Introduction
Connexins (Cxs) are a family of
structurally-associated transmembrane proteins that assemble to
form vertebrate gap junctions (1).
They are classified according to their predicted molecular mass,
for example, Cx26 for a predicted mass of 26kDa and Cx43 for a
predicted mass of 43kDa. Gap junctions are intercellular channels
that allow direct communication of ions, metabolites and secondary
messengers between neighboring cells that are essential for a
number of physiological processes (2). Cxs are assembled in groups of six to
form hemichannels or connexons, and two hemichannels then combine
to form a gap junction (3).
Mutation in Cx expression often results in functional or
developmental abnormalities.
Antisense oligodeoxynucleotides (AsODNs) are short
synthetic analogs of natural nucleic acids designed to specifically
bind to a target messenger RNA (mRNA) by Watson-Crick
hybridization, resulting in selective degradation of the mRNA or
prohibiting translation of the selected mRNA into protein.
Antisense agents are being studied as treatments for numerous
diseases known to be caused by particular genes. Antisense Cx43 and
antisense Cx31.1 have been developed to modulate Cx43 expression at
the cellular and tissue levels and have been identified to
accelerate skin wound healing in various animal models and clinical
trials (4). There are also studies
that indicate antisense Cx43 and Cx31.1 are novel therapeutic
candidates for ocular wounds, particularly corneal wounds (5,6).
The transparent cornea has a highly ordered
structure that transmits light and serves as a barrier between
external and intraocular environments. Once the cornea is injured,
visual deterioration is likely to be the ultimate consequence.
There are numerous causes of injury, the most commonly observed of
which include infectious diseases, chemical burns and
Stevens-Johnson syndrome (SJS). Corneal infectious diseases involve
inflammation of the cornea resulting from infection by bacteria,
fungi or viruses and are among the leading causes of blindness
worldwide. Chemical injuries, such as those caused by acid and
alkaline agents, may cause extensive damage resulting in visual
impairment through corneal scarring, melting and necrosis (7–10).
SJS is a rare, potentially life-threatening immune-complex
hypersensitivity disorder triggered by medications or infections.
Ocular surface involvement is present in 67–81% of patients and may
be potentially blinding in severe cases (11,12).
Severe dry eye, which ultimately results in corneal opacification
and eyelid deformities, is the most common long-term ocular
complication, which is found in 46% of patients (12). Corneal opacification significantly
hampers vision and there may be subsequent epithelial defects,
ulceration, inflammatory haze, neovascularization as well as limbal
stem cell deficiency in severe cases (13). To the best of our knowledge, Cx
expression patterns in diseased corneas have not been well studied.
In order to further explore the feasibility of using antisense Cx
treatment to improve corneal wound healing, changes in Cx gap
junction protein in terms of mRNA and protein expression levels and
distribution in human corneas injured by inflammation, chemical
burns and SJS were investigated in the present study.
In this study, the mRNA levels of various Cxs in
human cornea tissue with chemical burn, infectious diseases and SJS
were first examined; flow cytometry and immunostaining were
employed to study protein expression levels and localizations, in
the hope of unraveling the potential roles of CXs in the
pathophysiology of these diseases that result in corneal
injury.
Materials and methods
Sample collection
Approval for all human tissue-based research was
obtained from the ethics committee of Zhongshan Ophthalmic Center
(Sun Yat-Sen University, Guangzhou, China). Written consent was
obtained from every patient. Human tissue was handled according to
the tenets of the Declaration of Helsinki. Human corneal buttons
(n=18) were examined: Five normal corneas, which were not suitable
for corneal transplantation, were obtained from the Eye Bank of
Guangdong Province (Guangzhou, China); five chemically burned
corneas, five inflammatory corneas and three corneas affected by
SJS were obtained from patients who had undergone penetrating
keratoplasty. The demographic and clinical characteristics of all
donors of the diseased corneas are depicted in Table I. All corneas were divided into
four quadrant segments; one quadrant for quantitative polymerase
chain reaction (qPCR), the second quadrant for
immunohistochemistry, the third quadrant for flow cytometry, while
the fourth quadrant was stored at −80°C for further
investigation.
 | Table IDemographic and clinical data of the
donors of the diseased corneas. |
Table I
Demographic and clinical data of the
donors of the diseased corneas.
