Introduction
Morphological abnormalities of spermatozoa are often
identified in males with problematic fertility. Male infertility
may be classified as oligoasthenoteratozoospermia or azoospermia.
The quality of spermatozoa has an essential effect on the
fertilization of the oocyte and on the subsequent evolution of the
embryo. A direct correlation exists between abnormal sperm and
embryo morphology at the later stage of cleavage (1). The first two cycles of embryo cell
cleavage are controlled by maternal factors, whilst the paternal
effect begins to apply in the embryo from the four-cell stage
(2). The quality of DNA in sperm
is evaluated as an absence/incidence of fragmentations in late
embryonic development, which is the late paternal effect (3–5).
The first pregnancy and birth of a child following
the application of the intracytoplasmic sperm injection (ICSI)
method was recorded in 1992 (6).
ICSI has increased the success rates of in vitro
fertilization (IVF) treatment in couples with the male sterility
factor. The fertilization rate following ICSI has been reported to
be significantly higher compared with that of the subzonal
insemination method (7). ICSI has
been found to result in higher fertilization and pregnancy rates;
it has been shown to be successful in couples with unexplained
infertility (8), boundary
spermiogram values (9) and
immunological infertility (10),
and couples who have experienced repeated failures following
conventional treatment by IVF (11). Common indications for use of the
ICSI method are low parameters of spermatozoa in the ejaculate. The
ICSI method with ejaculated sperm may be successfully used in
patients with a low count of morphologically high-quality and
motile sperm in the ejaculate. Riedel et al (12) established minimum andrological
parameters for in vitro fertility in conventional IVF,
specifying a 5×106 cm−3 total count, 30%
progressive motility and 30% normal morphology; males exhibiting
worse parameters in the ejaculate had an very low prognosis of
successful medical treatment. The ICSI method represents an
effective procedure for this type of male infertility.
In the present study, fertilization, pregnancy and
take-home baby rates were analyzed following ICSI in males with a
high percentage of morphologically abnormal spermatozoa.
Teratospermia is one of the key parameters in the selection of
sperm suitable for ICSI. The aim of the present study was to
ascertain whether ICSI is a suitable treatment perspective for
infertile males with heavy teratospermia (≤1% normal
spermatozoa).
Materials and methods
Patients
ICSI was performed between January 2008 and February
2009 to assist reproduction in 174 infertile couples at the
Assisted reproduction clinic (Brno, Czech Republic). Success of the
treatment was evaluated from the male viewpoint, as the study
focused on male infertility. In total, 174 males were subjected to
ICSI cycles. The male patients were divided into a group of 137
individuals with mildly impaired or normal sperm morphology (>1%
of normal spermatozoa) and a group of 37 individuals with heavily
impaired sperm morphology (≤1% of normal spermatozoa). The mean age
of the male patients was 34.9±2.8 years and the age of their female
partners was 30.5±2.7 years. Informed consent was obtained from all
participants. The study was approved by the Ethics Committee of the
Faculty of Medicine (Masaryk University, Brno, Czech Republic).
Semen analysis
Spermatozoa were obtained through masturbation
following sexual abstinence for 3–5 days. Prior to analysis,
samples were incubated for 20 min at 37°C for fluidization. The
concentration, motility and morphology of the sperm were assessed
according to the guidelines of the World Health Organization (4th
edition, 1999). Samples were visually assessed under a
microscope.
Sperm processing
Spermatozoa gained from ejaculation were processed
using the swim-up method (13) and
incubated at a temperature of 37°C with 5% O2, 6%
CO2 and 89% N2 in Sydney IVF Sperm Medium
(Cook Medical, Brisbane, Australia).
