Introduction
There are >15 million fractures treated in the
United States annually and many more worldwide (1). While the vast majority of these
fractures heal with appropriate orthopedic management, 10–15% of
patients suffer complications that result in delayed- or non-union
(2). Fracture healing is a
multistage repair process that involves complex yet
well-established steps that are initiated in response to injury,
resulting in the repair and restoration of function (3). Numerous factors have been associated
with failure of normal fracture healing, including the fracture
location, the extent of soft tissue damage and interposition, the
degree of bone loss in anatomic criteria, infection, inadequate
reduction, poor stabilization/fixation factors that are exacerbated
by treatment, patient characteristics, comorbidities and drug use
(2).
Fracture repair involves the pathway of normal
embryonic development, which consists of several cell types
originating from the cortex, periosteum, surrounding soft tissue
and bone marrow space (4,5). Different biological factors, which
include recruitment, proliferation and differentiation of cell
types, vascular regeneration, expression of growth factors (e.g.
IGF, TGF-β and BMP) and appropriate biomechanical conditions, have
been considered to be critical for the healing of bone fractures.
Local imbalances of these different factors during conservative or
operative fracture treatment may lead to delay of fracture healing
or to fracture non-union (6).
According to radiological and histological criteria, non-unions are
generally classified into three types (7). Hypertrophic non-unions are often
linked with insufficient fracture stability and appear to have an
adequate blood, oxygen and nutrient supply, while atrophic
non-unions are generally poorly vascularized (7). In defect non-unions, the fracture
healing is affected by a lack of contact among fracture fragments
(6).
Although clinical experience in the treatment of
fracture non-unions is quite extensive, studies concerning the
high-throughput screening and function identification of
differential gene expression associated with fracture non-union are
limited. The objective of this study was to document the feature
genes and their interacting genes, also further explore their
potential functions associated with non-union fractures.
Materials and methods
Affymetrix microarray data
The gene chip GSE494 was downloaded from the gene
expression database Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) and is based on
three platforms: GPL92, [HG_U95B] Affymetrix Human Genome U95B
Array; GPL93, [HG_U95C] Affymetrix Human Genome U95C Array; and
GPL8300, [HG_U95Av2] Affymetrix Human Genome U95 Version 2 Array.
There were data for 12 bone samples of fractures in total in the
three platforms, with each platform containing data for two normal
healing fracture samples and two non-union fracture samples. All
the original files and the platform probe annotation information
files were also downloaded
Data preprocessing and gene differences
analysis
The original data were preprocessed using the R
language Affy software package (8,9). The
R language limma package (http://www.r-project.org/) was used to analyze for
differentially expressed genes between all the normal and non-union
samples (10), and Bayesian
methods were used to conduct multiple testing correction. The
threshold values were set as P<0.05 and | logFC |>1.
Predicting the interactions of
differentially expressed genes
A single gene is not able to regulate function; only
protein-protein interactions (PPIs) have been marked as the main
actors for all of the processes taking place in a cell and
therefore great efforts have been focused towards understanding
their biological function (11).
Hence, the software HitPredict (http://hintdb.hgc.jp/htp/) was used to analyze the
interactions between the differentially expressed genes (12,13).
The HitPredict database was built by collecting information from
the IntAct BIOGRID and HPRD databases through high-throughput or
small-scale experiments of protein interaction associations and,
according to the interaction score, estimating protein interactions
(the interaction score is obtained according to a likelihood
algorithm which uses a Bayesian network combining binding sequence,
structure and functional annotations of the PPI in the calculation)
(13). HitPredict collects PPI
data from high-throughput, small-scale experiments, and considers a
likelihood ratio of the resulting score of >1 as a high degree
of confidence interaction (12).
It has collected 239,584 PPIs from nine species, including humans
and mice, of which 168,458 are predicted to have a high degree of
confidence. The high degree of confidence interactions from the
database were used in this study (experimental and likelihood ratio
>1) to analyze the differential gene product.
Enrichment analysis of genes
Differentially expressing genes were screened using
enrichment analysis based on the hypergeometric distribution
algorithm of FuncAssociate (14).
The threshold value of P<0.05 was selected.
