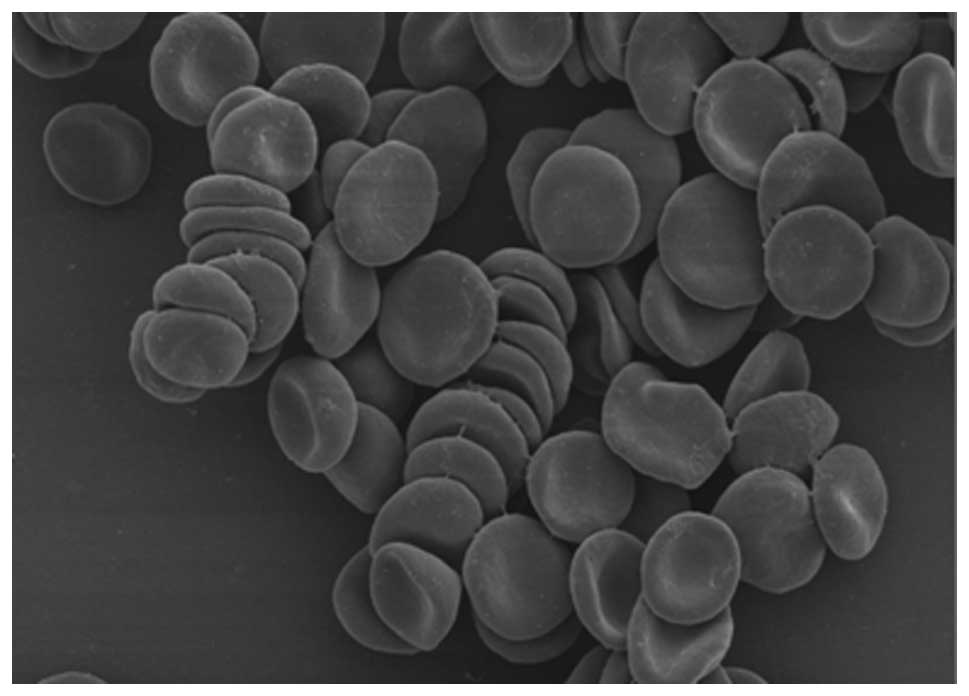
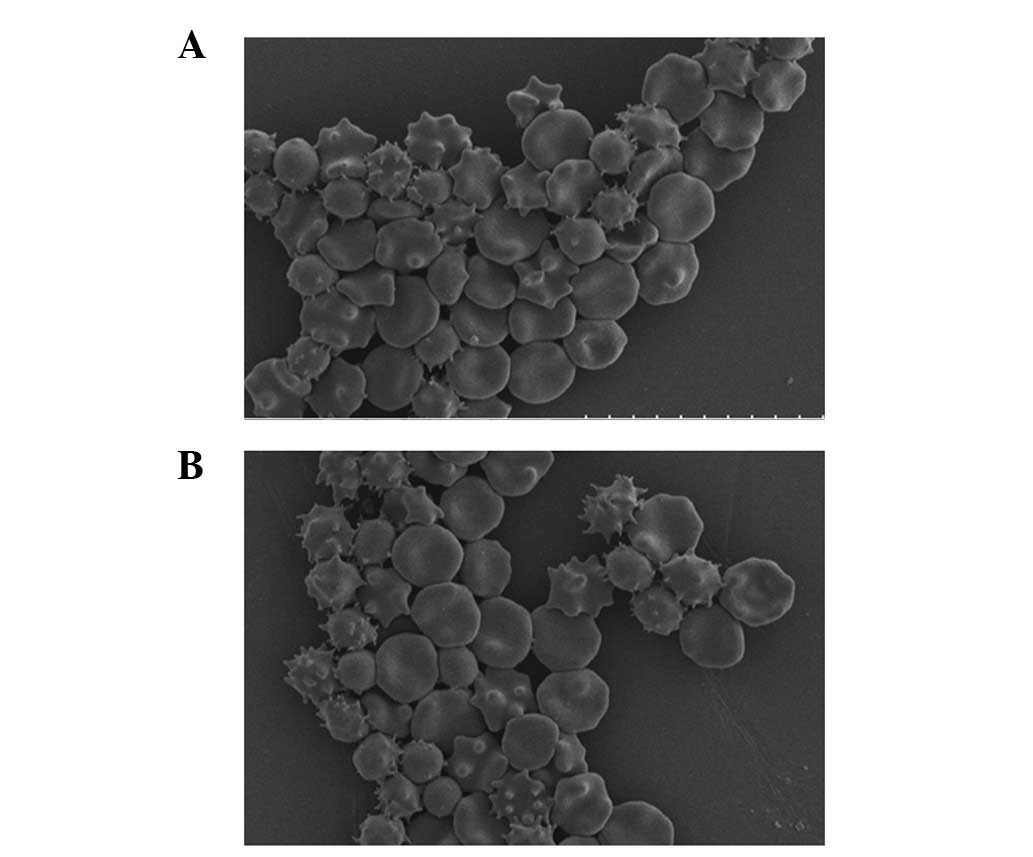
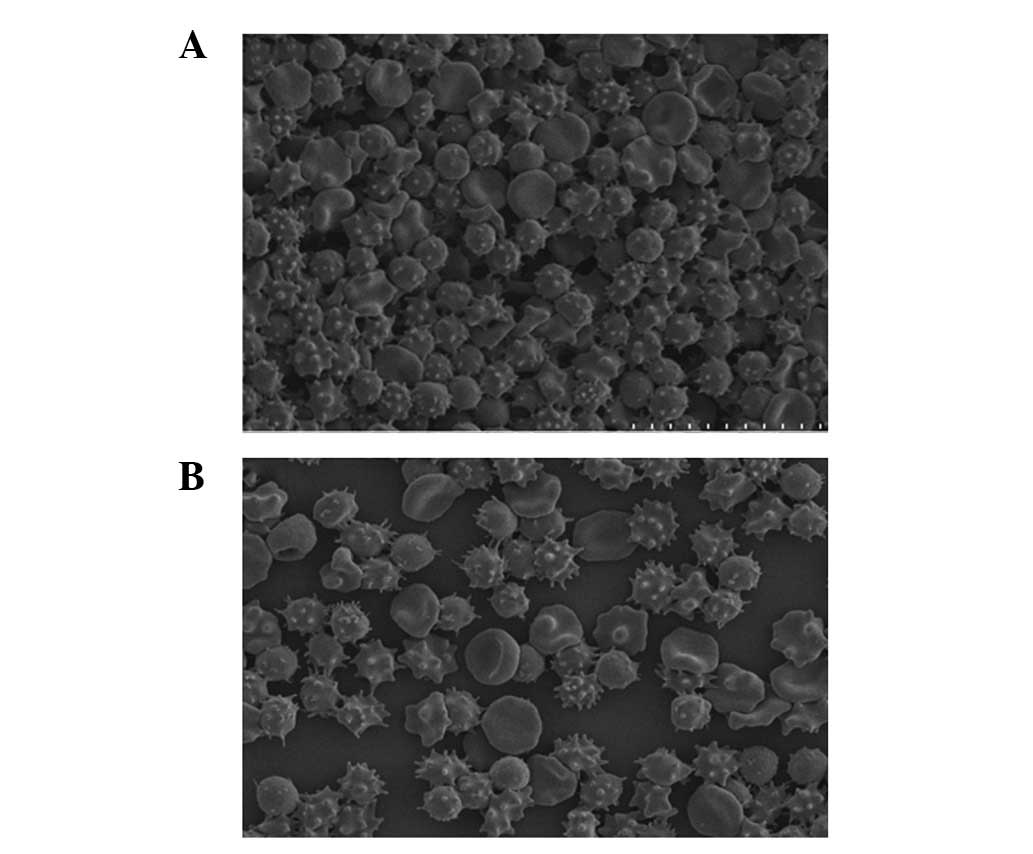
Spandidos Publications style
Yu Y, Ma C, Feng Q, Chen X, Guan X, Zhang X, Chen L, Lin Z, Pan J, Zhang T, Zhang T, et al: Establishment and performance assessment of preparation technology of internal quality control products for blood transfusion compatibility testing. Exp Ther Med 5: 1466-1470, 2013.
APA
Yu, Y., Ma, C., Feng, Q., Chen, X., Guan, X., Zhang, X. ... Wang, D. (2013). Establishment and performance assessment of preparation technology of internal quality control products for blood transfusion compatibility testing. Experimental and Therapeutic Medicine, 5, 1466-1470. https://doi.org/10.3892/etm.2013.994
MLA
Yu, Y., Ma, C., Feng, Q., Chen, X., Guan, X., Zhang, X., Chen, L., Lin, Z., Pan, J., Zhang, T., Luo, Q., Wang, D."Establishment and performance assessment of preparation technology of internal quality control products for blood transfusion compatibility testing". Experimental and Therapeutic Medicine 5.5 (2013): 1466-1470.
Chicago
Yu, Y., Ma, C., Feng, Q., Chen, X., Guan, X., Zhang, X., Chen, L., Lin, Z., Pan, J., Zhang, T., Luo, Q., Wang, D."Establishment and performance assessment of preparation technology of internal quality control products for blood transfusion compatibility testing". Experimental and Therapeutic Medicine 5, no. 5 (2013): 1466-1470. https://doi.org/10.3892/etm.2013.994