| Disease | Patient no. | Age | Gender | PK | Cause |
|---|
| Chemical burn | 1 | 23 | F | L | Alcohol |
| 2 | 41 | M | R | Hydrochloric
acid |
| 3 | 36 | M | R | Sodium hydroxide |
| 4 | 27 | M | R | Hydrogen cylinder
explosion |
| 5 | 50 | M | L | Sulfuric acid |
| Infection | 1 | 34 | M | R | Aspergillus
flavus |
| 2 | 54 | M | L | Pseudomonas
aeruginosa |
| 3 | 60 | F | R | Staphyloccocus
aureus |
| 4 | 25 | F | R | Herpes simplex
virus |
| 5 | 44 | M | L | Streptococcus
pneumoniae |
| SJS | 1 | 14 | M | L | Sulfonamide |
| 2 | 18 | M | L | Diclofenac |
| 3 | 12 | M | L | Sulfonamide |
RNA extraction and qPCR
Total RNA was extracted using an RNeasy Micro kit
according to the manufacturer’s instructions (Qiagen Inc.,
Valencia, CA, USA). DNase I (Qiagen Inc.) was used to exclude DNA
contamination. The total isolated RNA was quantified by its
absorption at 260 nm and then stored at 280°C until use. With the
housekeeping gene, GADPH, as an internal control, the
messenger RNA (mRNA) expression of eight Cx genes was analyzed in
normal and diseased human corneas by qPCR, which was performed
using ABI TaqMan MGB chemistries and an ABI Prism 7000 light
thermocycler (Applied Biosystems, Foster City, CA, USA). Briefly,
primers and TaqMan probes were designed using PrimerExpress
software 4.0 (Applied Biosystems) and qPCR reactions were optimized
to ensure high amplification efficiency (Table II). qPCR was established by
terminating reactions at the intervals of 20, 24, 28, 32, 36 and 40
cycles for each primer pair to ensure that PCR products were within
the linear portion of the amplification curve. All products were
separated by 2% agarose gel electrophoresis and visualized with 0.5
mg/ml ethidium bromide. The fidelity of the qPCR products was
verified by comparing their size with the size of cDNA bands and by
sequencing the PCR products, and expression levels in the diseased
cornea were normalized to the average level of respective mRNA in
normal cornea. All reactions were performed in triplicate.
 | Table IIPCR primers used. |
Table II
PCR primers used.
| Gene | Forward Primer
(5′-3′) | Reverse Primer
(5′-3′) | AT (°C) |
|---|
| Cx26 |
TCTTTTCCAGAGCAAACCGC |
GACACGAAGATCAGCTGCAG | 58 |
| Cx30.3 |
GCTACCTGCTGCTGAAAGTC |
CGTTGTGTATGAATGGAGCA | 58 |
| Cx31 |
GGCAAAGGATGAAAGCTCAG |
CAACCACAGAGCGAGTGAAA | 64 |
| Cx31.1 |
GTGGACATATGTCTGCAGCC |
CTATGAGAGATGCTAGAGC | 60 |
| Cx32 |
GCGAGGAGACAAGAGGAATG |
AAGCAGCATGCAAATCACAG | 64 |
| Cx43 |
AACTGGCATTCTTGGGTTTG |
CTCAGCATTTTCACCAGTCG | 64 |
| Cx45 |
GCACTGCCAGTAGCAAATCA |
CCAACAGCATCCCTGAAGAT | 62 |
| Cx50 |
GGGCTACCAAGAGACACTGC |
ACCTTCTCCTGCTCCTCCAT | 64 |
| GAPDH |
ACCAAGATCATCCATGACAAC |
GTCCACCACCCCGTTGCTGTA | 64 |
Flow cytometry
All the samples used for flow cytometry were
digested in 2 mg/ml collagenase type IV (Sigma-Aldrich, St. Louis,
MO, USA) and 0.05 mg/ml DNase I in RPMI-1640 for 90 min at 37°C
followed by trituration to form a single cell suspension. The
suspension was then passed through a 40-μm cell strainer and washed
in RPMI medium. Cell suspensions were incubated with antibodies
against Cx26, Cx31.1 and Cx43 (affinity purified mouse monoclonal
antibody; Sigma-Aldrich) in FACS buffer [phosphate-buffered saline
(PBS), 2% fetal bovine serum and 0.1% sodium azide]. After
staining, the cells were fixed in 1% paraformaldehyde and samples
were analyzed by flow cytometry (FACS Aria; BD Bioscience, San
Jose, CA, USA) with a 550-nm laser. Data were analyzed using Flowjo
version 8.7.1 software (TreeStar, Ashland, OR, USA). Analyses were
performed in triplicate and the results are displayed as the
average expression percentage of total cell number.
