Introduction
Patients with diabetes appear to have a higher risk
of hip and upper extremity fracture compared with non-diabetics
(1). Diabetes mellitus (DM)
influences bone metabolism and contributes to bone loss (2). A histomorphometric study in DM has
described low recruitment of osteoblasts and diminished mineral
apposition rates with no mineralization defects (3). Animal studies have also demonstrated
that bone formation was impaired in a diabetic mouse model during
tibial distraction osteogenesis (4). In a bone-loss mouse model, DM also
decreased osteoclastogenesis, reduced bone formation and enhanced
osteoblast apoptosis (5). The
mechanism of DM-related osteoporosis has yet to be elucidated.
Under hyperglycemia, the unbalanced coupling of bone
resorption and formation in remodeling promotes excess bone
resorption (6). Thus, DM causes a
more persistent response, greater loss of attachment and more
alveolar bone resorption and impaired new bone formation. The key
mechanism of osteoblast-osteoclast coupling imbalance is the
regulation of osteoblast cellular apoptosis (7). Under normal conditions, stress within
the ER triggers an adaptive cellular mechanism known as the
unfolded protein response (UPR) that attempts to return the cell to
homeostasis under hyperglycemia. If hyperglycemia persists and
cellular homeostasis is not restored, the ER stress response
initiates cell death stimuli that lead to ER stress-induced
apoptosis (8). Initiation of ER
stress-induced apoptosis through ER stress response signaling
involves transcriptional activation of the C/EBP-homologous protein
(CHOP) (9,10). CHOP is induced in response to
cellular stress, especially ER stress, and is involved in the ER
stress-induced apoptosis pathway (11). In this study, it was hypothesized
that hyperglycemia may induce CHOP-mediated ER stress apoptosis in
osteoblasts and result in unbalanced coupling of osteoblasts and
osteoclasts and ultimately lead to diabetic osteoporosis.
The aim of the present study was to explore the
pathological changes and the CHOP activity in diabetic rats femurs
and high-glucose cultured osteoblasts.
Materials and methods
Animal model induction and bone mineral
density (BMD) observation
Twenty male Sprague Dawley (SD) rats (purchased from
the Experiment Animal Center of Zhejiang University) were divided
into two groups (n=10) at random. The rats in the diabetic group
were fasted for 10 h and injected intraperitoneally with
streptozotocin (STZ) (Alexis Corporation, Switzerland) 65 mg/kg to
induce diabetes. The other 10 rats formed the control group and
they were fasted for 10 h and injected with 0.9% saline. The rats
with blood glucose >16 mmol/l 48 h after injection were
considered diabetic. All procedures were approved by the Zhejiang
University Institutional Animal Care and Use Committee.
Six weeks after diabetes was induced, all rats were
anesthetized, their thoracic cavities opened and perfused
intracardially with normal saline. Following saline perfusion, the
animals were perfused with 300–400 ml fixative containing 4%
paraformaldehyde in 0.1 mol/l phosphate buffer (pH 7.4). After
perfusion, both femurs were extracted. The BMD of the left femurs
was determined using a small animal radiophotography system
(Faxitron model MX20; Tucson, AZ, USA). The right femurs were used
for hematoxylin and eosin (H&E) staining and
immunohistochemistry assay.
Osteoblast culture and western blot
analysis
Primary osteoblasts were derived from newborn rat
calvaria as previously described (24). Cells were seeded in 6-, 24- or
96-well plates in Dulbecco’s modified Eagle’s medium (DMEM;
Invitrogen, Carlsbad, CA, USA) supplemented with antibiotics
(penicillin 100 U/ml and streptomycin 100 μg/ml) and 15%
fetal bovine serum (FBS) and incubated at 37°C in a humidified
atmosphere of 5% CO2 and 95% air. Cells were used
between the second and the fourth passages. The third passage cells
were divided into a normal medium group and a high-glucose medium
group (concentrations of glucose 100 mmol/l). The osteoblasts of
both groups were cultured for 48 h and washed twice with
phosphate-buffered saline (PBS). Ten wells of osteoblasts for each
group were used for CHOP western blot analysis.