Analysis for pathways involving genes in
the interaction network
Proteins in a PPI network and the same module
usually complete the same biological processes and functions by
co-expression. In the present study, enrichment analysis using
WebGestalt (15,16), which is based on the hypergeometric
distribution algorithm, was used to analyze the pathway of
interaction networks involving the differentially expressed gene
and its interactions (P<0.05).
Results
Screening for differentially expressed
genes
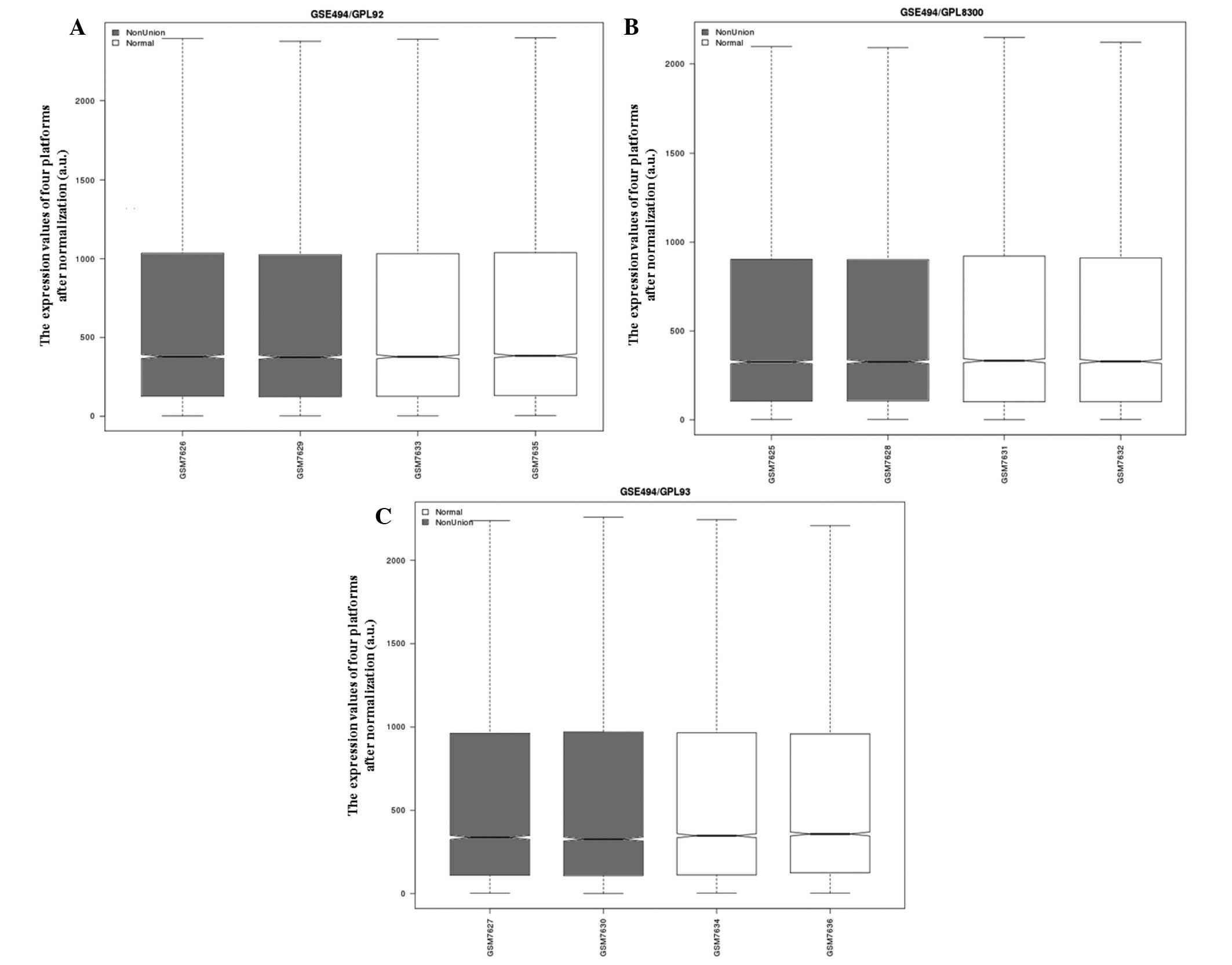