Immunofluorescence staining
Small tissue blocks (4×4 mm) from the central 6 mm
of every cornea were frozen in liquid nitrogen and then transferred
to a cryostat (Leica Jung CM 1500; Leica Microsystems, Wetzlar,
Germany) and cut into quadrants; one quadrant was cut orthogonally
to the corneal surface and mounted on an electrostatic slide
(Superfrost Plus; Menzel-Gläser, Braunschweig, Germany). They were
washed in PBS for 5 min prior to staining to remove optimal cutting
temperature (OCT) medium. All slides were fixed in cold 99%
methanol for 10 sec at −20°C, then washed three times for 5 min
each in PBS and blocked with 10% fetal calf serum (FCS) for 30
min.
Immunofluorescence staining of the tissue was
performed using a primary antibody against Cx26, Cx31.1 and Cx43
(Sigma-Aldrich). The primary antibody was diluted in 10% FCS
(1:100) and sections were incubated overnight at 4°C. After rinsing
the slides three times for 5 min each in PBS, the sections were
incubated for 30 sec with the secondary antibody (goat anti-mouse
Cy2-conjugated antibody; Jackson ImmunoResearch Laboratories, Inc.,
West Grove, PA, USA). Thereafter, slides were washed three times
for 5 sec each in PBS and finally mounted in anti-fading solution
for fluorescence microscopy (Mowiol; Merck KGaA, Darmstadt,
Germany) containing DAPI (Sigma-Aldrich) and observed with the aid
of a fluorescence microscope (Axiophot; Carl Zeiss Meditec AG,
Oberkochen, Germany). Control slides underwent all the
aforementioned procedures but without incubation with the primary