The cells were prepared in lysis buffer and
centrifuged (12,000 × g for 10 min at 4°C) to remove cellular
debris. The protein concentration was determined by the Lowry
method using a Bio-Rad DC protein assay kit (Bio-Rad, Hercules, CA,
USA). The lysates containing equal amounts of protein (50
μg) were resolved using 8–10% SDS-polyacrylamide gel
electrophoresis and transferred onto Millipore (Billerica, MA, USA)
nitrocellulose membranes. The reactions were stopped with a
solution containing 5% skimmed milk in Tris-buffered saline with
0.05% Tween-20 (TBST) for 1 h at room temperature and treated with
CHOP antibodies (1:2,000, Santa Cruz Biotechnology Inc., Santa
Cruz, CA, USA) in TBST overnight at 4°C, washed for 1 h with TBST
and further probed with secondary horseradish peroxidase (HRP;
1:2,000) in TBST for 1 h at room temperature. The immune complexes
were visualized using an enhanced chemiluminescence (ECL) detection
system according to the manufacturer’s protocols. The densitometric
analysis of the bands was assayed using Quantity One (Bio-Rad).
H&E staining and immunohistochemistry
assay
The remaining right femurs were fixed in the same
fixative for 4 h and placed in 30% phosphate-buffered sucrose until
the tissue sank. Sections 12 μm thick were cut on a freezing
microtome through the coronary planes of the proximal femur for
H&E staining and diaminobenzidine (DAB) immunohistochemical
staining.
Femur sections were rinsed in 0.01 M PBS and mounted
onto 0.02% poly-l-lysine-coated slides. The ABC system was used
with DAB as the chromagen. Tissue sections were first washed in PBS
and incubated in 1% bovine serum albumin (BSA) for 30 min. Tissues
were incubated overnight at 4°C in the medium of PBS with CHOP
antibody (1:100) plus 1% BSA. Control sections were incubated in
PBS and 1% BSA. The following day, the sections were incubated in a
biotinylated goat-anti-mouse secondary antibody (diluted 1:200 in
PBS, Boster Biotechnology Company, Wuhan, China) and subsequently
in an avidin-HRP solution. Immunolabeling was visualized with 0.05%
DAB plus 0.3% H2O2 in PBS. The sections were
dehydrated through ethanol and xylene before mounting.
Immunohistochemistry results were analyzed using
CHOP-positive osteoblast in femur per mm2 of two groups
of rats under a Nikon microscope (Nikon E600, Nikon Company, Japan)
at final magnifications of ×400.
Statistical analysis
CHOP-positive cells in each visual field under the
microscope were counted at ×200 magnification. The data represent
the means ± SD. The differences were evaluated by analysis of
two-tailed t-tests. P<0.05 was considered to indicate a
statistically significant result. All computations were performed
using SPSS 15.0 (SPSS Inc., Chicago, IL, USA).
