Introduction
Immune rejection-mediated failures of corneal
transplantation frequently occur (1). Although therapeutic strategies,
including treatment with corticosteroids or other agents have
improved the survival of grafts, patients with corneal
transplantation may have problems, such as chronic graft loss and
drug-associated adverse effects (2–4).
Therefore, the development of novel therapies and the understanding
of their therapeutic mechanisms are of great significance.
Activated T cells are known to be critical in
allograft rejection. T-cell activation depends on the transfer of
antigenic determinants presented by antigen-presenting cells (APCs)
and costimulation signals that involve the interactions between
costimulatory molecules on T cells and APCs, such as B7/CD28,
CD40/CD154 and B7RP1/ICOS. Both are indispensable in the production
of an effective immune response. Hence, therapeutic modulation of
costimulation signals may control T-cell activation and allograft
rejection (5,6). During the past 20 years, several
therapeutic antibodies against costimulation signaling molecules
have been developed and demonstrated to be effective in inhibiting
allograft rejection, including corneal allograft rejection
(7,8).
CD154 (CD40 ligand), mainly expressed on the surface
of activated CD4+ T cells, is an attractive therapeutic
target (9). Treatment with an
anti-CD154 mAb to block CD40 and CD154 interaction alone or
combined with additional approaches has been shown to be effective
in preventing experimental allograft rejection (10–13).
Administration of anti-CD154 mAb is also applied in the control of
corneal allograft rejection (14–16).
However, the effects of anti-CD154 mAb on allograft survival are
unsatisfactory and the mechanism by which anti-CD154 mAb exerts its
function in preventing allograft rejection remains unclear.
Therefore, investigation of the underlying mechanisms of anti-CD154
mAb may be of benefit.
Previous studies have suggested that the therapeutic
effects of anti-CD154 may be associated with the induction of
T-cell anergy, the deletion of alloreactive CD4+ T
cells, reductions in the levels of Th1 cytokine production or the
suppression of ocular chemokine gene expression (17–19).
Moreover, treatment with anti-CD154 mAb has been shown to enhance
Treg response in a mouse model of islet allograft transplantation
(20). Given that the rejection of
normal-risk corneal grafts is usually slow, the determination of
how anti-CD154 treatment affects the infiltration of alloreactive
effector T cells and Tregs into the grafts is challenging.
In the present study, a high-responsive mouse model
of corneal graft transplantation was employed to determine the
effects of anti-CD154 treatment on Th1 and Tregs response. Our
findings may provide novel insights into the mechanisms by which
anti-CD154 modulates the survival of corneal allografts.
Materials and methods
Animals
Male wild-type C57BL/6 (H-2b) and BALB/c
(H-2d) mice (age, 8–10 weeks) were obtained from the
Experimental Animal Center of Capital Medical University (Beijing,
China). The animals were housed in a specific pathogen-free
facility. Mice were housed at 24°C under 12 h light/dark cycles
with free access to food and water. The experimental procedures
were ethically approved by the Animal Care and Research Committee
of Capital Medical University (Beijing, China).
Corneal transplantation
Recipient BALB/c mice were subjected to orthotopic
penetrating transplantation of a corneal allograft from donor
C57BL/6 mice or syngeneic grafts from BALB/c mice. Surgery was
performed in the right eyes of individual mice, as described
previously (21). Briefly, the
donor corneas and the recipient graft bed were prepared by excising
a 2×2-mm site in the central cornea. The donor button was then
placed onto the recipient bed and secured with eight interrupted
11-0 nylon sutures. Following transplantation, the eyelids were
closed for 3 days with tarsorrhaphy using 8-0 nylon sutures. The
corneal sutures were not removed during the 4-week observation
period.
Experimental design and medical
interventions
Two sets of corneal transplants were designed
respectively. One set was used for clinical assessment of the
grafts and the other set was used for the immunological tests. In
each set, the allograft recipients were randomly injected
intraperitoneally with 250 μg monoclonal anti-CD154 antibody (clone
MR1) or control isotype hamster IgG antibody (both from Bio X Cell,
West Lebanon, NH, USA) on days 0 (immediately after grafting), 3
and 6 following transplantation. The dose and time of medical
intervention protocol used in this study were described previously
(14,22). The syngeneic graft recipients did
not receive anti-CD154 treatment. BALB/c mice transplanted with
corneal allografts were randomly treated with anti-CD154 mAb or
isotype IgG (n=10 per group). BALC/c mice that received syngeneic
corneal grafts were left untreated as controls (n=10).
Evaluation of corneal grafts
Corneal grafts were examined from day 3 post
surgery. The degrees of graft opacity were scored under a slit-lamp
biomicroscope (Topcon SL-1E; Topcon, Tokyo, Japan) twice per week
for up to 4 weeks following transplantation in a blinded manner.
The degrees of opacity were scored as follows: 0, clear; 1+,
minimal superficial opacity; 2+, mild stromal opacity with pupil
margin and iris vessels visible; 3+, moderate stromal opacity with
only pupil margin visible, but iris vessels obscured; 4+, complete
opacity with pupil and iris totally obscured. The onset of graft
rejection was diagnosed as the time when the corneal opacity score
increased to 3+ in a graft that was previously clear following
transplantation, as previously described (23,24).
Histopathology
Eyeballs were removed from individual recipients on
day 14 post surgery and fixed in 10% buffered formalin. The
paraffin-embedded tissue sections (5 μm) were stained with
hematoxylin and eosin, and examined under a light microscope
(Olympus BX51; Olympus, Tokyo, Japan).
Flow cytometric analysis
Ipsilateral draining submandibular lymph nodes,
cervical lymph nodes, spleens and corneal grafts were harvested
from the mice before surgery and at weekly intervals up to 4 weeks
after surgery. Splenic and lymph node single-cell suspensions were
prepared. Spleens and lymph nodes were harvested under sterile
condition and passed through a 200 mesh sieve to remove the tissue
fragments. The red cells were lysed in 5 ml of Tris-ammonium
chloride buffer (0.83% NH4Cl, 5 mM Tris buffer, pH 7.2)
at 37°C for 5 min. Then lymphocytes were washed twice at 500 × g
for 5 min and re-suspended in PBS at a concentration of
1×107 cells/ml. Th1 cells were identified by surface
staining with PerCP-anti-CD3, FITC-anti-CD4 and PE-anti-Tim-3.