The differences between the normalized expression
data were compared following data preprocessing (Fig. 1). A total of 531, 418 and 914 genes
from the three platforms that met the difference threshold
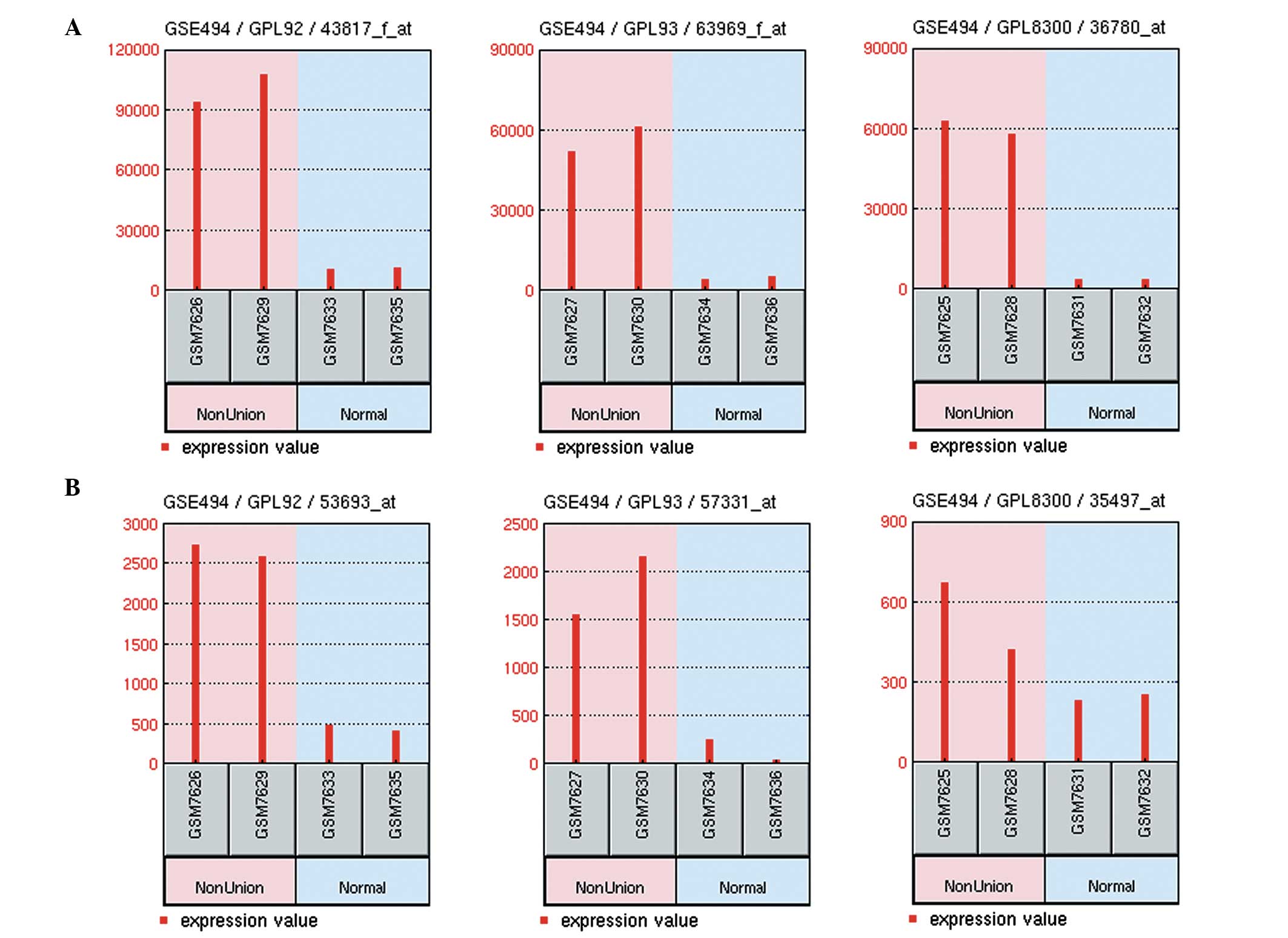
(P<0.05 and | logFC | >1) were screened. Two genes, CLU and
TSPAN2, were identified to be the commonly differentially expressed
genes in the three platforms. The expression values of these two
genes were upregulated in the delayed healing fracture sample
(Fig. 2).