Results
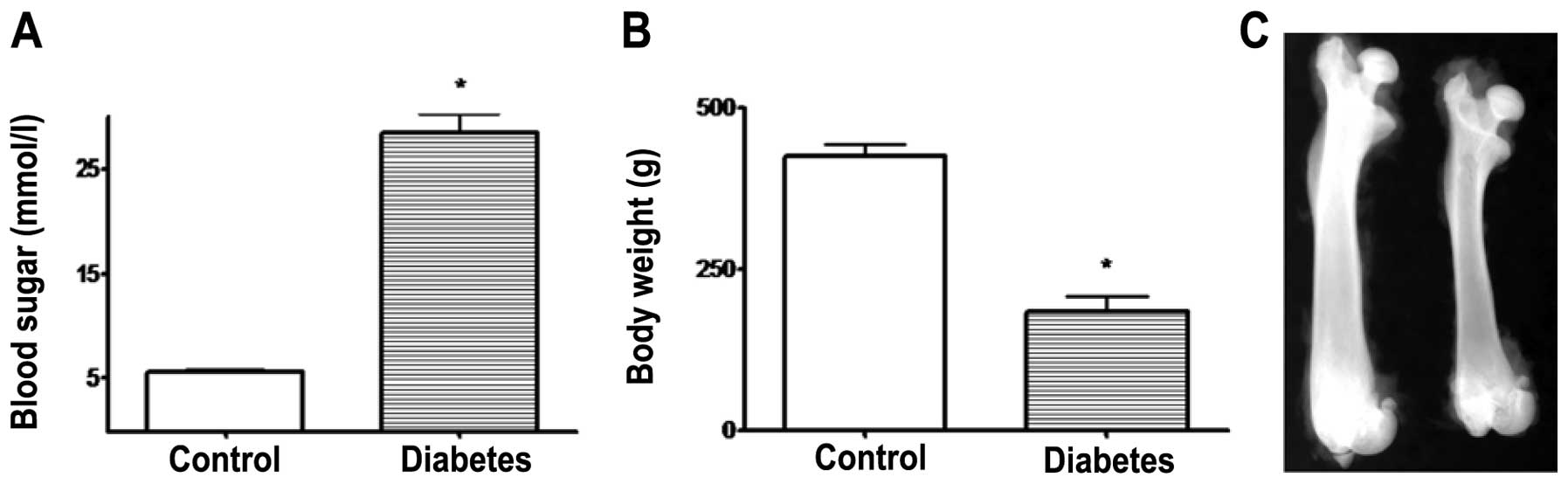
Body weight, blood glucose and proximal
femoral BMD analysis
Initially, the body weight and blood glucose showed
no significant differences (P>0.05) in both groups of rats
before STZ injection. After 6 weeks, the diabetic rats had a
significantly higher blood glucose and lower body weight when
compared with the control group rats (P<0.01). Compared with
non-diabetic control rats, diabetic rats showed a decrease in
femoral BMD (−15.4±2.3% vs. control, P<0.05, Fig. 1).
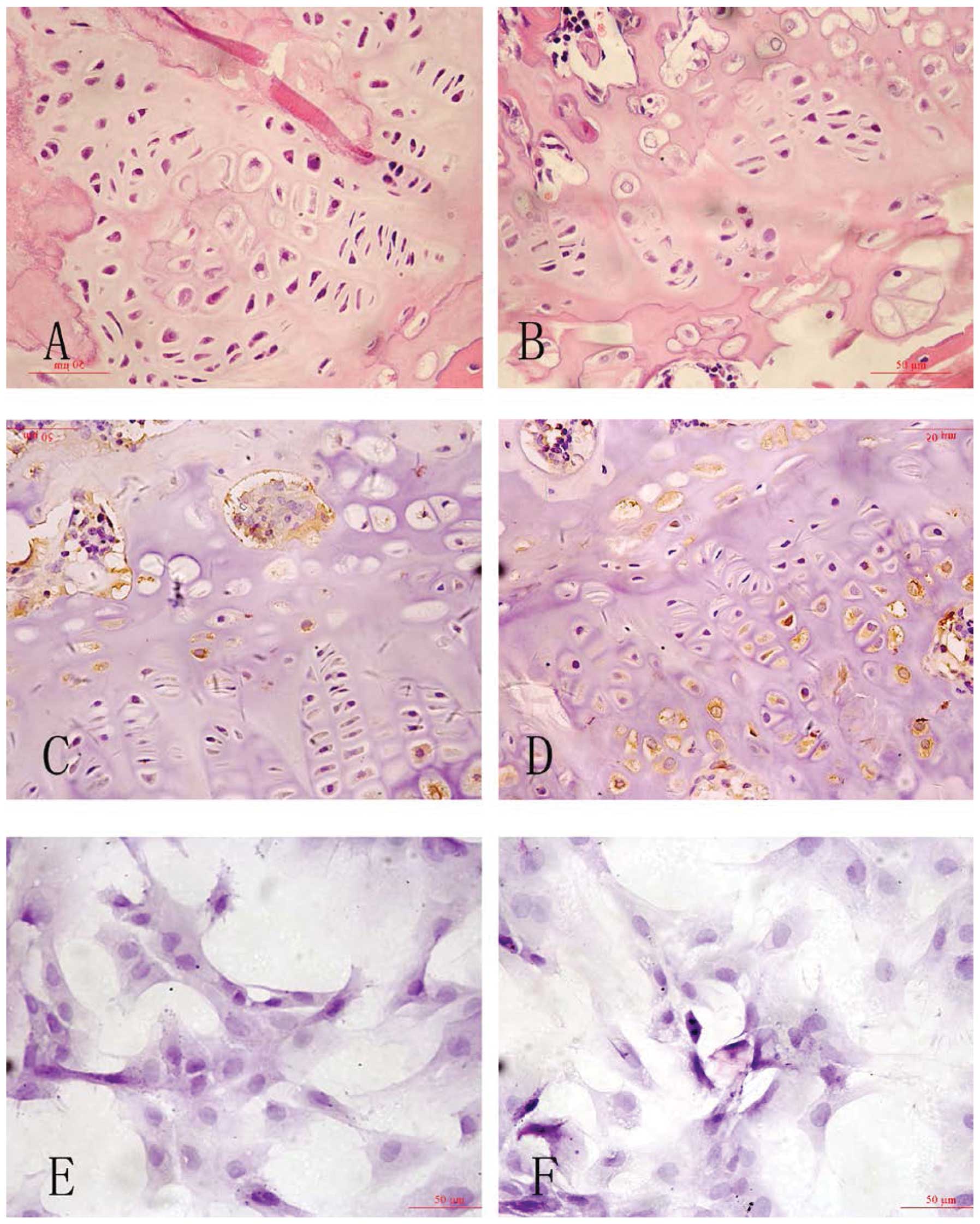
H&E staining and immunohistochemistry
assay
Osteoblasts were identified by a central lucency,
eccentric nucleus and basophilic cytoplasmic stain. Osteoclasts
were identified as single or multi-nucleated cells demonstrating
foamy cytoplasm. Six weeks after inducing diabetes, the proximal
femur H&E staining demonstrated a greater number of osteoclasts
that clumped together, reduced cortical bone and a deteriorated
bone micro-architecture in diabetic rats. The overall growth plate
architecture was dominated by hypertrophic chondrocytes, with fewer
proliferative and chondroblastic cells as compared with control
rats. The femur of control rats showed a normal shape with intact
cartilage, qualitatively equivalent cortical bone and a
well-organized bone matrix composed of trabecular bone compared
