Introduction
Gefitinib, an epidermal growth factor receptor
(EGFR) type 1 tyrosine kinase inhibitor, blocks the signal
transduction pathway implicated in the proliferation and survival
of cancer cells (1). The use of
gefitinib to treat patients with advanced non-small-cell-lung
cancer (NSCLC) has raised significant concern among physicians.
Gefitinib is better-tolerated and less toxic than conventional
cytotoxic drugs; however, gefitinib-induced interstitial lung
disease (ILD) has been demonstrated to be a serious adverse effect
(2). Phase II studies showed that
the objective response rates achieved after gefitinib treatment
were between 10 and 20%, with minimal toxicities; mainly an
acne-like rash and mild diarrhea, in patients with recurrent
non-small cell lung cancer (the IDEAL trials) (3). In subsequent phase III trials, the
addition of gefitinib to standard platinum-based chemotherapy
failed to demonstrate a survival advantage in patients with
untreated non-small cell lung cancer (the INTACT trials) (4,5). The
worldwide incidence of ILD was approximately 1% (2% in the Japanese
post-marketing experience and approximately 0.3% in a United States
expanded access program). The median time to onset of ILD was 24
days in the Japan group and 42 days in the United States group.
Approximately one-third of all ILD cases caused by gefitinib have
been fatal (6). In this study, a
case of gefitinib-induced interstitial pneumonia is described and
previous case reports between 2003 and 2011 are reviewed, with the
aims of summarizing the clinical features, mechanisms and treatment
strategies of gefitinib-induced interstitial pneumonia and
providing a reference for medication safety in the clinical
treatment of NSCLC.
Case report
A 62-year-old man, complaining of fever and
shortness of breath >1 year subsequent to surgery for
right-sided NSCLC, was admitted to the First Affiliated Hospital of
Xi’an Jiaotong University (Xi’an, China). In July 2010, the patient
was revealed to have a lesion in the right upper lobe of the lung
in a physical examination. In August 2010, upper right lobe
resection and mediastinal lymph node dissections were performed.
The tumor measured 3.5×3.0×2.0 cm. The pathological analysis of the
resected upper right lung showed that the patient was suffering
from differentiated adenocarcinoma and bronchioloalveolar