Tregs were surface stained first with APC-anti-CD4 and
PerCP-anti-CD25 (eBioscience, Inc., San Diego, CA, USA) and then,
following fixation and permeabilization, were intracellularly
stained with Alexa Fluor 488-conjugated anti-Foxp3 (Biolegend, San
Diego, CA, USA).
For intracellular cytokine staining, splenic single
cells (1×106/well) were stimulated in triplicate with
Cell Stimulation Cocktail (eBioscience, Inc.) in 10% fetal bovine
serum and RPMI-1640 medium (Gibco-BRL, Carlsbad, CA, USA) for 6 h.
The cells were surface-stained with APC-anti-CD4, fixed,
permeabilized, and then intracytoplasmically stained with
FITC-anti-IFN-γ and PerCP-anti-IL-10.
Corneal single cells were prepared as previously
described (25). Briefly, corneal
tissues were dissected and cut into small sections. The corneal
tissues were digested with 2 mg/ml collagenase type IV
(Sigma-Aldrich, St. Louis, MO, USA) at 37°C for 1 h. Subsequently,
the digested tissues were triturated through a 21-gauge needle and
passed through a 70 μm cell filter (BD Falcon; Becton Dickinson,
Franklin Lakes, NJ, USA). The isolated cells (5×104)
were surface stained with PerCP-anti-CD3, FITC-anti-CD4 and
PE-anti-Tim-3, fixed, permeabilized and then intracellularly
stained with Alexa Fluor 488-conjugated anti-Foxp3 (Biolegend).
All intracellular stains were performed using the
FixPerm kit (The Foxp3/Transcription Factor
Fixation/Permeabilization Concentrate and Diluent solutions;
eBioscience, Inc.). Unless otherwise specified, all anti-mouse
antibodies were obtained from eBiosciences. Proper isotype controls
were used in each set of experiments. Samples were acquired on a
FACS Calibur (BD Biosciences) and analyzed using Flowjo software
(Tree-Star, Inc., Ashland, OR, USA).
Suppression assay
For the suppression assay,
CD4+CD25+ Tregs were isolated from the
spleens of the allograft recipients that had been treated with
anti-CD154 or isotype IgG 14 days following transplantation.
CD4+CD25− T cells were isolated from the
spleens of naïve BALB/c mice. The isolation was conducted using a
CD4+CD25+ Regulatory T cell Isolation kit,
according to the manufacturer’s instruction (Miltenyi Biotec,
Bergisch Gladbach, Germany). The purity of the prepared cells was
>95%, as determined by flow cytometry. Fresh isolated
CD4+CD25− T cells were labeled with 0.5 μM
carboxyfluorescein diacetate succinimidyl ester (Invitrogen Life
Technologies, Carlsbad, CA, USA) and served as T effector (Teff)
cells. After washing, 1×105 Teff cells were co-cultured
in triplicate with 2×105 mitomycin-treated C57 BL/6
splenocytes in the presence or absence of Tregs for 72 h. The ratio
of Tregs to Teff cells varied from 1:1 to 1:8. The
alloantigen-stimulated Teff cell proliferation was determined by
flow cytometry and suppression was calculated using the following
formula: Suppression (%) = (Teff proliferation without Tregs - Teff
proliferation with Tregs)/(Teff proliferation without Tregs) ×
100.
Statistical analysis
Data are presented as the mean ± standard deviation.
The survival curves of corneal grafts in different groups of mice
were established by Kaplan-Meier analysis, and the difference among
the different groups of mice was determined by the log-rank test.
The difference in the frequency of T cells among different groups
of mice was determined by analysis of variance with Bonferroni
corrections or Student’s t-test. All analyses were performed using
GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
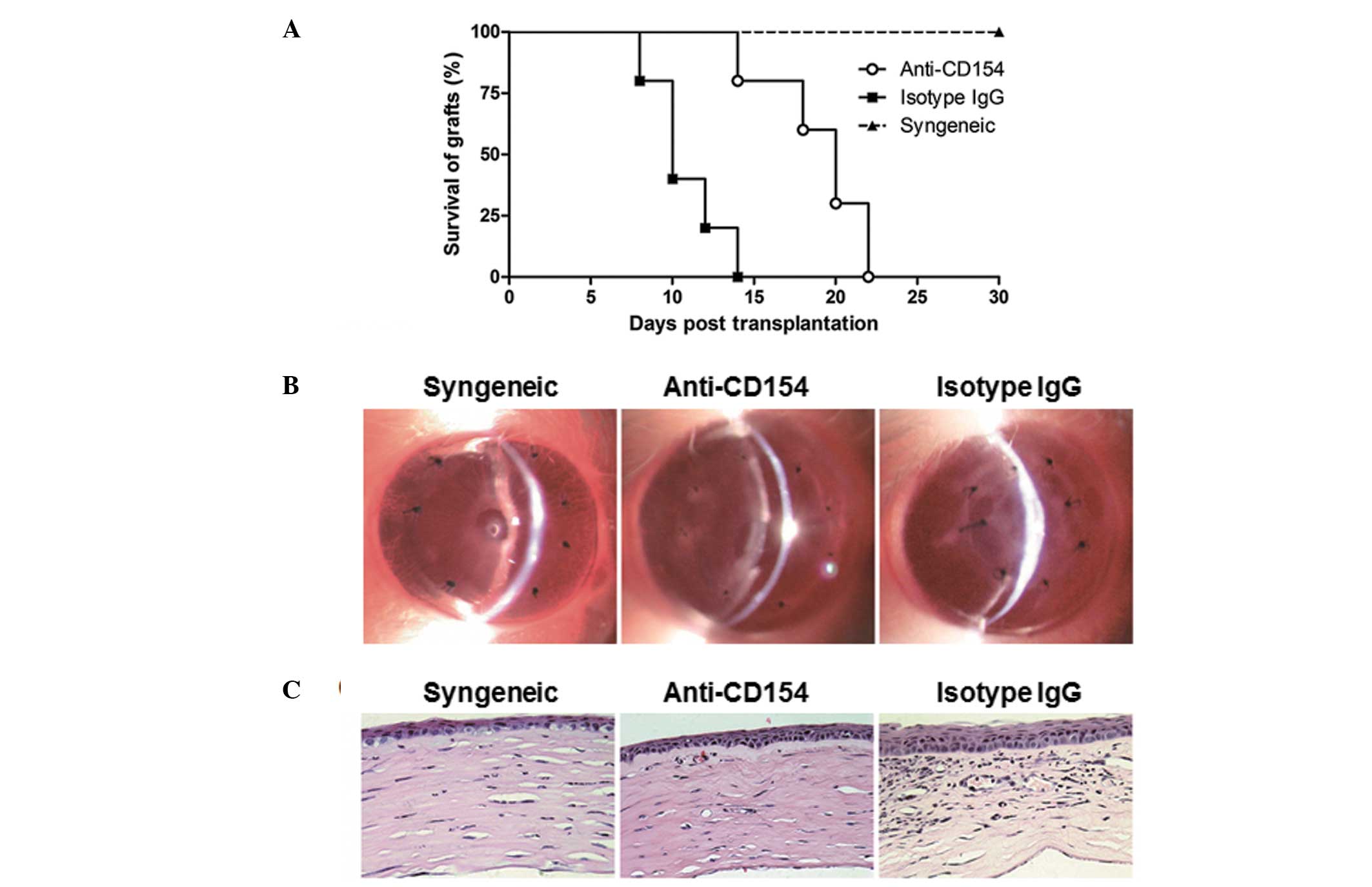
Anti-CD154 neutralization prolongs
survival of the corneal allograft and prevents corneal inflammation
in mice
The effects of short term treatment with anti-CD154
mAb on corneal allograft rejection were assessed. The survival time
of the transplanted corneal in each group was measured at various
time points following transplantation. It was observed that the
syngeneic grafts in the recipients without any special treatment
remained transparent throughout the 4-week observation period. All
the corneal allografts were rejected eventually, but the median
survival time of the grafts in anti-CD154 neutralization mice was
longer than that of the isotype IgG-treated mice (20 days vs. 10
days, P=0.0012; Fig. 1A). Corneal
grafts in the mice treated with isotype IgG began to lose their
transparency at day 8 following surgery and survived for 14 days at
most. By contrast, the onset of corneal allograft rejection in the
anti-CD154-treated mice was delayed to day 14 post surgery (1 week
following treatment withdrawal) and survived for >3 weeks post
surgery. Furthermore, the transparency of corneal grafts was
evaluated by slit-lamp biomicroscopy on day 14 following
transplantation. As shown in Fig.
1B, the corneal allografts in the anti-CD154-treated mice
appeared to have greater clarity than those of the isotype
IgG-treated mice. In addition, the transparency of the corneal
grafts was further analyzed by histopathology. As shown in Fig. 1C, the corneal allografts in the
anti-CD154-treated mice remained intact with a few inflammatory
infiltrations and neovessels in their stromas, which were similar
to those of the syngeneic grafts. By contrast, the allografts in
the isotype IgG-treated mice displayed edematous corneal stromas
with a large quantity of inflammatory cells and
neovascularizations. Given the significant difference between
anti-CD154-treated and isotype IgG-treated mice on day 14 following
transplantation, this time-point was selected for the following
experiments.
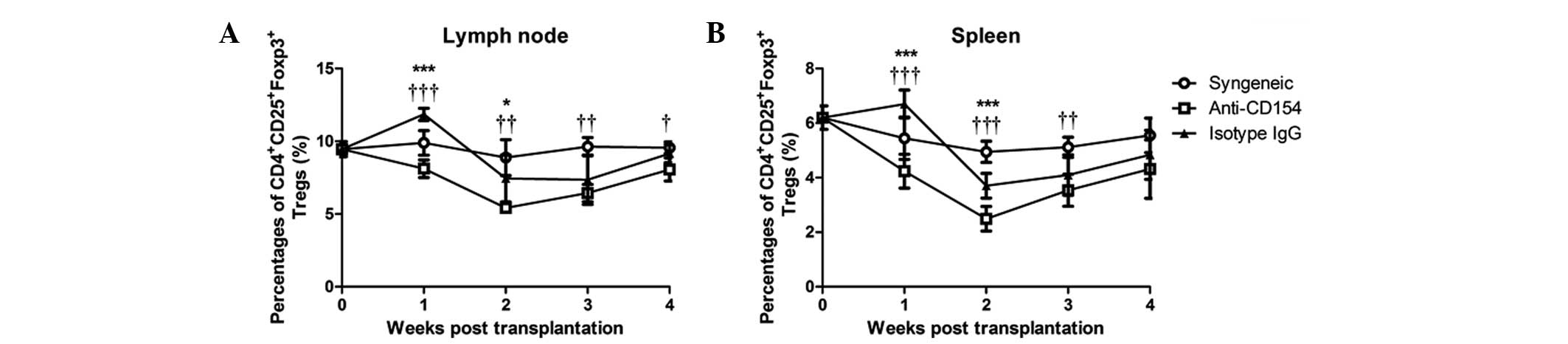
Anti-CD154 neutralization downregulates
the frequency of peripheral Tregs following corneal
transplantation
To investigate the role of Tregs in anti-CD154
mAb-mediated delayed rejection of the corneal allograft, the
frequency of Tregs in the spleens and lymph nodes were analyzed by
flow cytometry. Quantitative results are shown in Fig. 2. The concentrations of
CD4+CD25+Foxp3+ Tregs in the
spleens and lymph nodes in the syngeneic graft mice showed no
difference at any time-points. Following allogeneic corneal
transplantation, the concentration of Tregs marginally increased 1
week following surgery and decreased 2 weeks post surgery in the
isotype IgG-treated mice, followed by a gradual return to a
concentration similar to that prior to transplantation. By
contrast, the percentage of Tregs in the anti-CD154-treated mice
gradually deceased in the first 2 weeks and then increased to a
concentration similar to that prior to surgery. Statistically, the
percentage of Tregs in the spleens and lymph nodes in
anti-CD154-treated mice were significantly lower than those in the
isotype IgG-treated mice at 1 and 2 weeks post surgery (P<0.0001
at week 1, P<0.01 at week 2), and significantly lower than those
in syngeneic graft mice from 1 to 4 weeks post surgery (P<0.0001
at week 1, P<0.001 at week 2 and week 3, P<0.01 at week 4).