Predicting the interactions of the
differentially expressed genes
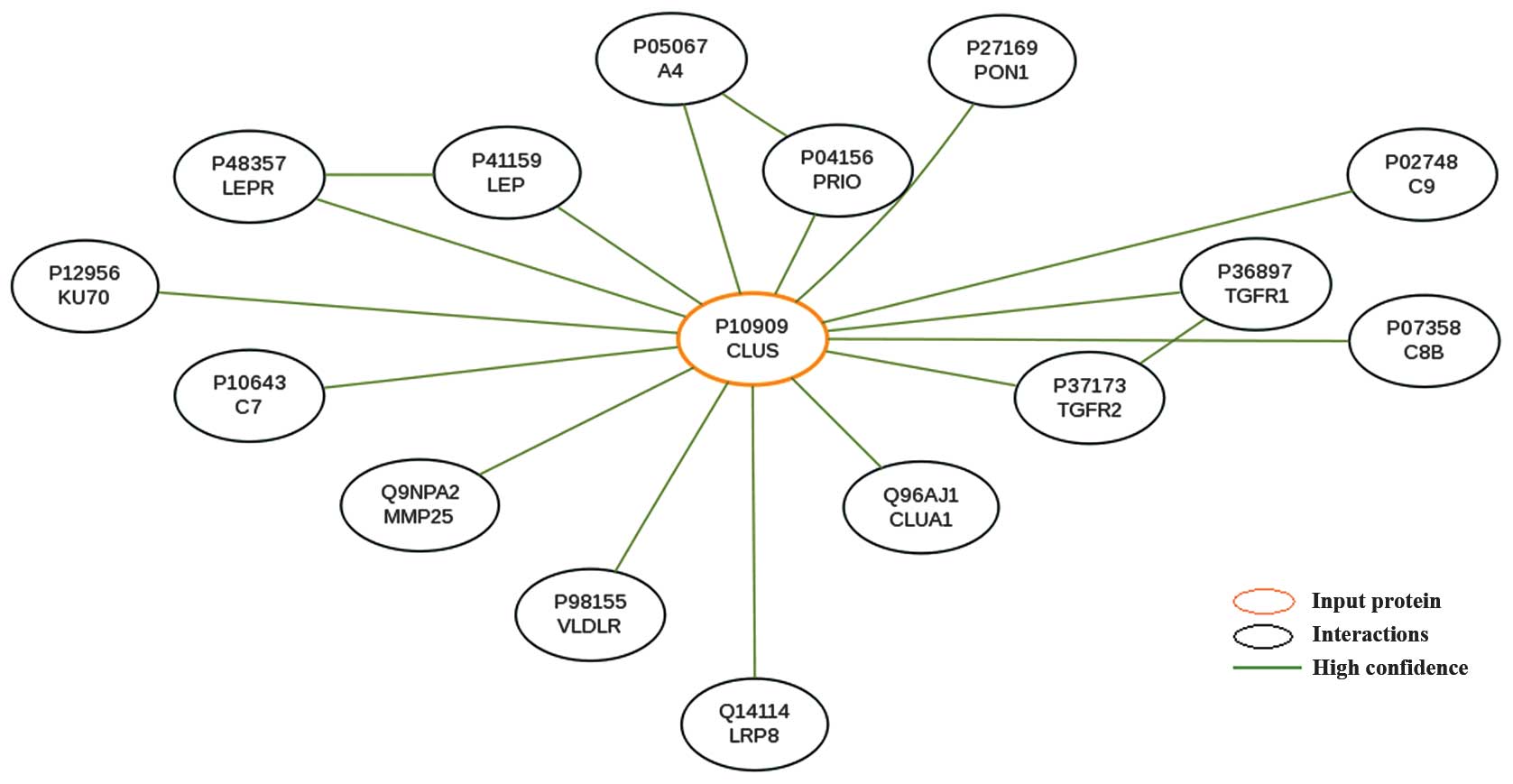
Using the software HitPredict to screen for all
differentially expressed genes and their product interactions, the
gene CLU and 44 interaction objects were obtained (Fig. 3; there were no interaction records
of TSPAN2 recorded in HitPredict). The interaction objects of CLU
and their likelihood of interaction scores are listed in Table I.