antibody. Photography was achieved digitally with a camera attached
to the microscope (AxioCam; Carl Zeiss Meditec AG). Multiple
immunostaining experiments were performed on each cornea (at least
four sets of independent experiments for each cornea). All
parameters during image acquisition were kept constant throughout
each experiment to allow direct comparison of all the 8-bit digital
images. Expression levels of Cx proteins were quantified by
counting Cx-positive pixels at the wound edges on binary images
with identical thresholds using NIH ImageJ software (version 1.44
for Windows). Cx pixels were counted per micron squared of cornea
in order to account for differences in corneal thickness.
Statistical analysis
Statistical analysis was performed with SPSS for
Windows, version 16.0 (SPSS, Inc., Chicago, IL, USA). The data of
each group were compared and are expressed as the means ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference. Statistical comparisons between the groups
were performed using analysis of variance.
Results
Cx mRNA expression in diseased
corneas
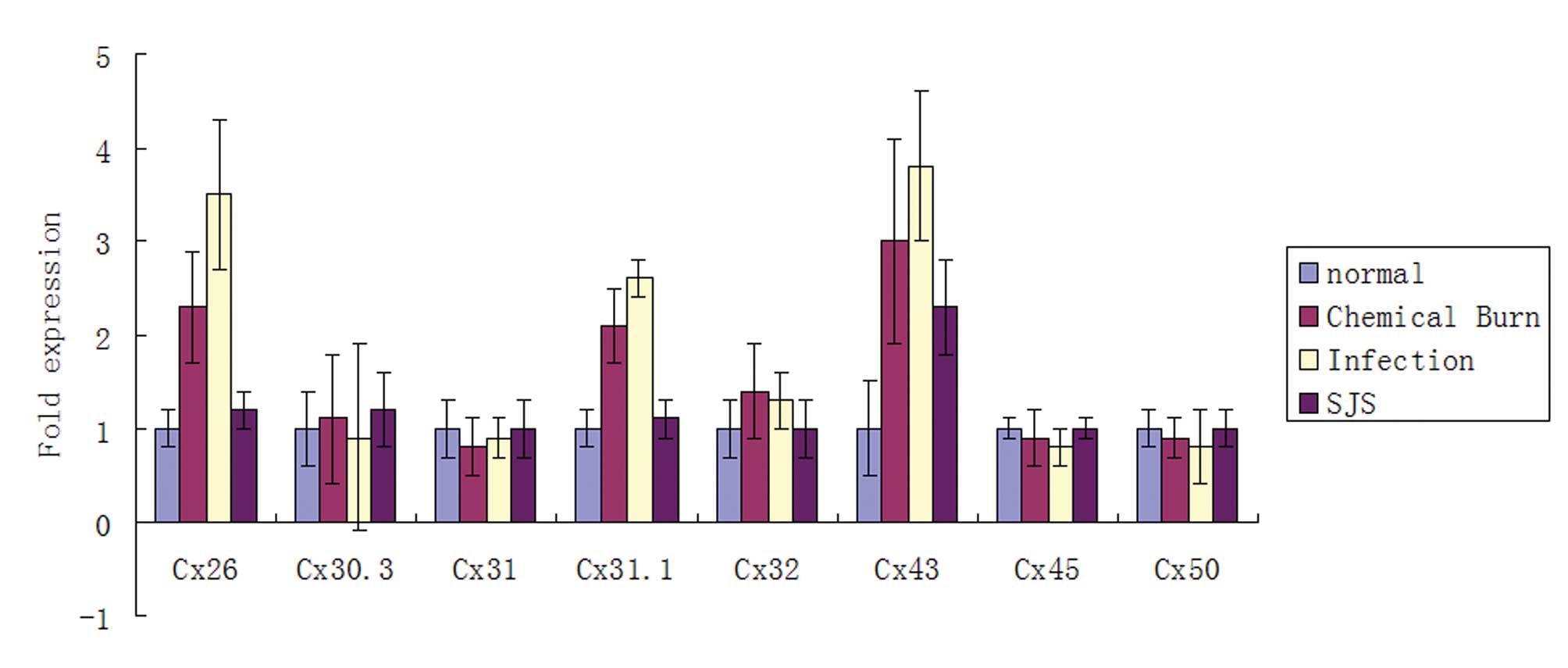
qPCR was performed to evaluate the mRNA expression
levels of Cxs. Of the eight cornea-associated Cx candidates, the
mRNA levels of Cx26, Cx31.1 and Cx43 were significantly higher
(P<0.05) in the diseased corneas than in the normal corneas
(Fig. 1). The remaining five Cx
candidates, Cx30.3, Cx31, Cx32, Cx45 and Cx50 were not observed to
be significantly different between the diseased and the normal
