.32±0.04b,d,e |
Discussion
A previous study identified that apoptosis plays an
important role in MIRI. Reperfusion injury leads to cell death
through necrosis and apoptosis (7). Laboratory and clinical data have
indicated that, due to the fact that myocardial apoptosis is
relatively severe during the reperfusion period, it contributes
greatly to myocardial infarction and ventricular remodeling
(8). Apoptosis is programmed cell
death and is also the final result of the cell death signal
regulation by stimulating factors. Currently, the most commonly
accepted theory is that apoptosis is co-regulated by pro-apoptotic
genes, which detect signals from pro-apoptotic factors through
signal transduction pathways and anti-apoptotic genes.
The mechanisms of MIRI-induced apoptosis are complex
and the details of the process are not entirely clear. However, one
study demonstrated that the myocardial infarction area is the
location of the integration of apoptosis and necrosis. Zhao et
al(9) identified that the
number of apoptotic cells in the surrounding tissue of the
infarcted myocardium is directly proportional to the reperfusion
time, which further proves the effect of apoptosis on the
myocardial infarction area. It has been proposed that apoptosis is
the major mode of cell death for early myocardial infarction. In an
animal study, decreasing the number of apoptotic cells decreased
the infarct area and improved the systolic function of the heart
(4). Therefore, inhibiting
apoptosis may be used as an effective measure in treating acute
myocardial infarction.
In the present study, LIPC significantly inhibited
reperfusion injury-induced apoptosis. The results demonstrated that
apoptosis was rare in the sham group and cardiomyocyte apoptosis in
the MIRI group increased. The number of apoptotic cells in the LIPC
group was significantly reduced in comparison to that of the MIRI
group and the number of apoptotic cells in the
LY294002-pretreatment group was the largest and was significantly
different from the other groups. These results suggest that
apoptosis plays an important role in MIRI.
LIPC inhibited apoptosis; however, the number of
apoptotic cells in the LY294002-pretreatment group was the largest
and the number of myocardial apoptotic cells in the LY294002+LIPC
co-treatment group was significantly reduced compared to that in
the LY294002-pretreated group. This further indicates that LIPC may
inhibit cardiomyocyte apoptosis through the ADP/PI3k/Akt signal
pathway. Under TEM, we observed that in the MIRI group, myocardial
cell structure was damaged with degeneration and necrosis of
myocardial cells. In the LIPC group, myocardial cells presented
mild edema with mild disordered arrangement of myofilaments
surrounding the nuclei. In the LY294002-pretreatment group,
myocardial cell structural damage was evident, with a large amount
of degenerated and necrotized myocardial cells. We observed that
cell damage in MIRI rats following co-treatment with LY294002 and
LIPC was clearly reduced compared to the group treated with
LY294002 only. This is consistent with the results discussed
above.
Apoptosis, one of the main pathological processes
induced by MIRI, involves a variety of cellular signal transduction
pathways. The Bcl-2 family is a group of important
apoptosis-regulating proteins that are expressed on the
mitochondrial outer membrane, endoplasmic reticulum membrane and
nuclear membrane. Overexpression of Bcl-2 proteins blocks the
pro-apoptosis signal transduction pathway, thereby inhibiting the
activation of caspase-3 and ultimately preventing apoptosis caused
by the caspase cascade (10).
Inhibition of apoptosis by Bcl-2 may be related to
the following mechanisms: i) overexpression of Bcl-2 may decrease
the production of oxygen radicals and the formation of lipid
peroxides; ii) overexpression of Bcl-2 prevents the increase of
mitochondrial permeability and reduces the release of proapoptotic
proteins, thus inhibiting apoptosis; iii) Bcl-2 may inhibit the
Ca2+ transmembrane flow and regulate apoptosis by
regulating intracellular Ca2+ concentration and iv)
Bcl-2 may bind to the apoptotic protease to achieve its
anti-apoptotic effects (11).
It has been suggested that members of the caspase
family are the key factors involved in apoptosis and caspase
activation and overexpression induces apoptosis; therefore, they
are also known as apoptotic proteases. When caspases are
translated, they exist in the cytoplasm in the form of inactive
zymogen. When the cells are stimulated by external physiological or
pathological factors, apoptosis is initiated and the caspases are
activated through a series of cleavage cascading reactions. Due to
cascade enlarging effects and positive feedback, a large number of
proteases are activated instantly, which causes simultaneous and
rapid decomposition of a variety of ‘cell death substrates’,
eventually leading to chromosome breakage, morphological changes of
cells and finally apoptosis (12).
Caspase-3 is an important member of the cysteine
protease family and one of the most important regulating genes in
the apoptotic pathway. It digests cell structure proteins and
directly causes cell apoptosis. Caspase-3 plays an important role
in apoptosis and is considered to be an apoptosis marker enzyme
(13). When the body is stimulated
by internal or external factors, caspase-3 zymogen is cleaved by
various proteases and then activated. Activation of caspase-3 leads
to apoptosis of a variety of cells and a number of
apoptosis-triggering factors ultimately induce apoptosis through
caspase-3-mediated signal transduction pathways. A previous study
reported that caspase-3 is involved in the genesis and development
of MIRI and the formation of apoptotic bodies (14).