 | Table IGenes interacting with CLU. |
Table I
Genes interacting with CLU.
| Interactors | Likelihood of
interaction |
|---|
| TGFR2 | 999 |
| KU70 | 999 |
| TGFR1 | 999 |
| PON1 | 999 |
| PRIO | 999 |
| VLDLR | 999 |
| C7 | 999 |
| LRP8 | 999 |
| MMP25 | 999 |
| A4 | 999 |
| C8B | 999 |
| LEPR | 999 |
| CLUA1 | 999 |
| C9 | 999 |
| LEP | 999 |
| COMD1 | 999 |
| LRP2 | 8.68 |
| DISC1 | 3.37 |
| TNIK | 3.37 |
| APOA1 | 3.37 |
| GRB2 | 3.37 |
| H2AX | 3.37 |
| NR4A1 | 3.37 |
| FOS | 3.37 |
| MDM2 | 3.37 |
| GCR | 3.37 |
| PPARG | 3.37 |
| ZNF24 | 3.37 |
| B2CL1 | 3.37 |
| MK09 | 3.37 |
| RL23 | 3.37 |
| BAT3 | 3.37 |
| CYP2E1 | 3.37 |
| RBBP8 | 3.37 |
| KLF11 | 3.37 |
| KEAP1 | 3.37 |
| T22D4 | 3.37 |
| SYVN1 | 3.37 |
| UBC | 3.37 |
| NFKB1 | 3.37 |
| IKBA | 3.37 |
| CUL1 | 3.37 |
| FBW1A | 3.37 |
| RAD21 | 3.37 |
Enrichment analysis of network genes
Enrichment analysis based on the hypergeometric
distribution algorithm was conducted using FuncAssociate. A
threshold value of P<0.05 was selected. As presented in Table II, five significantly enriched
features were obtained. The most significantly enriched genes in
the network were associated with sterol transport.
 | Table IIList of functions associated with
enriched genes in the network. |
Table II
List of functions associated with
enriched genes in the network.
| ID | Term | P-value | Genes |
|---|
| GO:0032371 | Regulation of
sterol transport | 0.00000703 | LEP, APOA1, PON1,
NFKB1, CLU |
| GO:0044421 | Extracellular
region part | 0.0000341 | LEP, C8B, APOA1,
LEPR, CLU, PON1, LRP8, LRP2, MMP25, VLDLR |
| GO:0005576 | Extracellular
region | 0.000481 | LEP, C8B, C7,
APOA1, C9, LEPR, CLU, PON1, LRP8, LRP2, MMP25, VLDLR |
| GO:0043233 | Organelle
lumen | 0.004086798 | FOS, APOA1, SYVN1,
CLU, UBC, NR4A1, MDM2, NFKB1, KEAP1, CUL1 |
| GO:0031974 | Membrane-enclosed
lumen | 0.004673635 | FOS, APOA1, SYVN1,
CLU, UBC, NR4A1, MDM2, NFKB1, KEAP1, CUL1 |
Analyzing gene functions in the
co-expression interaction network
By using WebGestalt, which is based on the
hypergeometric distribution algorithm, to analyze the pathway of
the interaction network involving the differentially expressed gene
and its interactors (threshold value P<0.05), four pathways with
significantly enriched genes were identified (Table III). One of the most significant
pathways was osteoclast differentiation, which involved the SYVN1,
MDM2, KEAP1 and CLU genes.
 | Table IIIList of pathways associated with
enriched genes in the network. |
Table III
List of pathways associated with
enriched genes in the network.