with the diabetic rats (Fig.
2).
CHOP immunoreactivity was visualized in a granular
immunostain pattern in the nucleus. Caspase-12 immunohistochemistry
staining positive cells with DAB staining, showed buff-colored
granules in the cytoplasm. Quantitative analysis for the number and
optical density of CHOP-positive cells with DAB immunostaining
showed significant increases in osteoblast cells in diabetic rats
and under high-glucose medium in vitro (P<0.05, Fig. 2 and Table I).
 | Table IComparison of C/EBP-homologous protein
(CHOP)-positive cells and optical density in both groups (mean ±
SD). |
Table I
Comparison of C/EBP-homologous protein
(CHOP)-positive cells and optical density in both groups (mean ±
SD).
| Group | No. of positive
cells | Optical density |
|---|
| Control rats |
8.3±2.1/mm2 | 102.4±12.1 |
| Diabetic rats |
25.4±7.6/mm2a | 180.3±17.4a |
| Osteoblasts in normal
medium |
3.9±1.2/mm2 | 112.1±13.8 |
| Osteoblasts in
high-glucose medium |
43.7±11.4/mm2b | 176.1±15.3b |
The number of CHOP-positive cells and the optical
density was significantly increased in the rats of the diabetic
group (P<0.05). Similarly, the number of positive cells and the
optical density was upregulated in the osteoblasts of high-glucose
medium compared with that of the normal medium group
(P<0.05).