In this study, RT-PCR was used to detect the effects
of LIPC on the mRNA expression level of caspase-3 and Bcl-2 in
myocardial cells. The results demonstrated that LIPC decreased the
mRNA expression level of caspase-3 and increased the mRNA
expression level of Bcl-2. However, LY290024 increased the mRNA
expression level of caspase-3 and decreased the expression level of
Bcl-2 mRNA, which was not significantly different from the results
of the MIRI group. Co-treating MIRI rats with LY294002 and LIPC
resulted in significant differences in mRNA expression levels of
caspase-3 and Bcl-2 from the LY294002-only group.
These results indicate that myocardial
ischemia-reperfusion-induced cardiac apoptosis is a result of
increased caspase-3 expression levels and decreased Bcl-2
expression levels. LIPC may reverse the above phenomenon, which
demonstrates that LIPC may play a role in protecting myocardial
cells by increasing the expression level of anti-apoptotic protein,
Bcl-2 and reducing the expression level of pro-apoptotic protein,
caspase-3. The present study demonstrated that at the molecular
level the expression level of apoptosis-related proteins in rat
myocardium following LIPC and before reperfusion may be changed
significantly; therefore, enhancing the ability of LIPC activates
the cardiac survival signaling pathway.
Acknowledgements
This study was supported by funding
projects for the Natural Science Foundation in Henan Province
(grant no. 2012B310016).
References
|
1.
|
Malmberg M, Parkka J, Vahasilta T, et al:
Cardiomyocyte apoptosis after cardioplegic ischemia: comparison to
unprotected regional ischemia-reperfusion. Eur Surg Res. 46:19–25.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Vilahur G, Juan-Babot O, Peña E, et al:
Molecular and cellular mechanisms involved in cardiac remodeling
after acute myocardial infarction. J Mol Cell Cardiol. 50:522–533.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Campisi J: Cellular senescence and
apoptosis: how cellular responses might influence aging phenotypes.
Exp Gerontol. 38:5–11. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Chen Z, Chua CC, Gao J, et al: Prevention
of ischemia/reperfusion induced cardiac apoptosis and injury by
melatonin is independent of glutathione peroxdiase 1. J Pineal Res.
46:235–241. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Wang J and Li J: Activated protein C: a
potential cardioprotective factor against ischemic injury during
ischemia/reperfusion. Am J Transl Res. 151:381–392. 2009.PubMed/NCBI
|
|
6.
|
Wu L, Qiao H, Li Y, et al: Protective
roles of puerarin and Danshensu on acute ischemic myocardial injury
in rats. Phytomedicine. 14:652–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Kawaguchi M, Takahashi M, Hata T, et al:
Inflammasome activation of cardiac fibroblasts is essential for
myocardial ischemia/reperfusion injury. Circulation. 23:594–604.
2011. View Article : Google Scholar
|
|
8.
|
Wang Y, Lau WB, Gao E, et al:
Cardiomyocyte-derived adiponectin is biologically active in
protecting against myocardial ischemia-reperfusion injury. Am J
Physiol Endocrinol Metab. 298:E663–E670. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Zhao L, Wang Y, Min X, et al:
Ischemia-reperfusion injury up-regulates Pim-3 gene expression in
myocardial tissue. J Huazhong Univ Sci Technolog Med Sci.
30:704–708. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Maiuri MC, Criolo A, Tasdemir E, et al:
BH3-only proteins and BH3 mimetics induce autophagy by
competitively disrupting the interaction between Beclin 1 and
Bcl-2/Bcl-X(L). Autophagy. 3:374–376. 2007. View Article : Google Scholar
|
|
11.
|
Baldi A, Abbate A, Bussani R, et al:
Apoptosis and post-infarction left ventricular remodeling. J Mol
Cell Cardiol. 34:165–174. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Suo GJ, Qin J, Zhong CP, et al: Suppressor
of cytokine signaling 1 inhibits apoptosis of islet grafts through
caspase 3 and apoptosis-inducing factor pathways in rats.
Transplant Proc. 42:2658–2661. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Penna C, Perrelli MG, Raimondo S, et al:
Postconditioning induces an antiapoptotic effect and preserves
mitochondrial integrity in isolated rat hearts. Biochim Biophys
Acta. 1787:794–801. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Jin YC, Kim KJ, Kim YM, et al:
Anti-apoptotic effect of magnolol in myocardial ischemia and
reperfusion injury requires extracellular signal-regulated
kinase1/2 pathways in rat in vivo. Exp Biol Med (Maywood).
233:1280–1288. 2008. View Article : Google Scholar : PubMed/NCBI
|