| ID | Term | P-value | Genes |
|---|
| hsa04380 | Osteoclast
differentiation | 0.011605668 | SYVN1, MDM2, KEAP1,
CUL |
| hsa04920 | Adipocytokine
signaling pathway | 0.022842413 | LEP, LEPR,
NFKB1 |
| hsa04610 | Complement and
coagulation cascades | 0.024136338 | C8B, C7, C9 |
| hsa04662 | B cell receptor
signaling pathway | 0.028195492 | FOS, GRB2,
NFKB1 |
Discussion
Non-union of a fracture is defined as the cessation
of all reparative processes of healing without bone-union (17). As previous data has demonstrated
that the diagnosis of non-union fractures is based on clinical
symptoms and physical findings, including pain at the fracture site
and evidence of pathologic motion (18), there are seldom studies on the
high-throughput screening and function identification of
differential gene expression in fracture non-unions. In the present
study, the upregulated gene CLU and its 44 interaction objects were
selected from a microarray chip composed of normal union and
non-union skeletal fracture samples. According to the function of
CLU and its interacting genes, the conclusion was reached that by
inhibiting the normal healing process following a fracture, the
selected genes regulated the healing of non-union skeletal
fractures through participating in sterol transport and the pathway
involved in the differentiation of osteoclasts.
CLU (also known as clusterin, apolipoprotein J,
TRPM-2 and SGP-2) is highly conserved in different species, with
approximately 70–80% protein homology in mammals with other
species. CLU consists of a 449-amino-acid primary polypeptide
chain. By its disulfide bridges, human CLU is cleaved into α and β
chains (19). CLU expression is
low in normal conditions but is induced by stress stimuli,
suggesting that its function may be directly or indirectly
associated with the stress response (20). In a number of studies, CLU has been
demonstrated to be antiapoptotic, protecting cells against a
variety of death signals (21–23).
Although there have been no direct studies indicating that CLU is
associated with fracture healing, a recent study has demonstrated
that the mRNA levels of CLU are increased in early osteoarthritic
articular (OA) cartilage, while they are decreased in advanced OA
(24).IL-1α-stimulated cartilage
explants have been demonstrated to produce decreased levels of CLU
compared with those in untreated cartilage (25) and treatment with IL-1β also
decreases the levels of CLU (26).
Synovial apoptosis inhibitor 1 (SYVN1), also known as DER3 and
HRD1, is an E3 ubiquitin ligase that is implicated in endoplasmic
reticulum-associated degradation (27). It is cloned from rheumatoid
synovial cells and is highly expressed in the synoviocytes of
patients with rheumatoid arthritis (RA). Through its antiapoptotic
effect, SYVN1 promotes the overproliferation of synoviocytes
(28,29). Murine double minute 2 (mdm2) was
first identified as the gene responsible for the spontaneous
transformation of 3T3 cells (30).
As an E3 ubiquitin ligase, mdm2 is a critical negative regulator of
p53 by targeting it for ubiquitination and proteasomal degradation
(31). Individuals carrying the
mdm2 SNP309 T/G or G/G have been identified to exhibit a
significantly earlier age of onset for osteosarcoma (32). In RA patients, the frequencies of
the mdm2 SNP309 are significantly reduced (33), while the mdm2 SNP 309G/G is
associated with higher levels of apoptotic activity in RA-derived
synoviocytes (34). Kelch-like ECH
associated protein 1 (Keap1) is a stress sensor and an adaptor
component of Cullin 3-based E3 ubiquitin ligase (35). Under normal (unstressed)
conditions, Keap1 activates and rapidly degrades Nrf2 through the
proteasome pathway. Upon cellular exposure, as an E3 ubiquitin
ligase component, Keap1 is inhibited, which provokes Nrf2
stabilization (36). A study has
reported that the Nrf2-Keap1 signaling cascade is conserved in
human skeletal muscle (37).
Fracture healing is a complex process that involves
osteoblasts, osteoclasts and a variety of other cells and cytokines
(38), which means it may be the
outcome of the interaction of multiple genes. Although there have
been only indirect studies that have indicating that CLU and its
interacting genes are involved in healing fractures, there is
significant evidence that they participate in the healing process.
In conclusion, the generally stronger inhibition of osteoblasts in
non-union fractures (39) combined
with the results of the present study indicating that CLU and its
interacting genes SYVN1, MDM2 and KEAP1 participate in the
osteoclast differentiation pathway suggest that all the genes which
were identified by screening may regulate the healing of fractures
through an involvement in osteoclast differentiation.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. C05030324).
