Introduction
Amiodarone (Amio), an agent with vasodilatory and
antiarrhythmic properties, has been used clinically in the
treatment of ventricular arrhythmias, including recurrent
ventricular tachycardia and fibrillation (1,2).
Amio is typically administered intravenously or orally in daily
high loading doses of 800–1,600 mg, until the arrhythmia is
controlled or as daily maintenance oral doses of 200–600 mg for
long term therapy. However, this iodine-containing compound tends
to accumulate in several organs, including the lungs and has been
associated with a variety of adverse events. Pulmonary toxicity is
the most serious complication of Amio and usually manifests as
acute or subacute pneumonitis (3,4).
Liver toxicity appears to be more common with higher doses
(5). Other adverse side-effects
include fatigue, tremor, involuntary movements, poor coordination,
peripheral neuropathy, nausea, vomiting, constipation, anorexia,
visual disturbances, corneal deposits, skin discoloration and rash,
photosensitivity, bradycardia and worsening of arrhythmias.
Uncommon side-effects include pneumonitis, pulmonary fibrosis,
optic neuropathy, blindness, thyroid dysfunction and liver injury
(5).
Diverse antioxidants have been shown to prevent
Amio-induced toxicity (6,7). A unique nutrient formulation (NM)
containing primarily ascorbic acid, lysine, proline,
N-acetyl cysteine and green tea extract has previously been
shown to exhibit a broad spectrum of pharmacological, therapeutic,
cardiovascular and chemoprotective properties (8). In previous studies, it was found that
NM significantly inhibited acetaminophen-induced and carbon
tetrachloride-induced hepatic and renal damage (9,10).
In the present study, the in vivo effects of
the NM diet were examined in mice treated with Amio, focusing on
cardiac enzyme levels.
Materials and Methods
Materials
Amio powder obtained from Sigma-Aldrich (St. Louis,
MO, USA) was diluted in warm saline (pH 7.4) to 50 mg/ml. The stock
solution of NM was composed of the following in the quantities
indicated: 700 mg vitamin C (as ascorbic acid and as Mg, Ca and
palmitate ascorbate); 1,000 mg L-lysine; 750 mg L-proline; 500 mg
L-arginine; 200 mg N-acetyl cysteine; 1,000 mg standardized
green tea extract (80% polyphenol); 30 μg selenium; 2 mg copper; 1
mg manganese; and 50 mg quercetin.
Animals
Male BALB/c mice, free of murine viruses, bacteria
and parasites, and ~6 weeks of age on arrival, were purchased from
Simonsen Laboratories (Gilroy, CA, USA) and maintained in
microisolator cages under pathogen-free conditions on a 12-h
light/12-h dark schedule for 1 week. All animals were cared for in
accordance with the institutional guidelines for the care and use
of experimental animals.
Experimental design
After one week of isolation, mice were divided into
four groups (A–D) of six animals per group. Mice in groups A and C
were fed a regular Purina mouse chow diet (Laboratory Rodent Diet
5001 from Purina Mills, LLC, purchased from Newco Distributing
Inc., Rancho Cucamonga, CA, USA) for three weeks, while mice in
groups B and D were fed the regular mouse chow diet supplemented
with 1% (w/w) NM during that period. During the study, the mice
consumed, on average, 4 g of their respective diets per day. Thus,
the supplemented mice received ~20 mg NM per day. After three
weeks, the mice in groups C and D received daily Amio injections of
50 mg/kg body weight intraperitoneally for 4 days and those in
groups A and B received saline alone. The respective diets were
continued for these 4 days. At 24 h after the final dose, mice were
sacrificed, blood was withdrawn, serum was collected for clinical
chemistry and livers, kidneys, hearts and lungs were excised and
weighed.
Serum analyses
Blood was collected and centrifuged at 13,000 × g
for 5 min at 4°C. The samples were stored at −80°C until sent for
analysis. Chemistry tests for serum creatine phosphokinase (CPK)
and aspartate aminotransferase (AST) were run on a Hitachi 747
Chemistry Analyzer (Tokyo, Japan) with reagents from Boehringer
Ingelheim (Ingelheim am Reine, Germany).
Statistical analysis
Results are expressed as mean ± SD for each group.