Ovarian stimulation and oocyte
retrieval
Ovarian stimulation was induced by a long protocol
in all 174 cycles using triptorelin [a gonadotropin-releasing
hormone agonist (GnRHa); Ferring Pharmaceuticals, Saint-Prex,
Switzerland], Metrodin [follicle-stimulating hormone (FSH); Serono,
Geneva, Switzerland] and Humegon [human menopausal gonadotropin
(hMG); Organon Laboratories Ltd., Hoddesdon, UK). Subcutaneous
triptorelin application was initiated from the mid-luteal phase of
the previous cycle and continued for 14 days until sufficient
downregulation of the pituitary was achieved. The development of
follicles was stimulated by FSH and hMG injections. The dose of
gonadotropins was individualized, and selected according to the age
of the female patient, previous stimulation and response to
stimulation. Ovulation was induced by injecting 5,000 IU human
chorionic gonadotropin (hCG) in the form of Pregnyl (Organon
Laboratories Ltd.) when the two largest follicles were >18 mm.
Oocytes were sampled 36 h following hCG application under general
anesthesia using ultrasound control.
Oocyte handling
Cumular cells were removed from the oocytes with a
denudation pipette (1–2 h following oocyte sampling by ovum pick
up) using 80 IU/ml hyaluronidase (in Sydney IVF Fertilization
medium) for 10–15 sec. Following the partial removal of cumular
cells, the oocytes were further denudated (Sydney IVF Fertilization
medium) until complete denudation. The denudated oocytes were
placed in a cultivation box (Heracell; Thermo Fisher Scientific,
Waltham, MA, USA) under conditions of 37°C, 5% O2, 6%
CO2 and 89% N2, until required for ICSI. The
sperm were injected into the oocytes 2–3 h following ovum pick up.
Oocytes used for ICSI were in metaphase II (MII) after extrusion of
the first polar body.
ICSI procedure
ICSI was conducted using an inverted microscope
(Olympus, Tokyo, Japan) with a micro-manipulator (Research
Instruments, Falmouth, UK) and injectors (Eppendorf, Hamburg,
Germany). During ICSI, oocytes were maintained in Sydney IVF
Fertilization medium, and Sydney IVF PVP (Cook Medical) was used
for spermatozoa. The oocytes were placed individually into 10-μl
micro-drops of Sydney IVF Fertilization medium and one micro-drop
with the Sydney IVF PVP medium was injected with a 2-μl suspension
of spermatozoa. Sperm were selected, immobilized, sucked into the
ICSI pipette and inserted into the oocyte cytoplasm under a
microscope at ×400 magnification, using Hoffman modulation
contrast. Prior to injection, the morphological structure of the
head, neck and tail of the sperm was assessed, as well as the
possible occurrence of vacuoles in the sperm head. Following ICSI,
the oocytes were transferred into Sydney IVF Cleavage medium (Cook
Medical) and deposited in the cultivation box.
Assessment of fertilization, embryo
cleavage and establishment of pregnancy
After 16–18 h, oocytes were checked to verify
fertilization. Fertilized oocytes were separated and tested for the
occurrence of the two-pronuclei (2PN) stage. The cleavage phase of
the embryo was established 25–27 h following oocyte fertilization
and early embryo cleavage was assessed (14). The early paternal effect of the
sperm is demonstrated prior to the main activation of embryonic
genome expression, as it starts between the fourth and the eighth
cell stage of embryo preimplantation development (3,4).
Embryos of the highest quality were transferred within 72–96 h from
the sampling of oocytes using a Wallace catheter (1816N; H.G.
Wallace Ltd., London, UK). Parameters evaluated in the embryos
included the number and regularity of blastomeres, as well as the
incidence or absence of fragments and vacuoles (15). The mean number of transferred
embryos was two per transfer. The luteal phase was supported by
progesterone in the form of Utrogestan (Besins Manufacturing
Belgium S.A., Drogenbos, Belgium) 2× 200 mg daily or by injecting
Agolutin (Biotika a.s., Slovenská L̓upča, Slovakia) 50 mg daily and
1,500 IU Pregnyl (N.V.Organon, Oss, The Netherlands on days 1, 4, 7
and 9 following embryo-transfer. In the case of positive chemical
gravidity, HCG was detected on day 14 following embryo-transfer.