carcinoma. The lung membrane, bronchial stump and lung hilar lymph
nodes were not invaded (stage IB, pT2aN0M0). Following surgery, the
patient did not receive any further treatment. In June 2011, the
patient started to cough with obvious incentive; white phlegm was
apparent, accompanied by intermittent bloody sputum. A chest
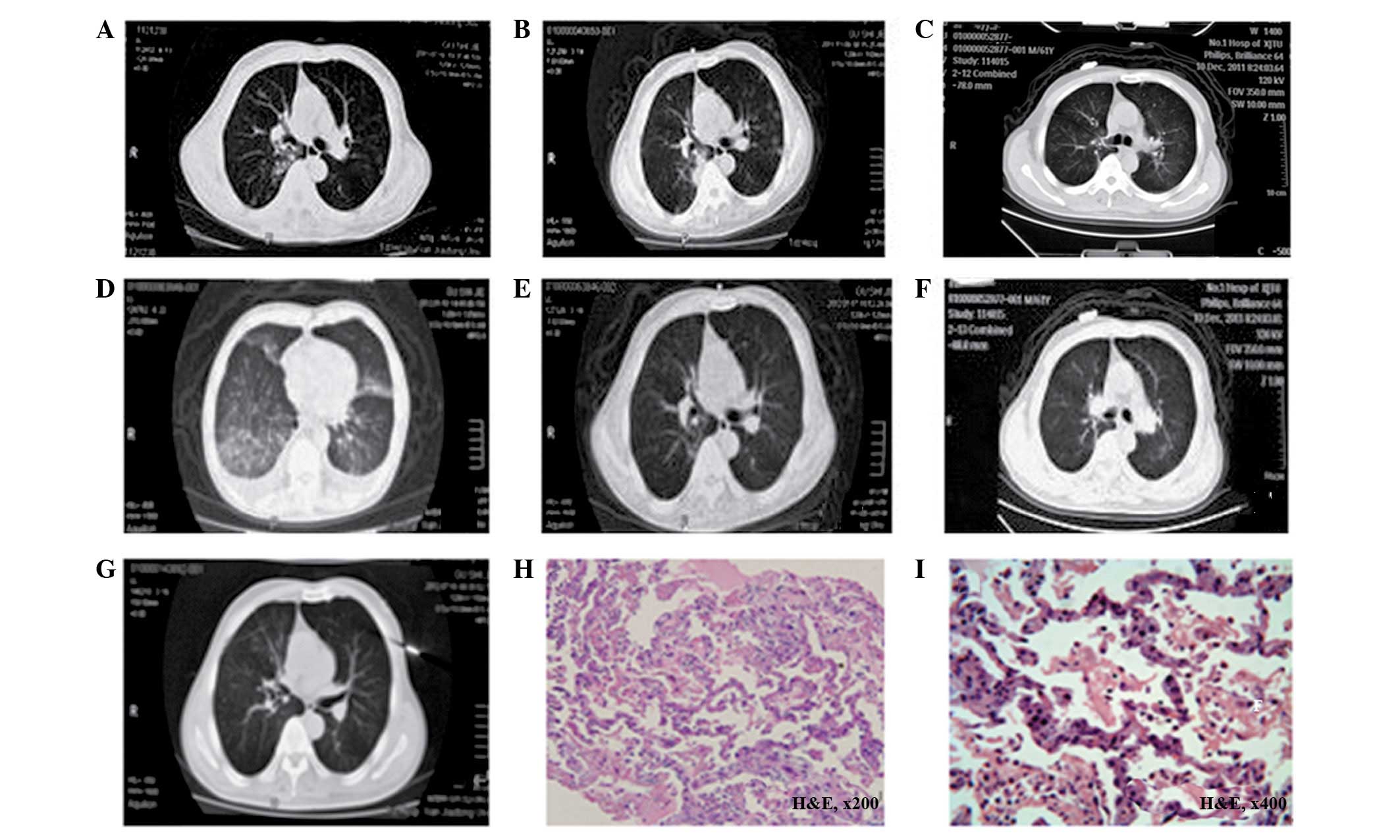
computed tomography (CT) scan (Fig.
1A) showed multiple small nodules scattered throughout the
patient’s lungs, suggesting pulmonary metastasis. The patient
developed a progressive disease following four cycles of
chemotherapy with a pemetrexed-cisplatin regimen, starting in July
2011 (Fig. 1B). The EGFR gene
mutation test showed a deletion in exon 19; however, there were no
mutations in exons 18, 20 or 21. The K-RAS gene mutation test
showed no mutations in exons 13 or 61. In November 2011, daily
treatment with oral gefitinib (250 mg/day) was initiated and the
patient achieved a partial response one month subsequently
(Fig. 1C). However, 60 days
subsequent to the initiation of the gefitinib treatment, the
patient developed fever, an aggravated dry cough and dyspnea. On
the visit to our clinic, the patient appeared acutely ill and
presented with dyspnea, with a body temperature fluctuating between
37.6 and 38.8°C. The patient was previously healthy and reported no
history of chronic diseases, such as hypertension, heart disease
and diabetes, and acute or chronic infection, such as hepatitis and
tuberculosis. He had a smoking history of >20 years (40
cigarettes a day) and had quit smoking for 13 years, with a smoking
index of 800. Physical examination showed that the patient had a
body temperature of 38.3°C, a pulse rate of 124 beats/min, a
respiratory rate of 41 breaths/min and a blood pressure of 130/80
mmHg. Hypoxemia and an oxyhemoglobin saturation of 88% were
detected using pulse oximetry and visible, large erythema and
pimples, with scales and itching, were observed around the ankles
and the dorsal surfaces of the elbow joint. The examination results
are listed in Table I. A chest CT
scan showed interstitial inflammation of the lungs, bilateral
pleural effusion and an adverse swelling of the lungs (Fig. 1D). The patient was admitted with
suspected gefitinib-induced interstitial pneumonitis.
 | Table ITest results prior to and following
treatment. |
Table I
Test results prior to and following
treatment.