Data was analyzed by independent sample t-tests. MedCalc Software
(Mariakerke, Belgium) was used. P<0.05 was considered to
indicate a statistically significant result.
Results
Body weights and food consumed
No significant difference in weight gain was
observed between the groups. The mean initial body weight of the
mice was 24.1±1.4 g and the mean final weights were 26.2±0.7,
26.4±1.4, 27.6±2 and 26.7±1.9 g for groups A, B, C and D,
respectively. The mean dietary intake for the supplemented groups
was 3.5±0.5 g, 83% of the mean intake for the mice fed a control
diet, which was 4.2±0.5 g (P=0.002).
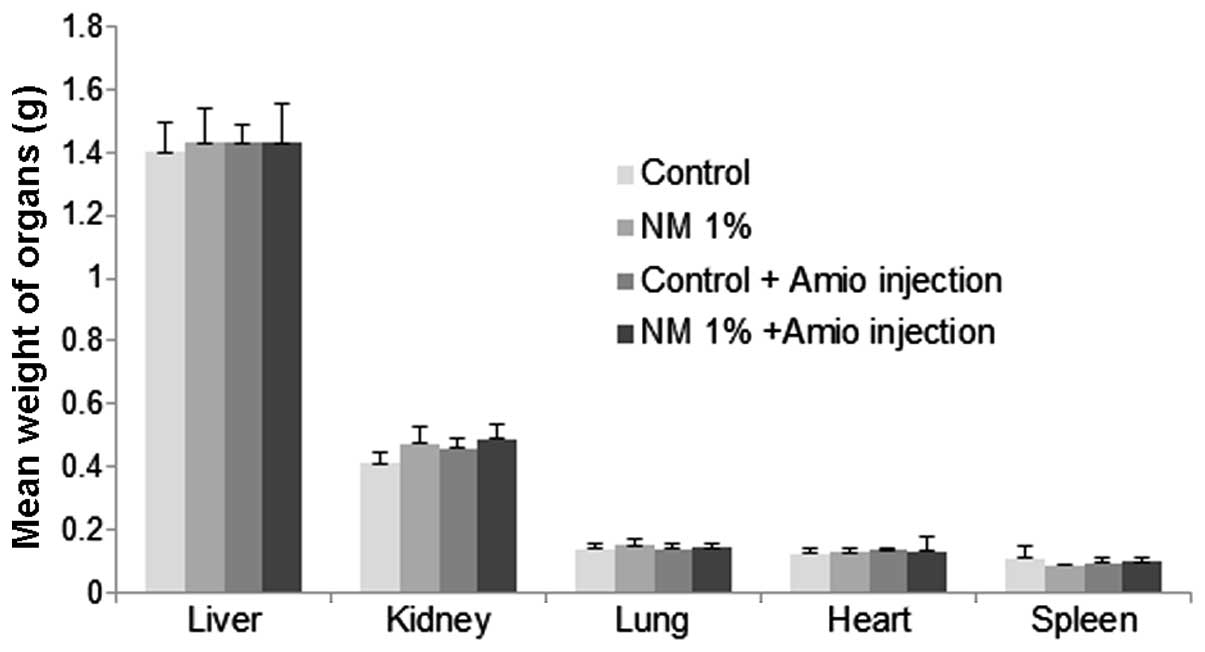
Vital organ weights
Liver, kidney, heart and lung weights were
comparable in all four groups, as shown in Fig. 1.