Clinical gravidity was defined as an intrauterine identification of
the gestational sac with a heart function. Abortion was defined as
gravidity terminated prior to week 20 of pregnancy.
Statistical analysis
Comparisons were made between pregnancy and
reproduction rates. The normality of data was tested by the
Anderson-Darling normality test and by visual inspection of
histograms. As specific parameters exhibited a non-normal
distribution, the Mann-Whitney U-test was used to compare continual
data. Fisher’s exact test was applied to compare categorical data.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Oocytes injected in the MII phase totaled 2,811 and
subsequent fertilization (2PN) was recorded in 2,303 oocytes (82%).
Transfer was implemented in all 174 couples and the total number of
transferred embryos amounted to 349 with an average count of two
embryos per transfer. Clinical gravidity per transfer was achieved
in 92 cases (53%). The resulting number of births was 83 (48%) with
108 live-born infants.
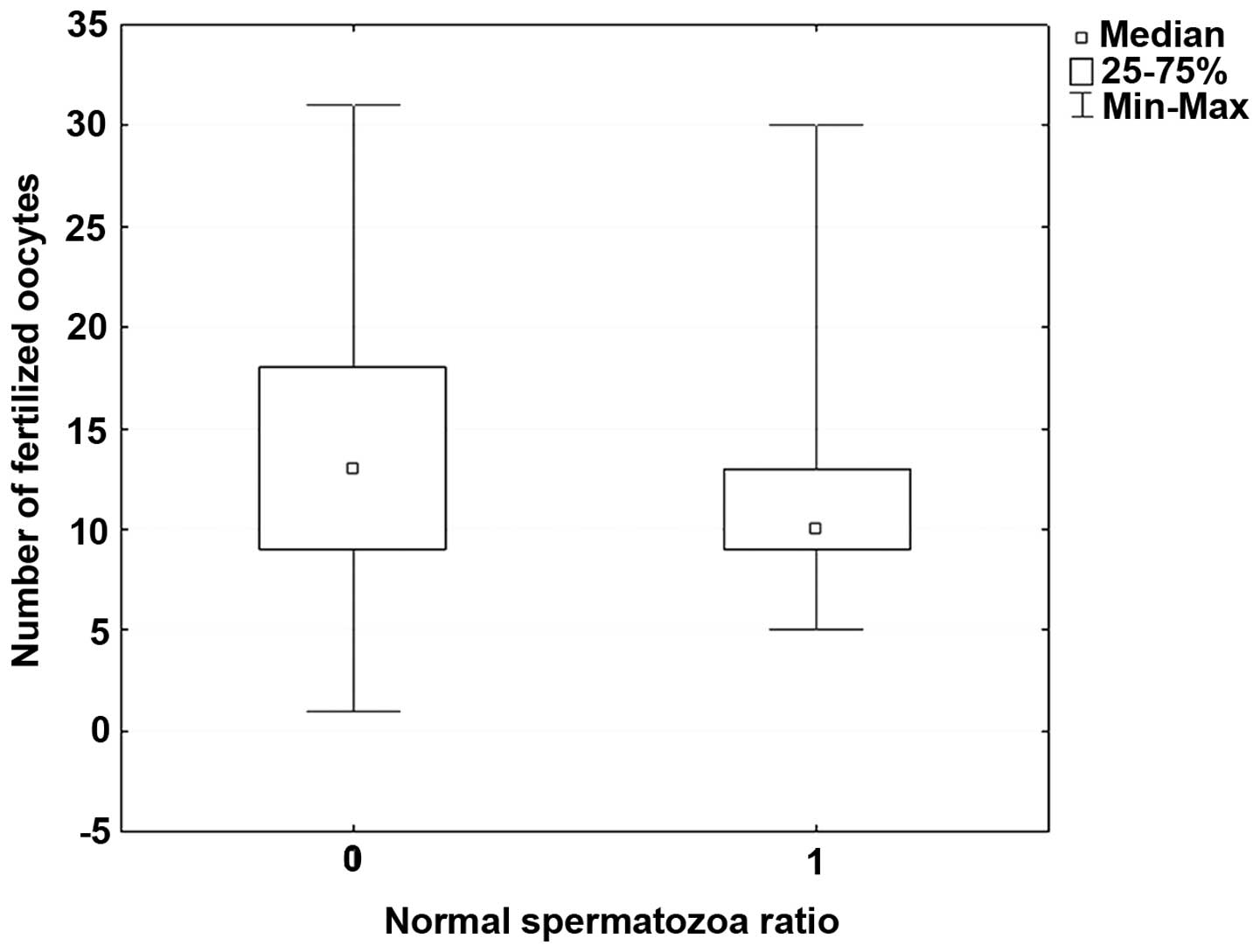
Table I summarizes
the characteristics of the patients. Table II summarizes characteristics and
outcomes of the patients and is divided into groups of males with
mildly and heavily impaired sperm morphology. Statistical
evaluations were performed of the age of male patients, mean count
of oocytes sampled from their female partners, number of oocytes in
the MII phase suitable for fertilization, number of fertilized
oocytes, number of clinical gravidities and number of deliveries of
live-born children. Table II
indicates that while the number of fertilized oocytes in the
patients with heavily impaired sperm morphology was significantly
lower (P=0.038), neither gravidity nor delivery of the partners
differed compared with those in the patients with mildly impaired
or normal spermatozoa. Trends opposite to that for fertilization
were recorded for gravidity [odds ratio (OR), 0.62; 95% confidence
interval (CI), 0.29–1.30] and delivery (OR, 0.55; 95% CI,