| Test | Prior to
treatment | Following
treatment |
|---|
| Complete blood
count |
| WBC
(x109/l) | 13.47 | 11.1 |
| NEUT (%) | 76.00 | 50.74 |
| Biochemical
examination |
| AST (U/l) | 131 | 63 |
| ALT (U/l) | 181 | 104 |
| GGT (U/l) | 56 | 49 |
| TBIL (μmol/l) | 23.7 | 19.2 |
| DBIL (μmol/l) | 9.8 | 4.4 |
| ALB (g/l) | 33 | 23 |
| Arterial blood |
| PO2
(mmHg) | 51.0 | 65.2 |
| PCO2
(mmHg) | 27.0 | 32.1 |
| pH | 7.440 | 7.415 |
| ECG | sinus
tachycardia | |
| Blood culture | | (−) |
| GM Test | (−) | |
| Sputum culture | (−) | (−) |
| PCT test (ng/ml) | <0.5 | |
| Phlegm fungi
smear | (−) | (−) |
| Sputum smear |
| Gram positive
coccus | ++/ | |
| GNB | +/ | |
| Phlegm fungi
training | (−) | (−) |
Following admission, gefitinib administration was
discontinued immediately. No evidence of infection or the presence
of pathological microorganisms was found in either the sputum or
blood culture (Table I), while
chest CT revealed a diffuse ground-glass opacity over the whole
right and left lungs. A lung biopsy revealed interstitial
pneumonia, with a widening of the alveolar septa and alveolar
epithelial hyperplasia. A small quantity of inflammatory exudate
was observed in the alveolar cavity (Fig. 1H and I). The patient was treated
with inhaled oxygen, electrocardiography, cefoperazone/sulbactam
(3.0 g twice daily; to provide resistance to infection),
doxofylline (0.2 g once daily; to relieve coughing), high-dose
methylprednisolone (1,000 mg daily for three days), inhalation of
expectorant and dexamethasone (5.0 mg twice daily), acetylcysteine
(600 mg/Tid; to prevent pulmonary fibrosis) and an infusion of
protein. Therapies for the protection of the liver and gastric
mucosa, improvement of the immune system, prevention of fungal
infections (fluorouracil mouthwash) and adjustment of the
intestinal flora were also administered to the patient. The
patient’s dyspnea and hypoxemia improved significantly in three
days. The results of the laboratory tests taken 10 days subsequent
to the treatments are presented in Table I. The follow-up high-resolution CT
scan (Fig. 1E) showed the total
resolution of the ground-glass lesions and the absorption of the
right-side pleural fluid. Based on the patient’s recovery,
gefitinib treatment was reinitiated, under close observation. The
gefitinib dosage was adjusted to 250 mg, taken once every two days.
In addition, acetylcysteine was administered to prevent pulmonary
fibrosis. No significant adverse response was detected in the
patient. One month later, the follow-up chest CT (Fig. 1F) showed no interstitial pneumonia
recurrence or tumor progression.
Literature review
Based on the case reports and literature reviews on
interstitial pneumonia published in Western countries and China
between 2003 and 2011, the clinical characteristics of
gefitinib-induced interstitial pneumonia were preliminarily
analyzed. The results are presented in Table II (7–29).
 | Table IIClinical characteristics of
gefitinib-induced interstitial pneumonia in case reports published
from 2003 to 2011. |
Table II
Clinical characteristics of
gefitinib-induced interstitial pneumonia in case reports published
from 2003 to 2011.