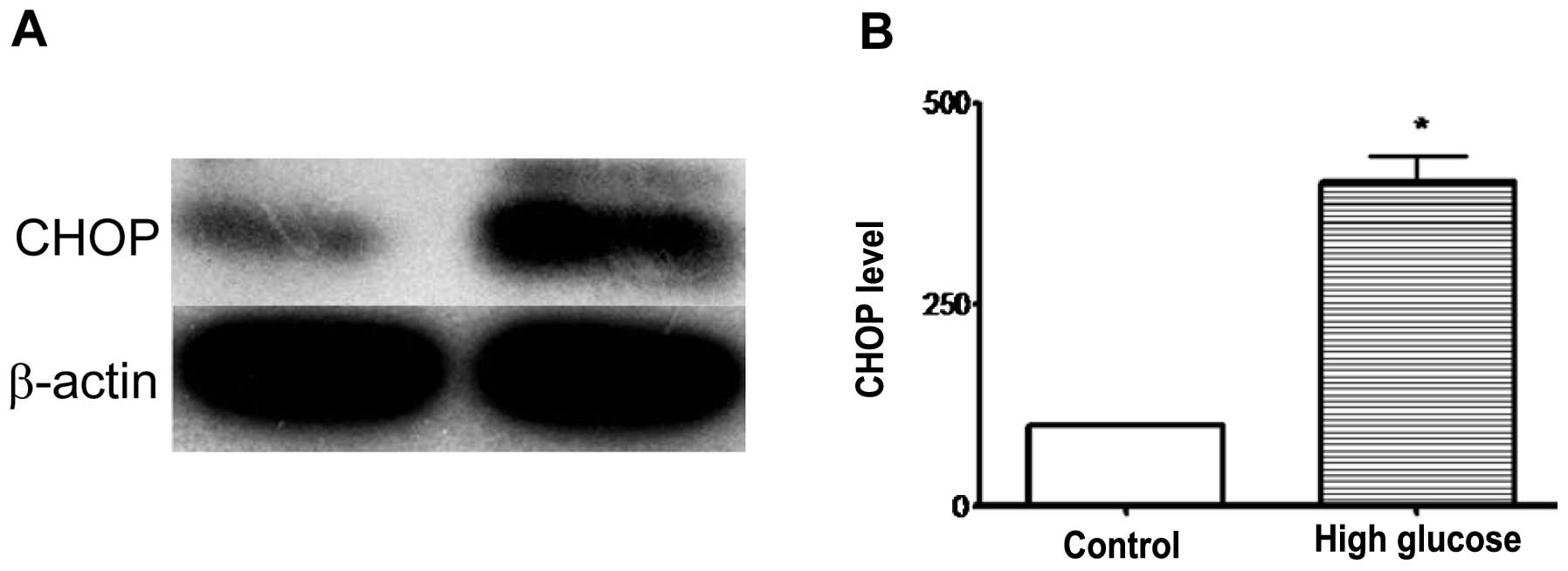
Western blot analysis
CHOP proteins in osteoblast cells were detected as
single bands migrating ∼27 kDa. The densitometric analysis of the
bands for CHOP revealed a significant increase in relative protein
content (451.4±32.6%) in high-glucose medium osteoblasts when
compared with those from the normal medium group (100.00%;
P<0.05, Fig. 3). This suggested
that CHOP was activated in osteoblasts under high-glucose
medium.
Discussion
DM is a group of pandemic debilitating metabolic
diseases featuring chronic hyperglycemia which results from
defective insulin secretion and/or insulin actions. Such chronic
hyperglycemia typically elicits dysfunction and failure of various
organs, particularly the eyes, kidneys, heart and nerves (12–15).
In addition, DM has been found to be associated with metabolic bone
diseases, osteoporosis and low-impact fractures (16). Patients with diabetes are at
greater risk of fractures due to their low BMD. Lower BMD is
explained by insulinopenia and hyperglycemia, which impair bone
formation (17). Despite the
discrepancies between BMD and fracture rates, clinical trials
uniformly support the fact that new bone formation and bone
microarchitecture and bone quality are altered in both types of
diabetes (18,19). In the present study, STZ-treated
rats showed a decrease in femoral BMD compared with normal control
rats. The femur of diabetic rats showed reduced cortical bone,
deteriorated bone micro-architecture, a decrease in the trabecular
width in the epiphysis and metaphysis and increased apoptosis of
osteoblasts when compared with the control rats.