human corneas (Fig. 1).
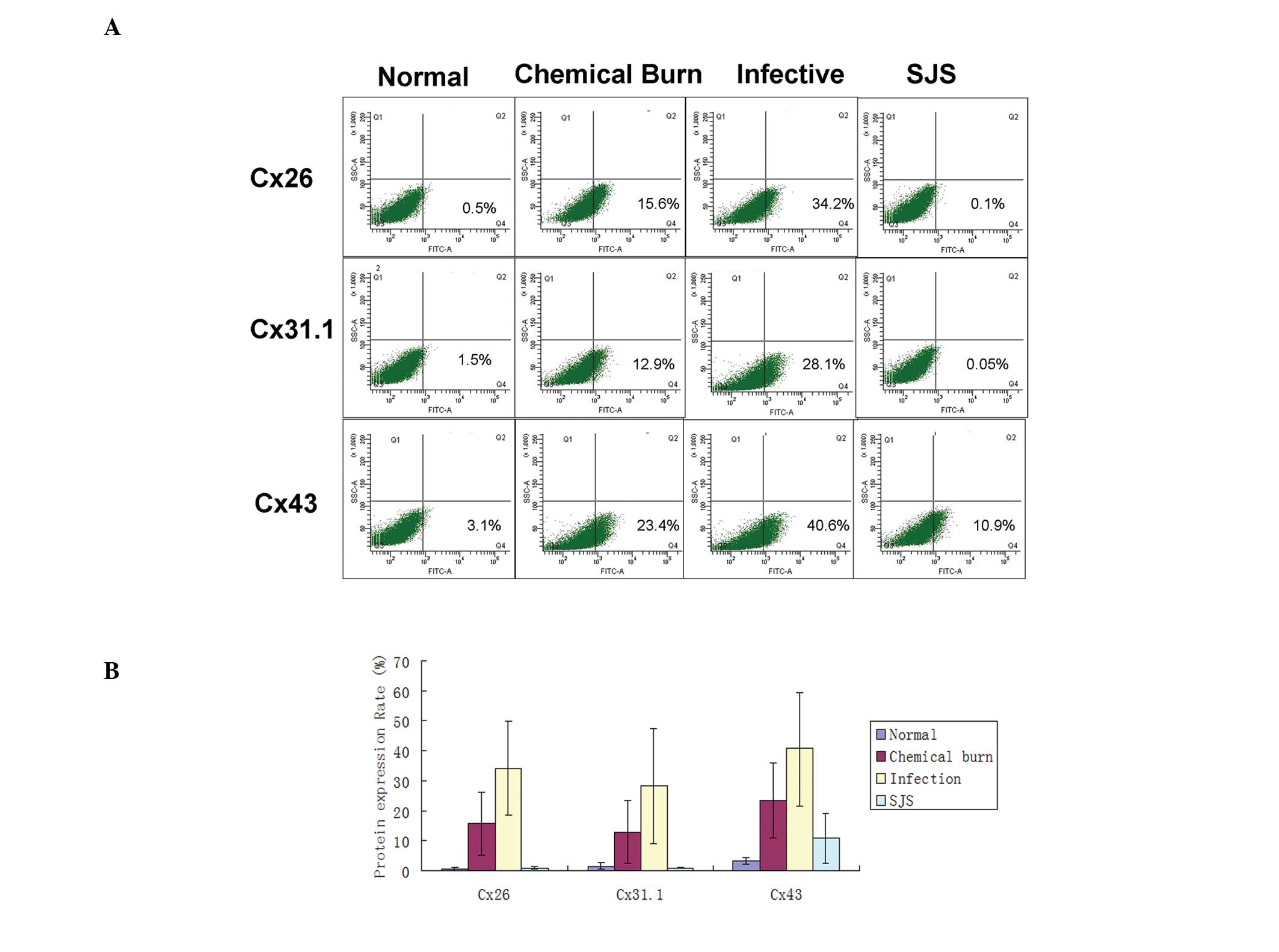
Flow cytometry
To further investigate the quantitative differences
in Cx26, Cx31.1 and Cx43 expression levels, one quadrant of the
diseased corneal tissue was evaluated by flow cytometry. As shown
in Fig. 2, only 0.5±0.5% cells in
normal corneal tissue expressed Cx26, but in the chemical burn,
infected and SJS-affected groups, the percentages of cells that
expressed Cx26 were 15.6±10.4, 34.2±15.6 and 0.1±0.06,
respectively. In addition, with the exception of SJS-affected
corneal tissue, all tissues showed a percentage of Cx26-expressing
cells that was significantly higher compared with that of the
normal corneas (P<0.05). For Cx31.1, the mean baseline
percentage of expression was 1.5±1.2, and the percentages of Cx31.1
expressed in the chemically burned, infected and SJS-affected
corneas were 12.9±10.6, 28.1±19.2 and 0.05±0.1, respectively.
Chemically burned corneas and infected corneas had significantly
higher percentages of Cx31.1-expressing cells compared with that of
the normal corneas (P<0.05), while SJS-affected corneas did not
show a significant difference from normal corneas. The baseline
proportion of cells that expressed Cx43 was 3.1±1.1%. In the
chemically burned, infected and SJS-affected corneas, the
percentages of Cx43-expressing cells were 23.4±12.5, 40.6±19.1 and
10.9±8.4, respectively. In all three injured cornea groups, the
percentages of cells that expressed Cx43 were significantly higher
than the percentage in the normal corneas (P<0.05).