| Case no. | Age (years) | Gender | Pathology | Smoking history
(years) | Onset time (days
post-gefitinib administration)[ref] | Radiation
history | Prognosis |
|---|
| 1 | 75 | Male | Adenocarcinoma | NA | 12[7] | No | Relieved |
| 2 | 60 | Male | Adenocarcinoma | NA | 40[8] | 8 Gy | Deceased |
| 3 | 55 | Male | Adenocarcinoma | NA | 210[9] | No | Relieved |
| 4 | 59 | Male | Adenocarcinoma | 20 | 23[10] | No | Relieved |
| 5 | 75 | Male | Adenocarcinoma | 14 | 2[11] | No | Deceased |
| 6 | 59 | Male | Adenocarcinoma | No | 60[12] | No | Deceased |
| 7 | 41 | Female | Adenocarcinoma | No | 20[13] | No | Relieved |
| 8 | 74 | Female | Adenocarcinoma | No | 5[14] | No | Relieved |
| 9 | 55 | Male | Adenocarcinoma | 35 | 42[15] | No | Relieved |
| 10 | 60 | Female | Adenocarcinoma | No | 34[16] | No | Relieved |
| 11 | 57 | Male | Adenocarcinoma | NA | 90[17] | No | Relieved |
| 12 | 74 | Female | Adenocarcinoma | No | 15[18] | No | Relieved |
| 13 | 77 | Female | Adenocarcinoma | NA | 20[19] | No | Relieved |
| 14 | 28 | Female | Adenocarcinoma | No | 25[20] | No | Relieved |
| 15 | 50 | Male | Adenocarcinoma | 30 | 38[21] | 60 Gy | Relieved |
| 16 | 65 | Male | Adenocarcinoma | 40 | 2[22] | No | Deceased |
| 17 | 73 | Male | Squamous
carcinoma | Long | 60[23] | No | Relieved |
| 18 | 81 | Male | Adenocarcinoma | Long | 13[24]a | No | Deceased |
| 19 | 66 | Female | Adenocarcinoma | No | 55[25] | No | Relieved |
| 20 | 51 | Female | Adenocarcinoma | No | 56[26] | No | Relieved |
| 21 | 70 | Male | Adenocarcinoma | No | 43[27] | 44 Gy | Relieved |
| 22 | 58 | Female | Adenocarcinoma | No | 38[28] | No | Relieved |
| 23 | 79 | Male | Adenocarcinoma | NA | 25[29] | No | Deceased |
Discussion
Gefitinib is an oral selective inhibitor of the EGFR
tyrosine kinase and may be effective in patients with advanced
non-small-cell lung, ovarian, breast, head and neck or colon
cancers. A significant survival benefit has been demonstrated for
patients of Asian origin and non-smokers. At present, gefitinib is
used as the second or third-line therapy for patients with locally
advanced or metastatic NSCLC, following the failure of platinum and
docetaxel-based chemotherapies, in a number of eastern Asian
countries (30).
The most common adverse effects associated with the
use of gefitinib are acneiform skin rashes, diarrhea and nausea,
which are usually mild in severity and manageable (31). However, in addition to these
effects, gefitinib-induced acute interstitial pneumonia is an
infrequent but potentially lethal adverse effect. The precise
mechanism of gefitinib-induced interstitial pneumonia remains
unknown. Members of the EGF family have been demonstrated to be
implicated in the repair of pulmonary damage (32). Therefore, inhibition of
EGFR-mediated signaling by gefitinib may impair the repair of the
bronchioloalveolar epithelium, thereby exacerbating lung injury,
particularly in patients with pulmonary comorbidities (33). This may be one of the causes of
gefitinib-induced ILD.
Although gefitinib-induced lung injury has a low
incidence, the number of patients with gefitinib-induced lung
injury is likely to increase. The incidence of ILD during gefitinib
treatment varies among different ethnicities. The highest
cumulative incidence of 4%, following 12 weeks of gefitinib
treatment, was described in a large Japanese cohort of >1,800
patients (34). In the rest of the
world, including Taiwan, the incidence of ILD has been reported to
be 1% (35).
The clinical manifestations of gefitinib-induced ILD
consist of chest tightness, shortness of breath, progressive
dyspnea, severe hypoxemia and respiratory failure.
Gefitinib-induced ILD may be diagnosed according to previously
published and generally accepted clinical criteria (36). In brief, a patient may be diagnosed
with gefitinib-induced ILD if the following conditions are met: (i)
There is a new onset of respiratory symptoms during gefitinib
treatment; (ii) there are characteristic signs on the chest
radiography or CT scan, such as non-specific areas with
ground-glass attenuation and extensive bilateral ground-glass
attenuation or airspace consolidations with traction bronchiectasis
(31); (iii) exclusion of
pulmonary infection and a progression of lung cancer, including
lymphangitis and carcinomatosis; and (d) exclusion of radiation
pneumonitis. To relieve the symptoms of gefitinib-induced ILD,
there is a requirement for timely judgment and gefitinib withdrawal
to be applied and for high-dose glucocorticoid (37), oxygen inhalation and anti-infective
therapies to be administered.