0.26–1.24). These results indicate that the lower number of
fertilized oocytes was not associated with the overall result.
 | Table IBasic characteristics of the male
subjects and their partners during intervention. |
Table I
Basic characteristics of the male
subjects and their partners during intervention.
| Characteristics | Value |
|---|
| Male age, years | 34.9±2.8 |
| Female age,
years | 30.5±2.7 |
| Normal spermatozoa,
% | 11 (3–21)a |
| Oocytes, n | 25±8 |
| Mature oocytes,
n | 16±6 |
| Fertilized oocytes,
n | 12 (9–17)a |
 | Table IICharacteristics and the outcome of
fertilization in patients with mildly and heavily defective
spermatozoa. |
Table II
Characteristics and the outcome of
fertilization in patients with mildly and heavily defective
spermatozoa.
| Morphology | Heavily defective
(≤1% normal spermatozoa) | Mildly defective
(>1% normal spermatozoa) | P-value |
|---|
| Age, years | 35.3±3.1 | 34.8±2.7 | 0.339 |
| Oocytes, n | 26±7 | 24±8 | 0.271 |
| Mature oocytes,
n | 15±6 | 16±6 | 0.192 |
| Fertilized oocytes,
n | 10 (9–13)a | 13 (9–17)a | 0.038 |
| Pregnancy rates,
present/absent | 23/14 | 69/68 | 0.266 |
| Delivery,
present/absent | 22/15 | 61/76 | 0.138 |
The present study indicates that there was a
significant difference in fertilization rates between the group of
males with heavily impaired sperm morphology and those with mildly
impaired sperm morphology. The result of a successful treatment was
pregnancy and the birth of a healthy child. Statistical evaluation
showed no significant difference between the two groups in this
respect (Fig. 1).
Discussion
The prospects of producing biological offspring, in
couples who previously had no likelihood of doing so, have
considerably increased since the ICSI method was introduced in 1992
(first gravidity and delivery of a child following ICSI applied in
a female). Following the first successful pregnancy and birth of a
healthy child using the ICSI method, the procedure began to be
widely used to assist fertilization, particularly in couples
affected by male infertility (6).