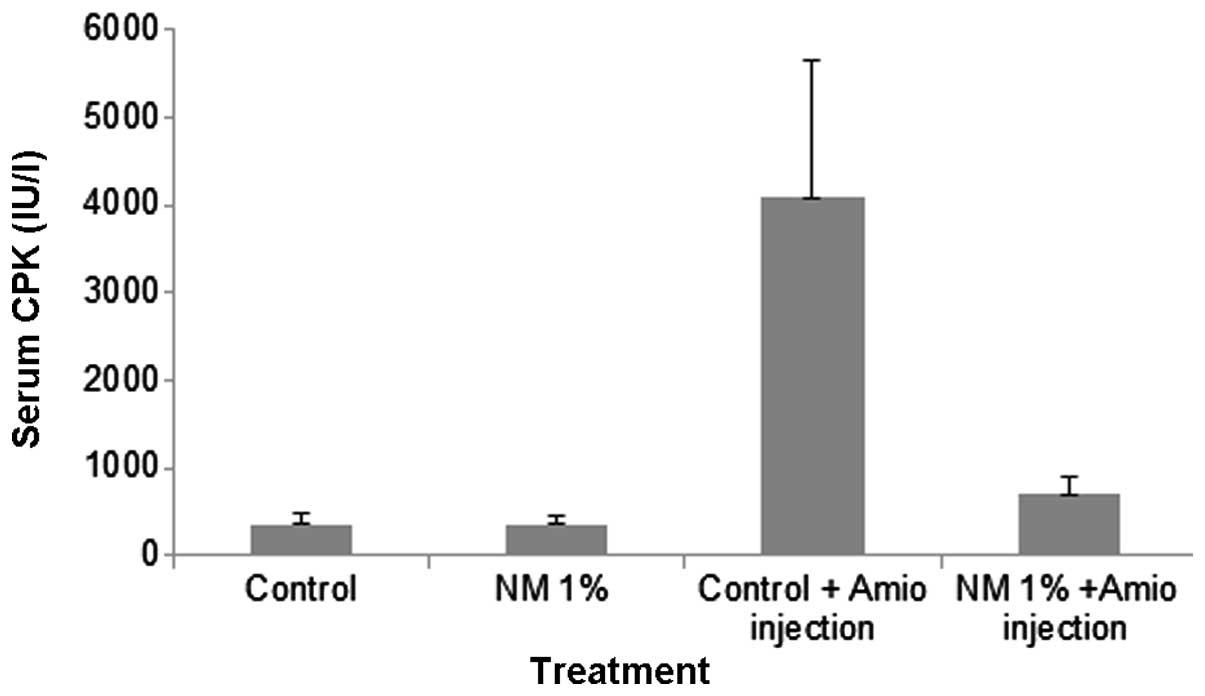
Serum CPK
Administration of Amio to the control diet group
resulted in a significant increase in the mean serum CPK level,
whereas in the 1% NM-fed mice, the mean serum CPK following Amio
administration was comparable to those in the saline injection
groups. The serum CPK levels in the control and NM 1% groups
(groups A and B) were 365±135 and 370±90 IU/l respectively.
Treatment of the mice in group C with Amio resulted in a marked
increase in the serum CPK level of 1,121% compared with that in
group A and a mean CPK level of 4,090±1,560 IU/l. Supplementation
with NM 1% prior to Amio administration, as performed with group D,
resulted in a significantly reduced level of CPK. The mean CPK
level of group D was 700±190 IU/l, a reduction of 83%, when
compared with that in group C (P=0.001), as shown in Fig. 2.
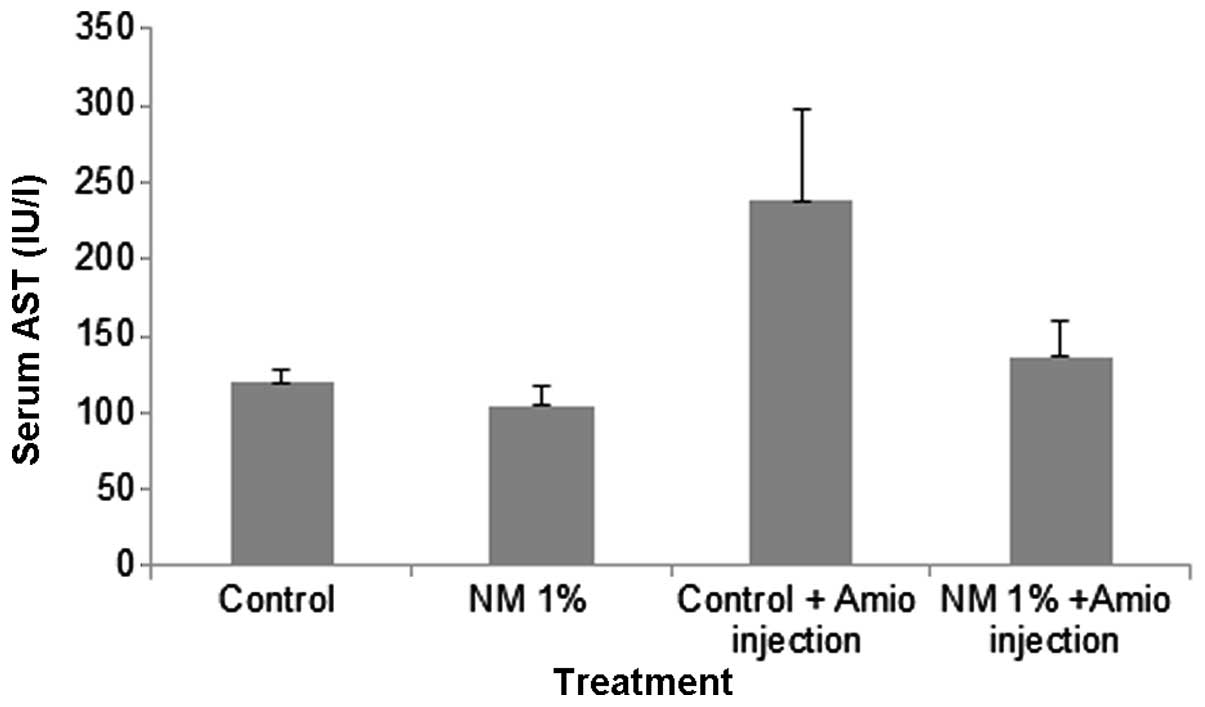
Serum AST
Amio administration also resulted in a significant
increase in the serum marker for AST in the mice fed with the
control diet, but not in mice fed with NM 1%, which exhibited
similar levels to those of the saline injection groups, as shown in
Fig. 3. Mean serum AST
concentrations for groups A and B were 120±8 and 105±12 IU/l,
respectively. Group C exhibited a marked increase with levels of
238±60 IU/l, which was a 198% increase compared with that of the
control. Group D demonstrated a significantly reduced level of AST
at 136±23 IU/l, which was a reduction of 43%, when compared with
the level in group C (P=0.003).
Discussion
The results of the present study demonstrate that
pretreatment for three weeks with a diet supplemented with 1% NM
reduced cardiac damage, as reflected in the enzyme levels of male
BALB/c mice injected with daily toxic doses (50 mg/kg body weight)
of Amio for four days. Amio treatment caused marked increases in
cardiac serum CPK and AST levels in unsupplemented mice; however,
supplementation with NM reversed the CPK and AST levels to near
normal limits. The levels of CPK, an enzyme found in the heart,