ER is the organelle where secretory and membrane
proteins are synthesized and folded. In certain severe situations
of ER stress, however, the protective mechanisms activated by the
UPR are not sufficient to restore normal ER function and cells die
by apoptosis (20). Hyperglycemia
accumulates the misfolded proteins and induces alterations in
Ca2+ homeostasis (21).
The UPR in osteoblasts alleviate ER stress by upregulation of
chaperones (such as BiP/GRP78) and degradation of misfolded
proteins (22). However, under
persistent hyperglycemia conditions, the UPR triggers apoptotic
cell death (23). Microarray
studies revealed that CHOP is one of the most highly inducible
genes during ER stress and increases during ER stress during
apoptosis (24). Overexpression of
CHOP and microinjection of CHOP protein have been reported to play
a key role in apoptosis by regulating the expression of
proapoptotic proteins (25).
Besides the possible mechanism that overexpression of CHOP in the
bone microenvironment may impair the function of osteoblasts
leading to osteopenia (26), CHOP
may act as a dominant-negative inhibitor of C/EBP and prevent
osteoblast differentiation (27).
In the current diabetic osteoporosis rat model, diabetes
significantly elevated the expression of CHOP in osteoblast cells.
CHOP promotes osteoblast reversal of translational repression
caused by the UPR to facilitate progress of apoptosis in
vivo and in vitro. It may be hypothesized, therefore,
that high blood sugar status, contributing to the high expression
of CHOP, facilitates the progress of apoptosis, which upsets the
osteoblast-osteoclast balance, leading to bone disorders and the
development of diabetic osteoporosis. The relationship between CHOP
expression and osteoclasts remains to be fully elucidated.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (No. 30971124) and
Zhejiang Provincial Natural Science Foundation of China (No.
Y2090120).
References
|
1.
|
Norris R and Parker M: Diabetes mellitus
and hip fracture: A study of 5966 cases. Injury. 42:1313–1316.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Villafán-Bernal JR, Sánchez-Enríquez S and
Muñoz-Valle JF: Molecular modulation of osteocalcin and its
relevance in diabetes (Review). Int J Mol Med. 28:283–293.
2011.PubMed/NCBI
|
|
3.
|
Aubia J, Serrano S, Mariñoso L, Hojman L,
Diez A, Lloveras J and Masramon J: Osteodystrophy of diabetics in
chronic dialysis: a histomorphometric study. Calcif Tissue Int.
42:297–301. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
He H, Liu R, Desta T, Leone C, Gerstenfeld
LC and Graves DT: Diabetes causes decreased osteoclastogenesis,
reduced bone formation, and enhanced apoptosis of osteoblastic
cells in bacteria stimulated bone loss. Endocrinology. 145:447–452.
2004. View Article : Google Scholar
|
|
5.
|
Lecka-Czernik B: Bone loss in diabetes:
use of antidiabetic thiazolidinediones and secondary osteoporosis.
Curr Osteoporos Rep. 8:178–184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Liu R, Bal HS, Desta T, et al: Diabetes
enhances periodontal bone loss through enhanced resorption and
diminished bone formation. J Dent Res. 85:510–514. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Martin TJ and Seeman E: Bone remodelling:
its local regulation and the emergence of bone fragility. Best
Pract Res Clin Endocrinol Metab. 22:701–722. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Van der Kallen CJ, van Greevenbroek MM,
Stehouwer CD and Schalkwijk CG: Endoplasmic reticulum
stress-induced apoptosis in the development of diabetes: is there a
role for adipose tissue and liver? Apoptosis. 14:1424–1434.