The patient in this case report was described as
being previously healthy, with a long history of smoking and a
smoking index of 800. The patient had ceased from smoking for 13
years, and was suffering from bronchioloalveolar carcinoma, which
had metastasized to the lungs. Following the failure of the
first-line treatments of pemetrexed plus cisplatin, EGFR and K-RAS
gene results showed that the patient was suitable for the targeted
therapy. One month subsequent to gefitinib treatment, the
double-lung metastatic carcinoma was significantly relieved. An
analysis of the domestic literature published over the past few
years has shown the importance of gender, pathological type and
smoking history among the factors affecting response rate and
prognosis. Female patients with adenocarcinoma (particularly with
alveolar cell carcinoma) and no smoking history have a good
prognosis and experience an improved quality of life following
gefitinib treatment. However, the present case showed that
satisfactory efficacy may also be achieved in male patients with
adenocarcinoma and a long-term smoking history, providing the
results of the genetic testing are in accordance with the
indications for gefitinib. This case report indicated that the EGFR
mutation status may be the ultimate decisive factor affecting the
response rate and prognosis for gefitinib treatment.
Adenocarcinoma and individual squamous cell
carcinoma have been revealed to be the most common types of
gefitinib-induced interstitial pneumonia. A total of 14 case
reports of gefitinib-induced interstitial pneumonia that have been
published in Western countries were reviewed. These cases each had
individual characteristics; however, the majority concerned male
smokers with adenocarcinoma. In addition, nine cases of
gefitinib-induced interstitial pneumonia were analyzed from the
studies published between 2004 and 2011 in China. The patients in
these cases shared certain features, such as predominantly being
female, with no history of smoking and with adenocarcinoma and a
radiation history. These features are correlated with a low risk of
ILD occurrence, a high response rate and a long survival. The
results of this analysis are consistent with the results of
analyses of gefitinib-induced interstitial pneumonia that have been
produced in Western countries (38). The time of ILD-onset has been shown
to range between 2 and 60 days following gefitinib treatment
(Table II).
Following treatment, the patient in the present case
recovered and continued to take gefitinib under careful medical
supervision. The patient was still alive, one year subsequent to
the restart of the EGFR tyrosine kinase inhibitor therapy. The
outcome of this case showed that the occurrence of controllable ILD
is not an absolute index for the discontinuation of gefitinib
administration. The benefits to the patient and the risks
associated with the use of the drug require comprehensive
consideration prior to the decision regarding the continuation or
discontinuation of gefitinib therapy being made.
Case 18 in Table
II continued gefitinib treatment following an interruption;
however, severe interstitial pneumonia occurred 13 days subsequent
to the restart of the treatment, and the patient died. It was
presumed that the gefitinib-induced ILD in this patient was
immune-related and that, following the first dose of gefitinib,
antibodies were induced in the patient. When the patient took the
same drug again, subsequent to the temporary discontinuation, an
antigen-antibody reaction took place, resulting in the formation of
immune complexes and the onset of ILD. Similar conditions were not
observed in the patient in the present case, which indicated that
individual differences exist in the response to gefitinib.
In conclusion, when gefitinib is used to treat
advanced NSCLC, it confers a high risk of ILD in patients with
progression-free survival and a significant clinical benefit in
non-smokers, females, patients with adenocarcinoma and patients
with no history of thoracic radiotherapy. Gefitinib therapy is an
important treatment option for patients with advanced NSCLC;
however, physicians should carefully decide on the indications for
the use of gefitinib and other cytotoxic agents, particularly for
patients with lung comorbidities. In addition, it is necessary for
careful attention to be paid to the clinical respiratory symptoms
of the patients and the radiographic results, particularly during
the first 1–2 months following the initiation of gefitinib
treatment.