Gradual improvement of procedures in the evaluation of spermatozoa
and micromanipulation techniques has opened new horizons for the
successful assistance of reproduction in cases of male infertility.
Compared with the conventional method of IVF, ICSI yields higher
fertilization rates, as well as higher counts of cleaved embryos
(16). One of the main differences
between conventional IVF and the ICSI method is the ability to
select just one sperm and insert it mechanically into the egg
cytoplasm (17). Studies by Aytoz
et al and Palermo et al found no significant
difference between the pregnancy rates for normal and abnormal
ejaculates using the ICSI method (18,19),
consistent with the results of the present study. The method of
selecting a suitable sperm markedly increased the count of
fertilized oocytes in IVF/ICSI treatment and provided a general
solution for the problem of heavy teratospermia in males who had no
likelihood of achieving a successful treatment result. Therefore,
the requirements for sperm donors have been markedly reduced.
The correlation between morphology and the low
success of conventional IVF treatment has been clearly ascertained
(20,21). The normal morphology of sperm is
important for binding and penetration through the zona pellucida.
Injection of one sperm through the zona pellucida and oolemma into
the egg cytoplasm facilitates penetration of the egg, even for
sperm previously incapable of overcoming these barriers during
normal IVF fertilization. A range of studies have indicated that
when using the ICSI method, the high percentage of morphologically
abnormal sperm in the ejaculate has no essential effect on the
outcome of fertilization, transfer of high-quality embryos and
gravidity (10,22–25).
A study by De Vos showed a lower fertilization rate of oocytes for
patients with abnormal spermatozoa but a comparable quality of
embryos. Lower implantation and clinical gravidity rates for
abnormal sperm were also observed (26). The present study using ICSI
observed that lower fertilization rates were obtained in males with
heavy teratospermia; however, this did not affect subsequent
implantation, clinical gravidity and delivery of a healthy child.
Fishel et al (27)
conducted a study to compare IVF and ICSI methods using partner
sperm and at the same time using donor sperm for IVF in couples
with unexplained infertility. The highest fertilization incidence
was observed in ICSI with the partner sperm. The conclusion of the
study was that ICSI is an effective method of achieving
fertilization in higher counts of oocytes, maximizing the number of
embryos and minimizing the risk of fertilization failure in the
treatment of infertility.
The results of the present study indicate that
fertilization of oocytes is less effective in patients with poor
morphological quality of sperm cells, compared with the
effectiveness in patients with mildly impaired morphology. However,
pregnancy and reproduction rates were independent of sperm
morphology when ICSI was used. These results are in concordance
with previously published studies. Microscopic selection of a sperm
with ‘normal’ morphology during the ICSI procedure achieves
excellent results, even in heavy teratospermias (28). Following introduction of the ICSI
method, 2,000 children conceived using this method have been born;
these individuals have not exhibited a higher incidence of
malformations compared with those in children born following
conventional IVF or in the normal population (29). However, this conclusion does not
correspond with later results published by Hansen et al and
Bonduelle et al (30,31)
or with a subsequent study by Davies et al (32). Hansen et al observed that
the incidence of inborn developmental defects diagnosed in the
first year of life in children conceived with ICSI was double that
in children conceived naturally (30). Factors that may increase the risk
include the relatively high age of infertile couples, causes of
infertility and the effect of drugs used to induce ovulation or to
maintain gravidity in the initial stages of embryonic development,
as well as factors connected with methods of assisted reproduction,
freezing and unfreezing of embryos, late fertilization of oocytes
and polyspermy. In the aforementioned study, a higher incidence of
cardiovascular, urogenital, chromosomal and musculoskeletal defects
was recorded when the ICSI method was used in assisted
reproduction. Following the adjustment for parental risk factors,