2009.PubMed/NCBI
|
|
9.
|
Endo M, Oyadomari S, Suga M, Mori M and
Gotoh T: The ER stress pathway involving CHOP is activated in the
lungs of LPS-treated mice. J Biochem. 138:501–507. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Zinszner H, Kuroda M, Wang X, et al: CHOP
is implicated in programmed cell death in response to impaired
function of the endoplasmic reticulum. Genes Dev. 12:982–995. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Liu G, Sun Y, Li Z, Song T, Wang H, Zhang
Y and Ge Z: Apoptosis induced by endoplasmic reticulum stress
involved in diabetic kidney disease. Biochem Biophys Res Commun.
370:651–656. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Xie XW, Xu L, Jonas JB and Wang YX:
Prevalence of diabetic retinopathy among subjects with known
diabetes in China: the Beijing Eye Study. Eur J Ophthalmol.
19:91–99. 2009.PubMed/NCBI
|
|
13.
|
Senior PA: Diabetic nephropathy, chronic
kidney disease and metabolic syndrome in Type 2 diabetes: answers
or more questions? Diabet Med. 25:1377–1379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Zhang XM, Shen F, Xv ZY, Yan ZY and Han S:
Expression changes of thrombospondin-1 and neuropeptide Y in
myocardium of STZ-induced rats. Int J Cardiol. 105:192–197. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Zhou J, Wang L, Ling S and Zhang X:
Expression changes of growth-associated protein-43 (GAP-43) and
mitogen-activated protein kinase phosphatase-1 (MKP-1) and in
hippocampus of streptozotocin-induced diabetic cognitive impairment
rats. Exp Neurol. 206:201–208. 2007. View Article : Google Scholar
|
|
16.
|
Kennedy RL, Henry J, Chapman AJ, Nayar R,
Grant P and Morris AD: Accidents in patients with insulin-treated
diabetes: increased risk of low-impact falls but not motor vehicle
crashes - a prospective register-based study. J Trauma. 52:660–666.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Follak N, Kloting I and Merk H: Influence
of diabetic metabolic state on fracture healing in spontaneously
diabetic rats. Diabetes Metab Res Rev. 21:288–296. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Abbassy MA, Watari I and Soma K: The
effect of diabetes mellitus on rat mandibular bone formation and
microarchitecture. Eur J Oral Sci. 118:364–369. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Yamaguchi T, Yamamoto M, Kanazawa I, et
al: Quantitative ultrasound and vertebral fractures in patients
with type 2 diabetes. J Bone Miner Metab. 29:626–632. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Rasheva VI and Domingos PM: Cellular
responses to endoplasmic reticulum stress and apoptosis. Apoptosis.
14:996–1007. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Wang S and Kaufman RJ: The impact of the
unfolded protein response on human disease. J Cell Biol.
197:857–867. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Schröder M: Endoplasmic reticulum stress
responses. Cell Mol Life Sci. 65:862–894. 2008.
|
|
23.
|
Karunakaran U, Kim HJ, Kim JY and Lee IK:
Guards and culprits in the endoplasmic reticulum: glucolipotoxicity
and beta-cell failure in type II diabetes. Exp Diabetes Res.
2012:6397622012. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Cheng WP, Hung HF, Wang BW and Shyu KG:
The molecular regulation of GADD153 in apoptosis of cultured
vascular smooth muscle cells by cyclic mechanical stretch.
Cardiovasc Res. 77:551–559. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Zhang X, He D, Xu L and Ling S: Protective
effect of tanshinone IIA on rat kidneys during hypothermic
preservation. Mol Med Report. 5:405–409. 2012.PubMed/NCBI
|
|
26.
|
Shirakawa K, Maeda S, Gotoh T, et al:
CCAAT/enhancer-binding protein homologous protein (CHOP) regulates
osteoblast differentiation. Mol Cell Biol. 26:6105–6116. 2006.
View Article : Google Scholar
|
|
27.
|
Pereira RC, Stadmeyer LE, Smith DL, et al:
CCAAT/Enhancer-binding protein homologous protein (CHOP) decreases
bone formation and causes osteopenia. Bone. 40:619–626. 2007.
View Article : Google Scholar : PubMed/NCBI
|