Reference
|
1
|
Arthritis and related conditions. United
States Bone and Joint Decade: The Burden of Musculoskeletal
Diseases in the United States. 1st edition. American Academy of
Orthopaedic Surgeons; Rosemont, IL: pp. 99–161. 2008
|
|
2
|
Einhorn TA: The cell and molecular biology
of fracture healing. Clin Orthop Relat Res. 355(Suppl): S7–S21.
1998. View Article : Google Scholar
|
|
3
|
Ai-Aql ZS, Alagl AS, Graves DT,
Gerstenfeld LC and Einhorn TA: Molecular mechanisms controlling
bone formation during fracture healing and distraction
osteogenesis. J Dent Res. 87:107–118. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gerstenfeld LC, Cullinane DM, Barnes GL,
Graves DT and Einhorn TA: Fracture healing as a post-natal
developmental process: molecular, spatial, and temporal aspects of
its regulation. J Cell Biochem. 88:873–884. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferguson C, Alpern E, Miclau T and Helms
JA: Does adult fracture repair recapitulate embryonic skeletal
formation? Mech Dev. 87:57–66. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hofmann A, Ritz U, Hessmann MH, et al:
Cell viability, osteoblast differentiation, and gene expression are
altered in human osteoblasts from hypertrophic fracture non-unions.
Bone. 42:894–906. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Megas P: Classification of non-union.
Injury. 36(Suppl 4): S30–S37. 2005. View Article : Google Scholar
|
|
8
|
Troyanskaya O, Cantor M, Sherlock G, et
al: Missing value estimation methods for DNA microarrays.
Bioinformatics. 17:520–525. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujita A, Sato JR, de Rodrigues LO,
Ferreira CE and Sogayar MC: Evaluating different methods of
microarray data normalization. BMC Bioinformatics. 7:4692006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smyth GK: Limma: Linear models for
microarray data. Bioinformatics and Computational Biology Solution
Using R and Bioconductor. Statistics for Biology and Health.
Gentleman R, Carey VJ, Huber W, Irizarry RA and Dudoit S: Springer;
New York, NY: pp. 397–420. 2005, View Article : Google Scholar
|
|
11
|
Tong AH, Drees B, Nardelli G, et al: A
combined experimental and computational strategy to define protein
interaction networks for peptide recognition modules. Science.
295:321–324. 2002. View Article : Google Scholar
|
|
12
|
Patil A and Nakamura H: Filtering
high-throughput protein-protein interaction data using a
combination of genomic features. BMC Bioinformatics. 6:1002005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Patil A, Nakai K and Nakamura H:
HitPredict: a database of quality assessed protein-protein
interactions in nine species. Nucleic Acids Res. 39:D744–D749.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Berriz GF, Beaver JE, Cenik C, Tasan M and
Roth FP: Next generation software for functional trend analysis.
Bioinformatics. 25:3043–3044. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang B, Kirov S and Snoddy J: WebGestalt:
an integrated system for exploring gene sets in various biological
contexts. Nucleic Acids Res. 33:W741–W748. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duncan D, Prodduturi N and Zhang B:
WebGestalt2: an updated and expanded version of the Web-based Gene
Set Analysis Toolkit. BMC Bioinformatics. 11(Suppl 4): P102010.
View Article : Google Scholar
|
|
17
|
Cleveland KB: Delayed union and nonunion
of fractures. Campbell’s Operative Orthopaedics. Crenshaw AH: 3.
7th edition. CV Mosby; St. Louis, MO: pp. 2053–2118. 1987
|
|
18
|
McKee MD: Aseptic non-union. AO Principles
of Fracture Management. Rüedi TP and Murphy WM: 1st edition. Georg
Thieme Verlag; Stuttgart and New York: pp. 748–762. 2000
|
|
19
|
Jones SE and Jomary C: Clusterin. Int J
Biochem Cell Biol. 34:427–431. 2002. View Article : Google Scholar
|
|
20
|
McLaughlin L, Zhu G, Mistry M, et al:
Apolipoprotein J/clusterin limits the severity of murine autoimmune
myocarditis. J Clin Invest. 106:1105–1113. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chaiwatanasirikul K and Sala A: The
tumour-suppressive function of CLU is explained by its localisation
and interaction with HSP60. Cell Death Dis. 2:e2192011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Trougakso IP, So A, Jansen B, Gleave ME
and Gonos ES: Silencing expression of the clusterin/apolipoprotein
J gene in human cancer cells using small interfering RNA induces
spontaneous apoptosis, reduced growth ability, and cell
sensitization to genotoxic and oxidative stress. Cancer Res.