Therefore, anti-CD154 neutralization downregulated the peripheral
frequency of Tregs in mice following corneal transplantation.
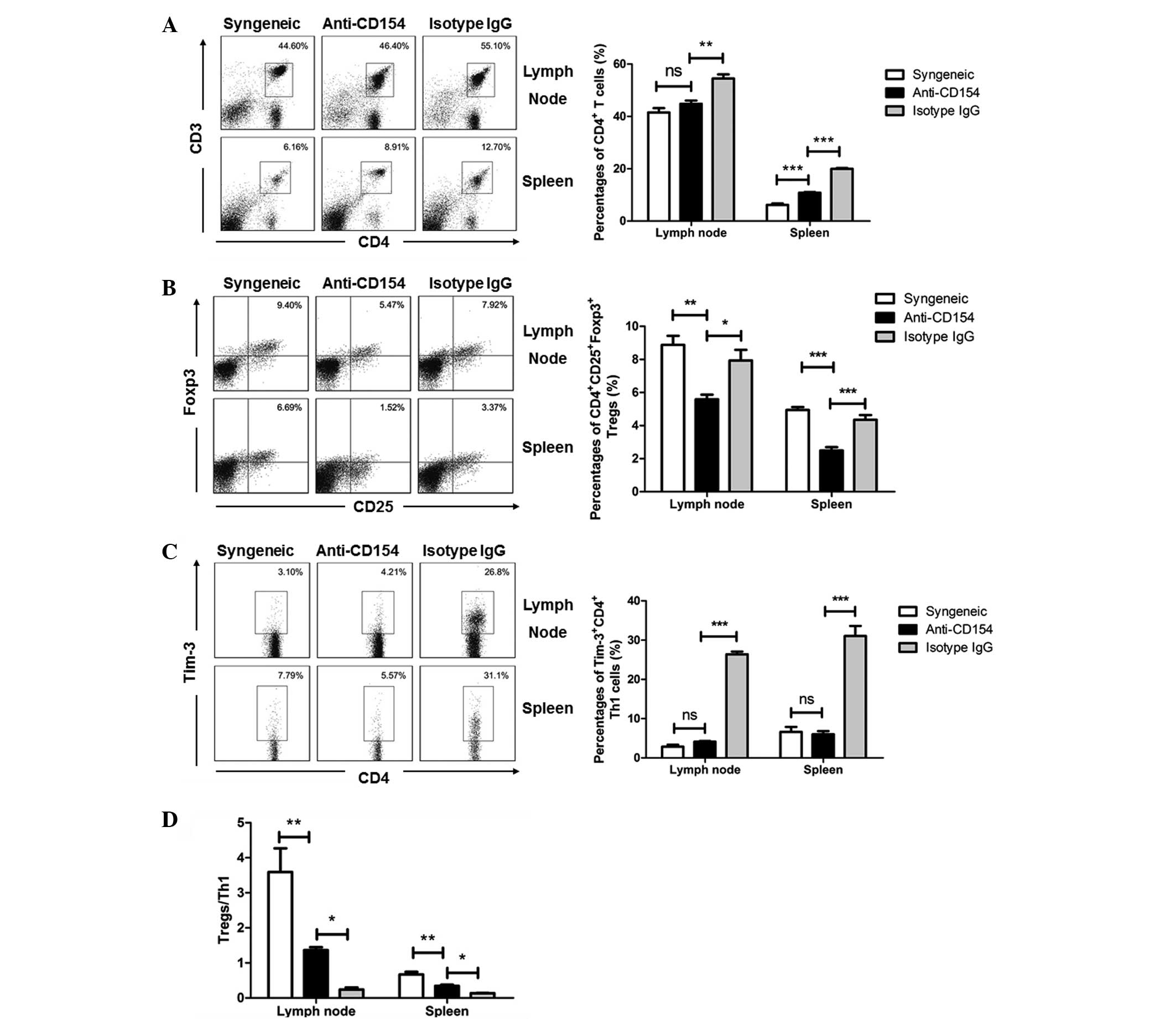
Regulatory effects of anti-CD154 on the
CD4+ T cells, Tregs and Th1 cells in mice following
corneal transplantation
The percentages of CD4+ T cells, Tregs
and Th1 cells in the spleens, lymph nodes and corneal grafts
following transplantation were analyzed by flow cytometry. Fig. 3 shows the flow cytometry results in
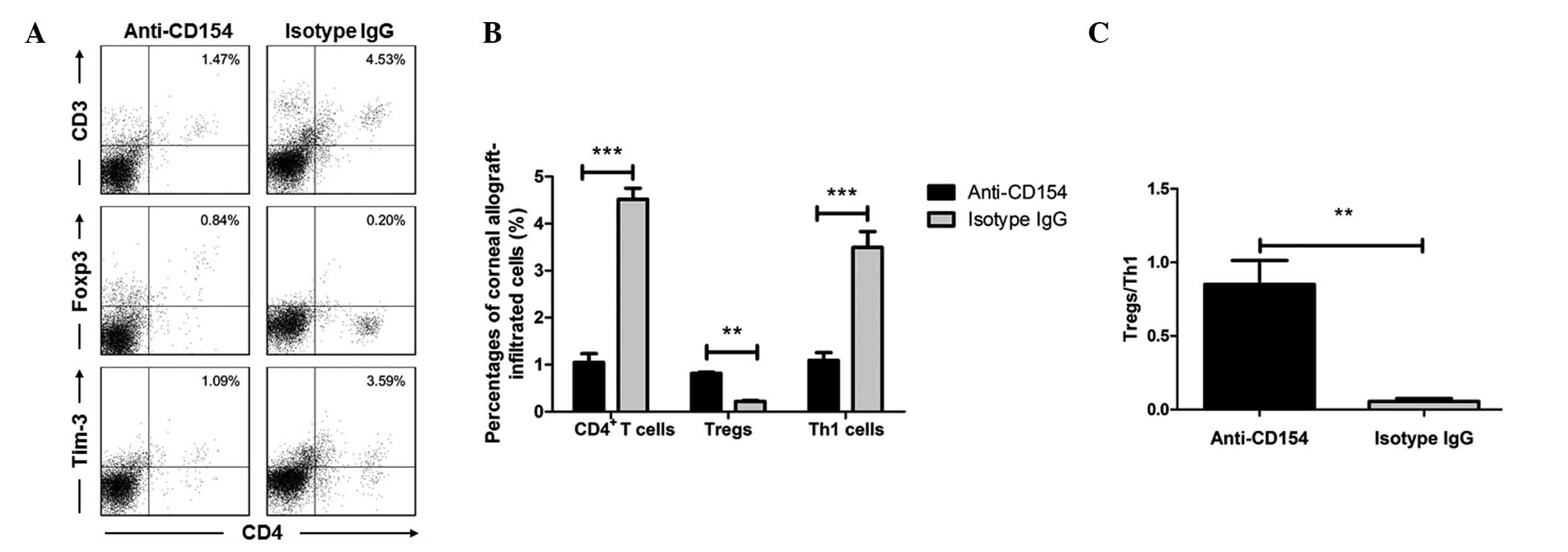
the spleens and lymph nodes, and Fig.