References
|
1
|
Von Pawel J: Gefitinib (Iressa, ZD 1839):
a novel targeted approach for the treatment of solid tumors. Bull
Cancer. 91:E70–E76. 2004.PubMed/NCBI
|
|
2
|
Cerosimo RJ: Gefitinib: an adverse effects
profile. Expert Opin Drug Saf. 5:469–479. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fukuoka M, Yano S, Giaccone G, et al:
Multi-institutional randomized phase II trial of gefitinib for
previously treated patients with advanced non-small-cell lung
cancer. J Clin Oncol. 21:2237–2246. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giaccone G, Herbst RS, Manegold C, et al:
Gefitinib in combination with gemcitabine and cisplatin in advanced
non-small-cell lung cancer: a phase III trial - INTACT 1. J Clin
Oncol. 22:777–784. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herbst RS, Giaccone G, Schiller JH, et al:
Gefitinib in combination with paclitaxel and carboplatin in
advanced non-small-cell lung cancer: a phase III trial - INTACT 2.
J Clin Oncol. 22:785–794. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ohyanagi F, Ando Y, Nagashima F, et al:
Acute gefitinib-induced pneumonitis. Int J Clin Oncol. 9:406–409.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Teramoto S, Yamamoto H and Ouchi Y:
Clinical efficacy and toxicity of gefitinib in patients with lung
cancer. Lancet. 361:1992–1993. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sumpter K, Harper-Wynne C, O’Brien M and
Congleton J: Severe acute interstitial pneumonia and gefitinib.
Lung Cancer. 43:367–368. 2004. View Article : Google Scholar
|
|
9
|
Umemura S, Kishino D, Tabata M, et al:
Systemic tumor embolism mimicking gefitinib (‘IRESSA’)-induced
interstitial lung disease in a patient with lung cancer. Intern
Med. 44:979–982. 2005.PubMed/NCBI
|
|
10
|
Suzuki M, Asahina H, Konishi J, et al:
Recurrent gefitinib-induced interstitial lung disease. Intern Med.
47:533–536. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aoe K, Hiraki A, Murakami T, et al: Sudden
onset of interstitial lung disease induced by gefitinib in a lung
cancer patient with multiple drug allergy. Anticancer Res.
25:415–418. 2005.PubMed/NCBI
|
|
12
|
Nagaria NC, Cogswell J, Choe JK and
Kasimis B: Side effects and good effects from new chemotherapeutic
agents. Case 1 Gefitinib-induced interstitial fibrosis. J Clin
Oncol. 23:2423–2424. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tian Q and Chen LA: Erlotinib achieved
partial response in a non-small cell lung cancer patient with
gefitinib-induced interstitial lung disease. Case Rep Oncol.
4:464–466. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kuo LC, Lin PC, Wang KF, et al: Successful
treatment of gefitinib-induced acute interstitial pneumonitis with
high-dose corticosteroid: a case report and literature review. Med
Oncol. 28:79–82. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seto T, Seki N, Uematsu K, et al:
Gefitinib-induced lung injury successfully treated with high-dose
corticosteroids. Respirology. 11:113–116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ohyanagi F, Ando Y, Nagashima F,
Narabayashi M and Sasaki Y: Acute gefitinib-induced pneumonitis.
Int J Clin Oncol. 9:406–409. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kitajima H, Takahashi H, Harada K, et al:
Gefitinib-induced interstitial lung disease showing improvement
after cessation: disassociation of serum markers. Respirology.
11:217–220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sakoda Y, Kitasato Y, Kawano Y, et al: A
case of alveolar hemorrhage caused by gefitinib. Nihon Kokyuki
Gakkai Zasshi. 49:506–510. 2011.(In Japanese).
|
|
19
|
Goto Y, Hojo M, Takeda Y, et al:
Gefitinib-induced interstitial lung disease - addition of
intravenous cyclophosphamide to corticosteroids is a valuable
treatment option: A case report. Med Oncol. 27:753–755. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fukui T, Otani S, Hataishi R, et al:
Successful rechallenge with erlotinib in a patient with EGFR-mutant
lung adenocarcinoma who developed gefitinib-related interstitial
lung disease. Cancer Chemother Pharmacol. 65:803–806. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Su Y, Peng L and Wu YM: A case of
gefitinib maintenance treatment of non-small cell lung cancer
caused by interstitial pneumonia. Chongqing Medical Journal.