the incidence of developmental defects was not significantly higher
in IVF, while the risk of ICSI-associated defects remained
significantly higher following adjustment. An analysis of perinatal
results from singleton pregnancies identified significant
differences in the gestation age, birth weight, body length, head
circumference and Apgar score in children conceived using ICSI when
compared those in children born following spontaneous pregnancies
(33). The international study by
Bonduelle et al focused on children born following ICSI and
their subsequent 5-year development (31). During these five years, these
children showed a greater probability of requiring physiotherapy
and speech therapy and showed a higher susceptibility to
respiratory, dermatological or gastrointestinal infections,
resulting in a higher need for various types of surgery. The
extensive observational study by Davies et al (32) corroborated the conclusions of the
two aforementioned studies (30,31)
with regard to the increased risk of defects in newly born children
conceived through assisted reproduction compared with the risk in
children from spontaneous conception. The study by Davies et
al is more comprehensive and includes 6,163 children conceived
through assisted reproduction and evaluated until the age of five
years. The increased risk of inborn IVF-associated defects is not
significant following adjustment for parental risk factors, while
in the ICSI method the higher risk persists. Notably, results from
a comparison between fresh and frozen embryos used in IVF and ICSI
cycles indicate that embryos transferred following thawing in the
two methods did not show a higher incidence of developmental
defects following birth than infants from natural conception. These
results may be explained by the theory that embryos are unlikely to
survive the demanding process of freezing and unfreezing unless
they are of high quality (34).
The process of mechanical injection of a selected sperm into the
egg cytoplasm in ICSI avoids all natural barriers of spontaneous
fertilization. As shown in the study by Fishel et al
(27), in conventional IVF using
high concentrations of donor sperm, when there is no intervention
in the process of sperm penetration into the egg, the fertilization
rate was significantly lower than that in the ICSI method with
partner sperm, despite the partner sperm not exhibiting the high
quality of the donor sperm.
The present study ended at the delivery of a
full-term infant. Developmental defects of the fetuses were not
observed, which may have been due to the low age of the females
included in the study (30.5±2.7 years). Data concerning the
subsequent development of the children are not available. Although
the ICSI method is often the only option for gravidity and delivery
of biological offspring, certain studies indicate that ISCI almost
doubles the risk of inborn developmental defects in children
(30,32). For males with
oligoasthenoteratospermia, ICSI treatment is often the only
potentially successful method of producing biological offspring.
Despite infertile parents having a number of age-associated
negative factors that may increase the risk of developmental
defects in the fetus, medication and ICSI remain the most efficient
method for males with heavy teratospermia to achieve pregnancy and
the birth of a child, as shown in the present study. The
development of a new technique of continual monitoring in the
cultivation of embryos may provide an improved method for the
selection of the embryos that are most suitable for transfer, which
may subsequently decrease the incidence of fetal developmental
defects (35).
References
|
1
|
Parinaud J, Mieusset R, Vieitez G, Labal B
and Richoilley G: Influence of sperm parameters on embryo quality.
Fertil Steril. 60:888–892. 1993.PubMed/NCBI
|
|
2
|
Braude P, Bolton V and Moore S: Human gene
expression first occurs between the four-and eight-cell stages of
preimplantation development. Nature. 332:459–461. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tesarik J, Greco E and Mendoza C: Late,
but not early, paternal effect on human embryo development is
related to sperm DNA fragmentation. Hum Reprod. 19:611–615. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tesarik J: Paternal effects on cell
division in the human preimplantation embryo. Reprod Biomed Online.
10:370–375. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cohen-Bacrie P: Sperm quality and
selection. J Gynecol Obstet Bio Reprod (Paris). 37(Suppl 1): S4–S8.