4 shows the flow cytometry results in the corneal grafts (data
of the syngeneic graft group were not shown due to lack of cells).
It was identified that anti-CD154 treatment decreased the systemic
total CD4+ T-cell frequency. The percentages of
CD4+ T cells in the anti-CD154-treated mice were similar
to those in the syngeneic graft mice, and significantly lower than
those in the isotype IgG-treated mice (Fig. 3A, P<0.001 in lymph node and
P<0.0001 in spleen; Figs. 4A and
B, P<0.0001). Th1 cells have been considered as critical for
allograft rejection; therefore, the correlations between Th1 cells
and Tregs in the spleens, lymph nodes and corneal grafts of
different groups of mice were determined. Following anti-CD154
neutralization, the percentages of lymph nodes and splenic Tregs
were significantly lower than those in the syngeneic graft and the
isotype IgG-treated allogeneic graft groups of mice (Fig. 3B). However, the concentration of
Tregs in the corneal grafts from the anti-CD154-treated mice was
upregulated (Fig. 4A and B). The
percentages of lymph node and splenic Th1 cells following
neutralization were similar to those in the syngeneic graft group
of mice, but significantly lower than those in the isotype
IgG-treated allogeneic graft group of mice in all three tissues
(Figs. 3C, 4A and 4B; P<0.0001). Furthermore, the
ratios of Tregs to Th1 cells in the lymph nodes, spleens and
corneal grafts from the anti-CD154-treated mice were significantly
lower than those of the syngeneic graft mice, but significantly
greater than those of the isotype IgG-treated mice (Fig. 3D, anti-CD154 group vs. syngeneic
group, P<0.001; anti-CD154 group vs. isotype IgG group,
P<0.01. Fig. 4C, P<0.001).
These results indicate that a low Treg:Th1 ratio may have
contributed to the inhibition of corneal allograft rejection in
mice.
Anti-CD154 neutralization modulates Treg-
and Th1-associated cytokines in mice following corneal
transplantation
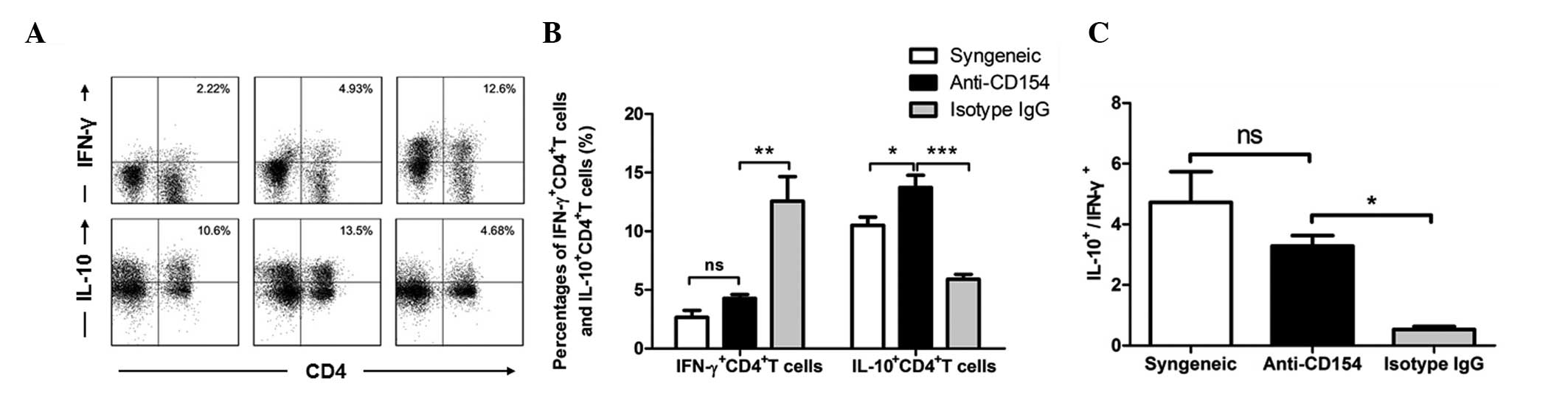
The frequencies of IFN-γ- and IL-10-expressing
CD4+ T cells in the spleens from different groups of
mice were determined by flow cytometry. Representative and
quantitative flow cytometry results are shown in Fig 5. The percentage of splenic
IFN-γ+CD4+ T cells in the anti-CD154-treated
mice was not significantly different from that in the syngeneic
graft group of mice, but significantly lower than that in the
isotype IgG-treated group of mice (Fig. 5A and B; P<0.001). By contrast,
the concentration of IL-10+CD4+ T cells in
mice following anti-CD154 neutralization was significantly higher
than those in the other two groups of mice (anti-CD154 group vs.
syngeneic group, P<0.01; anti-CD154 group vs. isotype IgG group,
P<0.0001). As shown in Fig. 5C,
the ratio of IL-10+CD4+ T cells to
IFN-γ+CD4+ T cells in anti-CD154-treated mice
was significantly greater than that in the isotype IgG-treated mice
(P<0.01) In addition, there was no significant difference in
this ratio between the anti-CD154-treated and syngeneically grafted
mice. Therefore, anti-CD154 treatment significantly increased the
expression levels of IL-10+ by CD4+ T cells,
whereas it downregulated the allograft-mediated expression of
IFN-γ+ by CD4+ T cells. Anti-CD154 treatment
increased the ratio of IL-10+CD4+ T cells to
IFN-γ+CD4+ T cells in mice with allogeneic
corneal transplants.