40:3102011.(In Chinese).
|
|
22
|
Peng W, Gao J and Miao LY: Death from
pulmonary interstitial fibrosis and liver damage after taking
gefitinib. Adverse Drug Reactions Journal. 11:429–430. 2009.(In
Chinese).
|
|
23
|
Feng ZZ, Cao Y, Sun LM and Sun P: A cases
of Gefitinib treatment of non-small cell lung cancer with finger
bone metastasis caused interstitial pneumonia. Journal of Dalian
Medical University. 30:588–589. 2008.(In Chinese).
|
|
24
|
Cui H, Huang Q and Chen Y: A case report
of Gefitinib-induced interstitial pneumonia. J Clin Oncol.
12:235–237. 2007.
|
|
25
|
Liang H and Ynag H: A case of
Gefitinib-induced interstitial pneumonia. Practical Journal of
Medicine. 1:1622009.
|
|
26
|
Huang JH, Zhang Y and Zheng J:
Interstitial pneumonia caused by gefitinib in one patient. Chinese
Journal of New Drugs and Clinical Remedies. 26:878–879. 2007.(In
Chinese).
|
|
27
|
Wang WL and Sun Y: One case of
interstitial pneumonia caused by oral gefitinib and radiotherapy.
Pharmaceutical and Clinical Research. 16:506–507. 2008.(In
Chinese).
|
|
28
|
Zhou Y, Dai J, Zhu YZ and Chen FL:
Gefitinib-induced interstitial pneumonia CT image analysis. China
Modern Doctor. 12:61–62. 2010.(In Chinese).
|
|
29
|
Cao SF, Deng QN and Zhang DM: One case of
epidermal growth factor receptor inhibitors-IRESA induced
interstitial pneumonia. Journal of Guangdong College of Pharmacy.
20:304–305. 2004.(In Chinese).
|
|
30
|
Ciardiello F and Tortora G: EGFR
antagonists in cancer treatment. N Engl J Med. 358:1160–1174. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hotta K, Kiura K, Takigawa N, et al:
Comparison of the incidence and pattern of interstitial lung
disease during erlotinib and gefitinib treatment in Japanese
patients with non-small cell lung cancer: the Okayama Lung Cancer
Study Group experience. J Thorac Oncol. 5:179–184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Min JH, Lee HY, Lim H, et al: Drug-induced
interstitial lung disease in tyrosine kinase inhibitor therapy for
non-small cell lung cancer: a review on current insight. Cancer
Chemother Pharmacol. 68:1099–1109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Danson S, Blackhall F, Hulse P and Ranson
M: Interstitial lung disease in lung cancer: separating disease
progression from treatment effects. Drug Saf. 28:103–113. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kudoh S, Kato H, Nishiwaki Y, et al:
Interstitial lung disease in Japanese patients with lung cancer: a
cohort and nested case-control study. Am J Respir Crit Care Med.
177:1348–1357. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Armour A: Gefitinib in advanced non-small
cell lung cancer: Clinical experience in patients of Asian origin.
Asia Pac J Clin Oncol. 3:66–78. 2007. View Article : Google Scholar
|
|
36
|
American Thoracic Society; European
Respiratory Society. American Thoracic Society/European Respiratory
Society International Multidisciplinary Consensus Classification of
the Idiopathic Interstitial Pneumonias. This joint statement of the
American Thoracic Society (ATS), and the European Respiratory
Society (ERS) was adopted by the ATS board of directors, June 2001
and by the ERS Executive Committee, June 2001. Am J Respir Crit
Care Med. 15:277–304. 2002.
|
|
37
|
Zhang Y, Yang H, Zhao M and He J:
Successful treatment of gefitinib-induced acute interstitial
pneumonitis with corticosteroid and non-invasive BIPAP-ventilation.
J Thorac Dis. 4:316–319. 2012.PubMed/NCBI
|
|
38
|
Shih YN, Chiu CH, Tsai CM and Perng RP:
Interstitial pneumonia during gefitinib treatment of non-small-cell
lung cancer. J Chin Med Assoc. 68:183–186. 2005. View Article : Google Scholar : PubMed/NCBI
|