2008.(In French).
|
|
6
|
Palermo G, Joris H, Devroey P and Van
Steirteghem AC: Pregnancies after intracytoplasmic injection of
single spermatozoon into an oocyte. Lancet. 340:17–18. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Van Steirteghem AC, Nagy Z, Joris H, Lui
J, Staessen C, Smitz J, Wisanto A and Devroey P: High fertilization
and implantation rates after intracytoplasmic sperm injection. Hum
Reprod. 8:1061–1066. 1993.
|
|
8
|
Aboulghar MA, Mansour RT, Serour GI,
Sattar MA and Amin YM: Intracytoplasmic sperm injection and
conventional in vitro fertilization for sibling oocytes in cases of
unexplained infertility and borderline semen. J Assist Reprod
Genet. 13:38–42. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aboulghar MA, Mansour RT, Serour GI and
Amin YM: The role of intracytoplasmic sperm injection (ICSI) in the
treatment of patients with borderline semen. Hum Reprod.
10:2829–2830. 1995.PubMed/NCBI
|
|
10
|
Nagy ZP, Liu J, Joris H, Verheyen G,
Tournaye H, Camus M, Derde MC, Devroey P and Van Steirteghem AC:
The result of intracytoplasmic sperm injection is not related to
any of the three basic sperm parameters. Hum Reprod. 10:1123–1129.
1995.PubMed/NCBI
|
|
11
|
Cohen J, Alikani M, Munné S and Palermo G:
Micromanipulation in clinical managment of fertility disorders.
Semin Reprod Endocrinol. 12:151–168. 1994. View Article : Google Scholar
|
|
12
|
Riedel HH, Hübner F, Ensslen SC, Bieniek
KW and Grillo M: Minimal andrological requirements for in-vitro
fertilization. Hum Reprod. 4(8 Suppl): 73–77. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Enginsu ME, Dumoulin JC, Pieters MH, Evers
JL and Geraedts JP: Predictive value of morphologically normal
sperm concentration in the medium for in-vitro fertilization. Int J
Androl. 16:113–120. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Petersen CG, Mauri AL, Ferreira R, Baruffi
RL and Franco Júnior JG: Embryo selection by the first cleavage
parameter between 25 and 27 hours after ICSI. J Assist Reprod
Genet. 18:209–212. 2001.PubMed/NCBI
|
|
15
|
Gardner DK and Lane M: Culture and
selection of viable blastocysts: a feasible proposition for human
IVF? Hum Reprod Update. 3:367–382. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lucas H, Lammers J, Pfeffer J, Aknin I,
Carré-Pigeon F, Jafou N, Paulus JM and Sifer C: Conventional IVF
versus ICSI in sibling oocytes: a French experience analysis for
BLEFCO. Gynecol Obstet Fertil. 38:515–520. 2010.(In French).
|
|
17
|
Shoukir Y, Chardonnens D, Campana A and
Sakkas D: Blastocyst development from supernumerary embryos after
intracytoplasmic sperm injection: a paternal influence? Hum Reprod.
13:1632–1637. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aytoz A, Camus M, Tournaye H, Bonduelle M,
Van Steirteghem A and Devroey P: Outcome of pregnancies after
intracytoplasmic sperm injection and the effect of sperm origin and
quality on this outcome. Fertil Steril. 70:500–505. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Palermo GD, Schlegel PN, Hariprashad JJ,
Ergün B, Mielnik A, Zaninovic N, Veeck LL and Rosenwaks Z:
Fertilization and pregnancy outcome with intracytoplasmic sperm
injection for azoospermic men. Hum Reprod. 14:741–748. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Coetzee K, Kruge TF and Lombard CJ:
Predictive value of normal sperm morphology: a structured
literature review. Hum Reprod Update. 4:73–82. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Duran EH, Gürgan T, Günalp S, Enginsu ME,
Yarali H and Ayhan A: A logistic regression model including DNA
status and morphology of spermatozoa for prediction of
fertilization in vitro. Hum Reprod. 13:1235–1239. 1998. View Article : Google Scholar
|
|
22
|
Mansour RT, Aboulghar MA, Serour GI, Amin
YM and Ramzi AM: The effect of sperm parameters on the outcome of
intracytoplasmic sperm injection. Fertil Steril. 64:982–986.