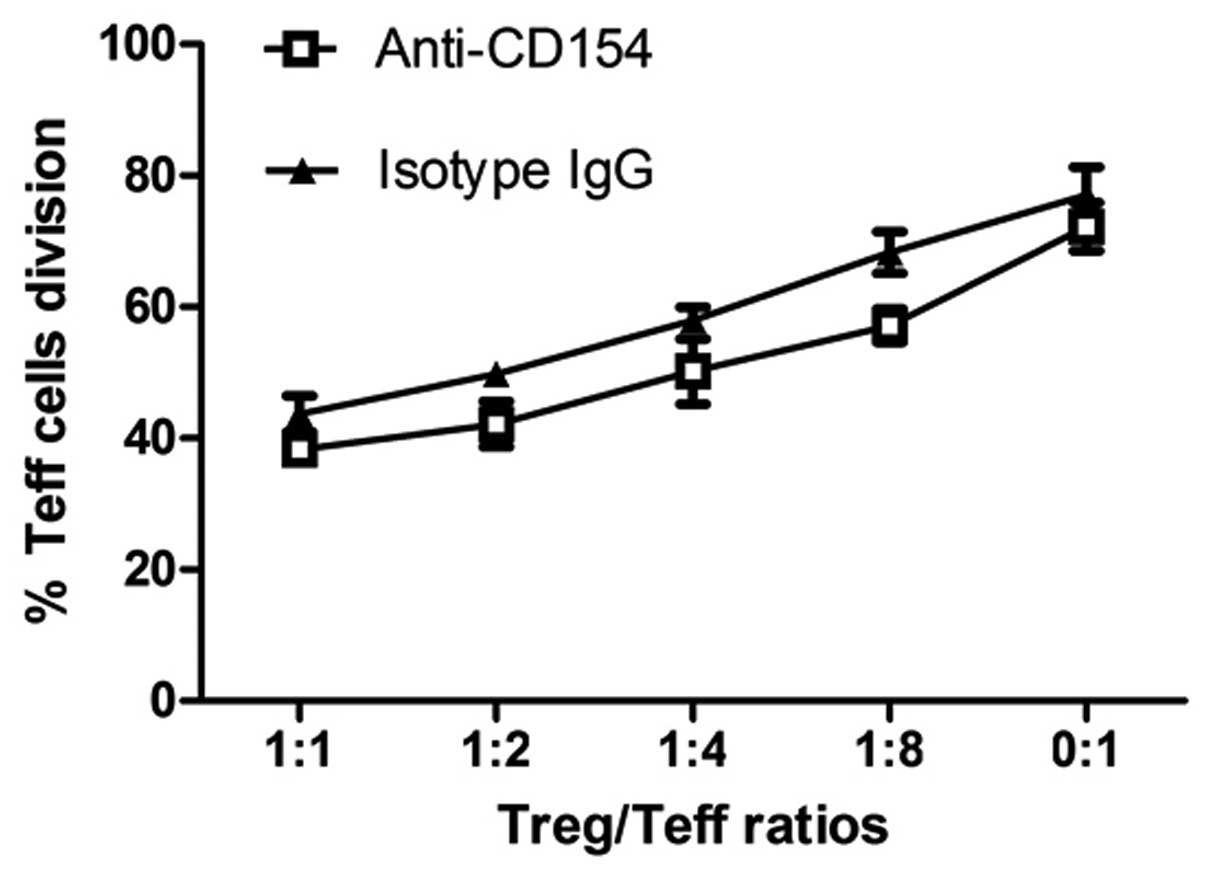
Anti-CD154 neutralization does not
enhance the suppressive activity of Tregs
Since Tregs are able to suppress the proliferation
of effector T cells, the effects of anti-CD154 neutralization on
the suppressive activity of Tregs were further examined. Although
the presence of Tregs in the isotype IgG-treated mice appeared to
have marginally increased the proliferation of Teff cells compared
with that for the Tregs from the anti-CD154-treated mice, no
statistically significant difference was identified in the
suppressive activity of Tregs from the two groups of mice (Fig. 6). These results indicate that
treatment with anti-CD154 mAb does not enhance the suppressive
activity of Tregs in mice with allogeneic corneal
transplantation.
Discussion
Antibody-based immunotherapies have been widely used
for protecting organ transplants from immune rejection. However,
there are only a small number of studies concerning the application
of antibodies in corneal transplantation (26,27).
In the present study, it was demonstrated that treatment with
anti-CD154 mAb (three times) following allogeneic corneal
transplantation prolonged the survival of corneal allografts in
mice, and the survival rate at 2 weeks following transplantation
was 80%. These findings suggest that therapy with anti-CD154 mAb
may not have induced permanent transplant tolerance but rather
prolonged immune suppression. Anti-CD154 treatment inhibited T-cell
activation and prolonged the survival of allografts in the
recipients (28). Hence, the
modest prolonged effects of graft survival were expected.
In the present study, the immunosuppressive effects
of anti-CD154 mAb were confirmed by the findings of CD4+
T cells and their subsets following corneal transplantation.
CD4+ T cells are important in controlling the different
mechanisms involved in corneal graft rejection and are thus a
target for evaluating therapeutic interventions designed to prevent
corneal allograft rejection (29).