64:1834–1842. 2004. View Article : Google Scholar
|
|
23
|
Zellweger T, Chi K, Miyake H, Adomat H,
Kiyama S, Skov K and Gleave ME: Enhanced radiation sensitivity in
prostate cancer by inhibition of the cell survival protein
clusterin. Clin Cancer Res. 8:3276–3284. 2002.PubMed/NCBI
|
|
24
|
Connor JR, Kumar S, Sathe G, et al:
Clusterin expression in adult human normal and osteoarthritic
articular cartilage. Osteoarthritis Cartilage. 9:727–737. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wilson R, Belluoccio D, Little CB, Fosang
AJ and Bateman JF: Proteomic characterization of mouse cartilage
degradation in vitro. Arthritis Rheum. 58:3120–3131. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Clutterbuck AL, Smith JR, Allaway D,
Harris P, Liddell S and Mobasheri A: High throughput proteomic
analysis of the secretome in an explant model of articular
cartilage inflammation. J Proteomics. 74:704–715. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamasaki S, Yagishita N, Sasaki T, et al:
Cytoplasmic destruction of p53 by the endoplasmic
reticulum-resident ubiquitin ligase ‘Synoviolin’. EMBO J.
26:113–122. 2007.PubMed/NCBI
|
|
28
|
Amano T, Yamasaki S, Yagishita N, et al:
Synoviolin/Hrd1, an E3 ubiquitin ligase, as a novel pathogenic
factor for arthropathy. Genes Dev. 17:2436–2449. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsuchimochi K, Yagishita N, Yamasaki S, et
al: Identification of a crucial site for synoviolin expression. Mol
Cell Biol. 25:7344–7356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cahilly-Snyder L, Yang-Feng T, Francke U
and George DL: Molecular analysis and chromosomal mapping of
amplified genes isolated from a transformed mouse 3T3 cell line.
Somat Cell Mol Genet. 13:235–244. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Harris SL and Levine AJ: The p53 pathway:
positive and negative feedback loops. Oncogene. 24:2899–2908. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bond GL, Hu W, Bond EE, et al: A single
nucleotide polymorphism in the MDM2 promoter attenuates the p53
tumor suppressor pathway and accelerates tumor formation in humans.
Cell. 119:591–602. 2004. View Article : Google Scholar
|
|
33
|
Assmann G, Voswinkel J, Mueller M, et al:
Association of rheumatoid arthritis with Mdm2 SNP309 and genetic
evidence for an allele-specific interaction between MDM2 and p53
P72R variants: a case control study. Clin Exp Rheumatol.
27:615–619. 2009.PubMed/NCBI
|
|
34
|
Heyne K, Huwer J, Zimmer V, Pfreundschuh
M, Ney JT and Assmann G: Different apoptotic responses of RA
synoviocytes depending on different genotypes of the mdm2 SNP
T309G. Apoptosis. 17:424–427. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kobayashi A, Kang MI, Okawa H, et al:
Oxidative stress sensor Keap1 functions as an adaptor for
Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2.
Mol Cell Biol. 24:7130–7139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Seibenhener ML, Geetha T and Wooten MW:
Sequestosome 1/p62 - more than just a scaffold. FEBS Lett.
581:175–179. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Safdar A, deBeer J and Tarnopolsky MA:
Dysfunctional Nrf2-Keap1 redox signaling in skeletal muscle of the
sedentary old. Free Radic Biol Med. 49:1487–1493. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lories RJ, Daans M, Derese I, et al:
Noggin haploinsufficiency differentially affects tissue responses
in destructive and remodeling arthritis. Arthritis Rheum.
54:1736–1746. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kloen P, Lauzier D and Hamdy RC:
Co-expression of BMPs and BMP-inhibitors in human fractures and
non-unions. Bone. 51:59–68. 2012. View Article : Google Scholar : PubMed/NCBI
|