1995.PubMed/NCBI
|
|
23
|
Küpker W, Schulze W and Diedrich K:
Ultrastructure of gametes and intracytoplasmic sperm injection: the
significance of sperm morphology. Hum Reprod. 13(Suppl 1): 99–106.
1998.PubMed/NCBI
|
|
24
|
Lundin K, Söderlund B and Hamberger L: The
relationship between sperm morphology and rates of fertilization,
pregnancy and spontaneous abortion in an in-vitro
fertilization/intracytoplazmic sperm injection programme. Hum
Reprod. 12:2676–2681. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Svalander P, Jakobsson AH, Forsberg AS,
Bengtsson AC and Wikland M: The outcome of intracytoplasmic sperm
injection is unrelated to ‘strict criteria’ sperm morphology. Hum
Reprod. 11:1019–1022. 1996.
|
|
26
|
De Vos A: Intracytoplasmic sperm injection
(ICSI). Hum Reprod. 15(Suppl 4): 59–64. 2000.PubMed/NCBI
|
|
27
|
Fishel S, Aslam I, Lisi F, Rinaldi L,
Timson J, Jacobson M, Gobetz L, Green S, Campbell A and Lisi R:
Should ICSI be the treatment of choice for all cases of in-vitro
conception? Hum Reprod. 15:1278–1283. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
French DB, Sabanegh ES Jr, Goldfarb J and
Desai N: Does severe teratozoospermia affect blastocyst formation,
live birth rate, and other clinical outcome parameters in ICSI
cycles? Fertil Steril. 93:1097–1103. 2010. View Article : Google Scholar
|
|
29
|
Bonduelle M, Camus M, De Vos A, Staessen
C, Tournaye H, Van Assche E, Verheyen G, Devroey P, Liebaers I and
Van Steirteghem A: Seven years of intracytoplasmic sperm injection
and follow-up of 1987 subsequent children. Hum Reprod. 14(Suppl 1):
243–264. 1999.PubMed/NCBI
|
|
30
|
Hansen M, Kurinczuk JJ, Bower C and Webb
S: The risk of major birth defects after intracytoplasmic sperm
injection and in vitro fertilization. New Engl J Med. 346:725–730.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bonduelle M, Wennerholm UB, Loft A,
Tarlatzis BC, Peters C, Henriet S, Mau C, Victorin-Cederquist A,
Van Steirteghem A, Balaska A, Emberson JR and Sutcliffe AG: A
multi-centre cohort study of the physical health of 5-year-old
children conceived after intracytoplasmic sperm injection, in vitro
fertilization and natural conception. Hum Reprod. 20:413–419.
2005.
|
|
32
|
Davies MJ, Moore VM, Willson DK, Van Essen
P, Priest K, Scott H, Haan EA and Chan A: Reproductive technologies
and the risk of birth defects. New Engl J Med. 366:1803–1813. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Katalinic A, Rösch C and Ludwig M; German
ICSI Follow-Up Study Group. Pregnancy course and outcome after
intracytoplasmic sperm injection: a controlled, prospective cohort
study. Fertil Steril. 81:1604–1616. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wennerholm UB, Söderström-Anttila V, Bergh
C, Aittomäki K, Hazekamp J, Nygren KG, Selbing A and Loft A:
Children born after cryopreservation of embryos or oocytes: a
systematic review of outcome data. Hum Reprod. 24:2158–2172. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kirkegaard K, Agerholm IE and Ingerslev
HJ: Time-lapse monitoring as a tool for clinical embryo assessment.
Hum Reprod. 27:1277–1285. 2012. View Article : Google Scholar : PubMed/NCBI
|