In the present study, it was observed that treatment with
anti-CD154 mAb significantly reduced the frequency of total
CD4+ T cells that infiltrated the ipsilateral draining
lymph nodes, spleens and corneal grafts. Engagement of T-cell
receptor on naïve CD4+ T cells by antigen determinant
presented by major histocompatibility complex molecules, may have
activated the differentiation of T cells into different subsets.
Th1 cells are the sole mediators of delayed type hypersensitivity
response, which has been identified to be closely associated with
corneal allograft rejection (30).
In the present study, treatment with anti-CD154 mAb significantly
reduced the frequency of splenic, lymph node and corneal
graft-infiltrating Th1 cells in mice. In parallel, the
concentration of splenic IFNγ+CD4+ T cells
was significantly reduced. These results support the theory that
Th1 cells are the primary, if not sole T-cell population required
for corneal allograft rejection, and the inhibition of Th1
responses by CD154 neutralization is associated with long-term
survival of the corneal allograft.
The role of Tregs in establishing and maintaining
immune tolerance is increasingly appreciated (31–33).
Previous studies in mice have shown that treatment with anti-CD154
mAb enhanced the Treg response and contributed to its inhibitory
effect on allograft rejection (34,35).
Notably, anti-CD154 mAb treatment reduced the concentration of
Tregs in the spleen and lymph nodes at the early stage of corneal
allograft rejection in the present. These results are consistent
with findings in CD154-knockout mice (36). Furthermore, the kinetics of
peripheral Tregs during the entire observation period were
investigated. The levels of Tregs were observed to be reduced
following CD154 blockade and restored at the end of treatment.
Moreover, the frequency of splenic IL-10+CD4+
T cells was increased at the early stage of corneal allograft
rejection. Given that IL-10 may be produced by Tregs and other
regulatory T cells, such as Th2 and Tr1, and that IL-10 is a potent
anti-inflammatory cytokine, the increased concentration of
IL-10+CD4+ T cells may have contributed to
the inhibition of corneal allograft rejection. Notably, no
significant differences in the suppressive activity of Tregs from
the anti-CD154-treated and isotype IgG-treated control mice were
observed. These results re-confirm the immunosuppressive effects of
anti-CD154 mAb on corneal allograft rejection and indicate that the
CD40/CD40L pathway may have marginal regulatory effects on Treg
suppressive activity.
However, the effects of Tregs should not be excluded
as the balance between pro-inflammatory Th1 cells and
anti-inflammatory Tregs may have determined the survival of the
allograft. Accordingly, in the present study was found that the
inhibitory effects of anti-CD154 mAb treatment on the Th1 response
were stronger than that on Tregs. As a result, the ratio of Tregs
to Th1 cells in the anti-CD154-treated mice was higher than that in
the isotype IgG-treated control mice. Therefore, anti-CD154 mAb
treatment preferentially inhibited the pro-inflammatory Th1
responses and regulated the balance of anti-inflammatory Tregs and
pro-inflammatory Th1 responses, resulting in the prolonged survival
time of the corneal allografts in mice.
The cornea has been considered to be blood-free and
possibly lymph vessel-free, and the corneal infiltrating cells are
mainly derived from neovascularized vessels, which reflects the
change in the peripheral cells (37). However, treatment with anti-CD154
mAb increased the frequency of Tregs in corneal grafts. It is
possible that following the blockade of CD40 and CD40L interaction,
Tregs redistribute in the recipients and tended to migrate into the
corneal grafts. Therefore, these results suggest that CD40 and
CD40L interaction not only regulates Treg differentiation, but also
modulates Treg migration in vivo.
In conclusion, our data indicate that treatment with
anti-CD154 mAbs inhibited acute corneal allograft rejection in mice
by increasing the ratio of Tregs to Th1 cells in the peripheral
lymphoid organs and the corneal graft. Our findings also suggest
that modulation of the balance between anti-inflammatory Tregs and
the inflammatory Th1 response may have determined the survival of
the corneal allograft. Therefore, we speculate that the effects of
anti-CD154 mAb on graft survival may be improved by alternatively
adding an additional agent that may strengthen the Treg response.
It should be addressed that, besides Tregs and Th1 cells, other
subsets of T cells, such as Th2, Th9, Th17 and Th22 may be
recruited to regulate allograft rejection. Therefore, further
studies are required to investigate the role of anti-CD154 mAb on
these factors in corneal allograft rejection.
Acknowledgements
This study was supported by grants from the Beijing
Health Systems High-level Health and Technical Talent Training Plan
(grant no. 2009-2-05) and the National Natural Science Foundation
of China (grant nos. 30801264 and 81170824).
References
|
1
|
Watson MP, George AJ and Larkin DF:
Differential effects of costimulatory pathway modulation on corneal
allograft survival. Invest Ophthalmol Vis Sci. 47:3417–3422. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Raizman M: Corticosteroid therapy of eye
disease. Fifty years later. Arch Ophthalmol. 114:1000–1001.
1996.PubMed/NCBI
|
|
3
|
Shimazaki J, Den S, Omoto M, Satake Y,
Shimmura S and Tsubota K: Prospective, randomized study of the
efficacy of systemic cyclosporine in high-risk corneal
transplantation. Am J Ophthalmol. 152:33–39. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Birnbaum F, Reis A, Böhringer D, et al: An
open prospective pilot study on the use of rapamycin after
penetrating high-risk keratoplasty. Transplantation. 81:767–772.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamada J, Kurimoto I and Streilein JW:
Role of CD4+ T cells inimmunobiology of orthotopic corneal
transplants in mice. Invest Ophthalmol Vis Sci. 40:2614–2621.
1999.
|
|
6
|
Bromley SK, Iaboni A, Davis SJ, et al: The
immunological synapse and CD28-CD80 interactions. Nat Immunol.
2:1159–1166. 2001.PubMed/NCBI
|
|
7
|
Thiel MA, Kaufmann C, Coster DJ and
Williams KA: Antibody-based immunosuppressive agents for corneal
transplantation. Eye (Lond). 23:1962–1965. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thiel MA, Coster DJ, Standfield SD, et al:
Penetration of engineered antibody fragments into the eye. Clin Exp
Immunol. 128:67–74. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ford ML and Larsen CP: Translating
costimulation blockade to the clinic: lessons learned from three
pathways. Immunol Rev. 229:294–306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kirk AD, Burkly LC, Batty DS, et al:
Treatment with humanized monoclonal antibody against CD154 prevents
acute renal allograft rejection in nonhuman primates. Nat Med.