brain and skeletal muscles, increase with heart muscle damage;
levels rise 4–8 h following an acute myocardial infarction, peaking
at 16–30 h and returning to baseline within 4 days (11,12).
AST, an enzyme that is normally present in heart and liver cells,
is released into the blood when the liver or heart is damaged. The
amount of AST in the blood is directly associated with the extent
of tissue damage (11).
A study of the role of free radicals in the toxicity
of Amio by Verikei et al revealed that Amio generates free
radicals, under in vitro and in vivo conditions, that
may play a role in the pathogenesis of Amio toxicity, as well as in
other well-established mechanisms. The study also revealed that
antioxidants may have a partial protective effect against Amio
toxicity (13). Vitamin C has been
shown to decrease Amio-induced toxicity in rat thymocytes by
restoring cellular glutathione content (7). The antioxidants vitamin C and
N-acetyl cysteine were shown to protect mouse fibroblasts
from Amio-induced cytotoxicity (6). In a literature review, Harling et
al found that vitamin C and E significantly decreased
postoperative atrial fibrillation (14).
The NM tested in the present study was formulated
based on the targeting of various physiological processes involved
in a wide spectrum of pathological conditions at the cellular
level. Based on our own studies and published data, it was
hypothesized that metabolic effects are likely to result from the
synergy of ascorbic acid, lysine, proline, green tea extract,
arginine, N-acetyl cysteine, quercetin, selenium, copper and
manganese. Combining these micronutrients expands metabolic
targets, maximizing biological impact with lower doses of
components. A previous study of the comparative effects of NM,
green tea extract and epigallocatechin gallate (EGCG) on the
inhibition of MMP-2 and MMP-9 secretion in various cancer cell
lines with varying MMP secretion patterns, revealed the superior
potency of NM over green tea extract and EGCG at equivalent doses
(15).