5:686–693. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kirk AD, Blair PJ, Tadaki DK, Xu H and
Harlan DM: The role of CD154 in organ transplant rejection and
acceptance. Philos Trans R Soc Lond B Biol Sci. 356:691–702. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arefanian H, Tredget EB, Rajotte RV, Gill
RG, Korbutt GS and Rayat GR: Short-term administrations of a
combination of anti-LFA-1 and anti-CD154 monoclonal antibodies
induce tolerance to neonatal porcine islet xenografts in mice.
Diabetes. 59:958–966. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J, Li H, Jiang N, et al: Effects of
gene transfer CTLA4Ig and anti-CD40L monoclonal antibody on islet
xenograft rejection in mice. Transplant Proc. 42:1835–1837. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qian Y, Boisgerault F, Benichou G and Dana
MR: Blockade of CD40-CD154 costimulatory pathway promotes survival
of allogeneic corneal transplants. Invest Ophthalmol Vis Sci.
42:987–994. 2001.PubMed/NCBI
|
|
15
|
Qian Y and Dana MR: Effect of locally
administered anti-CD154 (CD40 ligand) monoclonal antibody on
survival of allogeneic corneal transplants. Cornea. 21:592–597.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ardjomand N, McAlister JC, Rogers NJ, Tan
PH, George AJ and Larkin DF: Modulation of costimulation by CD28
and CD154 alters the kinetics and cellular characteristics of
corneal allograft rejection. Invest Ophthalmol Vis Sci.
44:3899–3905. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Snyder JT, Shen J, Azmi H, Hou J, Fowler
DH and Ragheb JA: Direct inhibition of CD40L expression can
contribute to the clinical efficacy of daclizumab independently of
its effects on cell division and Th1/Th2 cytokine production.
Blood. 109:5399–5406. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Burrell BE, Lu G, Li XC and Bishop DK:
OX40 costimulation prevents allograft acceptance induced by
CD40-CD40L blockade. J Immunol. 182:379–390. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qian Y, Hamrah P, Boisgerault F, et al:
Mechanisms of immunotherapeutic intervention by anti-CD154 (CD40L)
antibody in high-risk corneal transplantation. J Interferon
Cytokine Res. 22:1217–1225. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen M, Xiao X, Demirci G and Li XC: OX40
controls islet allograft tolerance in CD154 deficient mice by
regulating Foxp3+ Tregs. Transplantation. 85:1659–1662.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huq S, Liu Y, Benichou G and Dana MR:
Relevance of the direct pathway of sensitization in corneal
transplantation is dictated by the graft bed microenvironment. J
Immunol. 173:4464–4469. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Muller YD, Mai G, Morel P, et al:
Anti-CD154 mAb and rapamycin induce T regulatory cell mediated
tolerance in rat-to-mouse islet transplantation. PLoS one.
5:e103522010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cunnusamy K, Chen PW and Niederkorn JY:
IL-17 promotes immune privilege of corneal allografts. J Immunol.
185:4651–4658. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cunnusamy K, Paunicka K, Reyes N, Yang W,
Chen PW and Niederkorn JY: Two different regulatory T cell
populations that promote corneal allograft survival. Invest
Ophthalmol Vis Sci. 51:6566–6574. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen L, Jin Y, Freeman GJ, Sharpe AH and
Dana MR: The function of donor versus recipient programmed
death-ligand 1 in corneal allograft survival. J Immunol.
179:3672–3679. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fu H, Larkin DF and George AJ: Immune
modulation in corneal transplantation. Transplant Rev (Orlando).
22:105–115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rodrigues EB, Farah ME, Maia M, et al:
Therapeutic monoclonal antibodies in ophthalmology. Prog Retin Eye
Res. 28:117–144. 2009. View Article : Google Scholar
|
|
28
|
Rothstein DM and Sayegh MH: T-cell
costimulatory pathways in allograft rejection and tolerance.
Immunol Rev. 196:85–108. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thiel MA, Coster DJ and Williams KA: The
potential of antibody-based immunosuppressive agents for corneal
transplantation. Immunol Cell Biol. 81:93–105. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nickerson P, Steurer W, Steiger J, Zheng
X, Steele AW and Strom TB: Cytokines and the Th1/Th2 paradigm in
transplantation. Curr Opin Immunol. 6:757–764. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hall BM, Tran G and Hodgkinson SJ:
Alloantigen specific T regulatory cells in transplant tolerance.
Int Immunopharmacol. 9:570–574. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gorantla VS, Schneeberger S, Brandacher G,
et al: T regulatory cells and transplantation tolerance. Transplant
Rev (Orlando). 24:147–159. 2010. View Article : Google Scholar
|
|
33
|
O’Connell PJ, Yi S, Carrington EM and Lew
AM: Role of regulatory T cells in xenotransplantation. Curr Opin
Organ Transplant. 15:224–229. 2010.
|
|
34
|
Jiang X, Sun W, Guo D, et al: Cardiac
allograft acceptance induced by blockade of CD40-CD40L
costimulation is dependent on CD4+CD25+
regulatory T cells. Surgery. 149:336–346. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ferrer IR, Wagener ME, Song M, Kirk AD,
Larsen CP and Ford ML: Antigen-specific induced Foxp3+
regulatory T cells are generated following CD40/CD154 blockade.
Proc Natl Acad Sci USA. 108:20701–20706. 2011.PubMed/NCBI
|
|
36
|
Guiducci C, Valzasina B, Dislich H and
Colombo MP: CD40/CD40L interaction regulates
CD4+CD25+ T reg homeostasis through dendritic
cell-produced IL-2. Eur J Immunol. 35:557–567. 2005.PubMed/NCBI
|
|
37
|
Niederkorn JY: High-risk corneal
allografts and why they lose their immune privilege. Curr Opin
Allergy Clin Immunol. 10:493–497. 2010. View Article : Google Scholar : PubMed/NCBI
|