In conclusion, the present study demonstrated that
pretreatment for three weeks with a diet supplemented with 1% NM
reduced the cardiac damage in BALB/c mice caused by the
administration of multiple toxic doses of Amio. Supplementation
with dietary NM reduced the Amio-induced elevated cardiac enzymes
in the mice. Although clinical studies are required, the results
indicate the therapeutic potential of using NM adjunctively with
Amio to protect against Amio-induced heart damage.
Acknowledgements
Cardiac enzyme analyses were provided by IDEXX
Laboratories, Inc. (Westbrook, ME, USA). The research study was
funded by the Dr. Rath Health Foundation (Santa Clara, CA, USA), a
non-profit organization. The abstract was presented at the 51st
Annual Meeting of the Society of Toxicology, March 11–15, 2012 in
San Francisco, CA and published as abstract no. 2413 in
Toxicologist 126: 521, 2012.
References
|
1
|
Zimetbaum P: Amiodarone for atrial
fibrillation. N Engl J Med. 356:935–941. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siddoway LA: Amiodarone: guidelines for
use and monitoring. Am Fam Physician. 68:2189–2196. 2003.PubMed/NCBI
|
|
3
|
Ernawati DF, Stafford L and Hughes JD:
Amiodarone - induced pulmonary toxicity. Br J Clin Pharmacol.
66:82–87. 2008. View Article : Google Scholar
|
|
4
|
Wolkove N and Baltzan M: Amiodarone
pulmonary toxicity. Can Respir J. 16:43–48. 2009.
|
|
5
|
LiverTox Amiodarone. http://livertox.nih.gov/Amiodarone.htmluri.
Accessed August 7, 2013
|
|
6
|
Durukan AB, Erdem B, Durukan E, Sevim H,
Karaduman T, Gurbuz HA, Gurpinar A and Yorgancioglu C: May toxicity
of amiodarone be prevented by antioxidants? A cell-culture study. J
Cardiothorac Surg. 7:612012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cekic S, Pavlovic D, Sarac M, Kamenov B,
Dimic A and Pavlovic V: The effect of vitamin C on
amiodarone-induced toxicity in rat thymocytes. Cent Eur J Med.
6:58–63. 2011. View Article : Google Scholar
|
|
8
|
Niedzwiecki A, Roomi MW, Kalinovsky T and
Rath M: Micronutrient synergy - a new tool in effective control of
metastasis and other key mechanisms of cancer. Cancer Metastasis
Rev. 29:529–542. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roomi MW, Kalinovsky T, Ivanov V, Rath M
and Niedzwiecki A: A nutrient mixture prevents acetaminophen
hepatic and renal toxicity in ICR mice. Hum Exp Toxicol.
27:223–230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roomi MW, Kalinovsky T, Roomi NW, Ivanov
V, Rath M and Niedzwiecki A: A nutrient mixture suppresses carbon
tetrachloride-induced acute hepatic toxicity in ICR mice. Hum Exp
Toxicol. 27:559–566. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moses S: Family Practice Notebook. Serum
cardiac marker. http://www.fpnotebook.com/cv/Lab/SrmCrdcMrkr.htmuri.
Accessed August 7, 2013
|
|
12
|
Medline Plus. Creatinine Phosphokinase
test. http://www.nlm.nih.gov/medlineplus/ency/article/003503.htmuri.
Accessed August 7, 2013
|
|
13
|
Vereckei A, Blazovics A, Gyorgy I, Feher
E, Toth M, Szenasi G, Zsinka A, Foldiak G and Feher J: The role of
free radicals in the pathogenesis of amiodarone toxicity. J
Cardiovasc Electrophysiol. 4:161–177. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harling L, Rasoli S, Vecht JA, Ashrafian
H, Kourliouros A and Athanasiou T: Do antioxidant vitamins have an
anti-arrhythmic effect following cardiac surgery? A meta-analysis
of randomised controlled trials. Heart. 97:1636–1642. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roomi MW, Monterrey JC, Kalinovsky T, Rath
M and Niedzwiecki A: Comparative effects of EGCG, green tea and a
nutrient mixture on the patterns of MMP-2 and MMP-9 expression in
cancer cell lines. Oncol Rep. 24:747–757. 2010.PubMed/NCBI
|