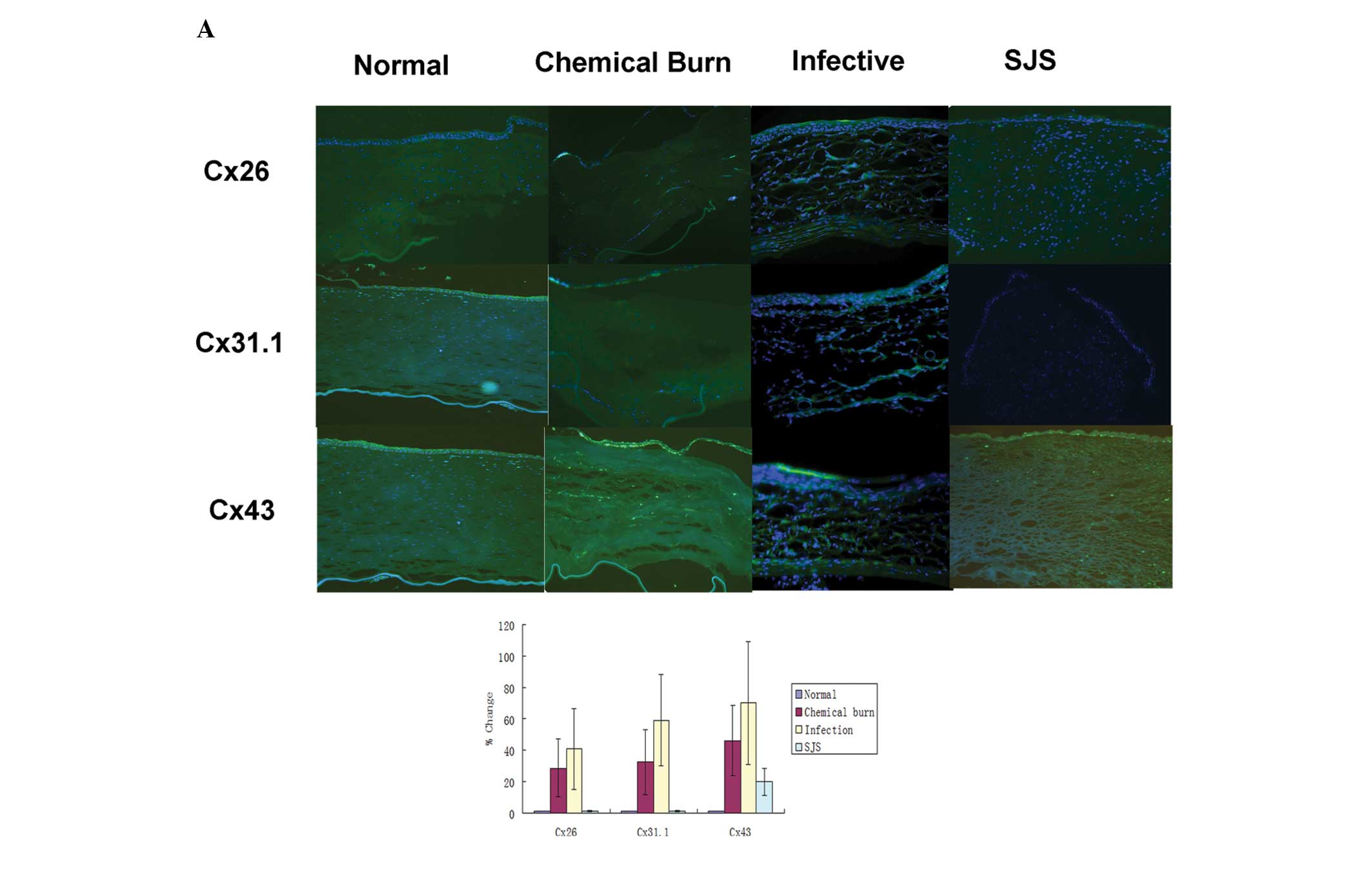
Immunofluorescence staining
Cx31.1 and Cx43 were mainly expressed in the corneal
epithelium and the upper side of the corneal stroma in the normal
corneas, whereas Cx26 showed marginal expression in the corneal
epithelium. There were stronger expression levels of Cx26, Cx31.1
and Cx43 in the chemically burned corneas, but the distribution
varied: Cx26 and Cx43 were mainly localized in the epithelium as
well as the stroma, whereas Cx31.1 was mainly localized in the
epithelium and the upper stroma. Infected corneas showed the
strongest Cx26, Cx31.1 and Cx43 expression levels of all the
corneal samples and expression was throughout the whole area. The
infected corneas did not show a regular lamellar corneal shape due
to the presence of ulcer lesions. In SJS-affected corneas, only the
expression levels of Cx43 were increased in the corneal epithelium
compared with those of the normal corneas; however, the expression
levels of Cx26 and Cx31.1 were slightly downregulated compared with
their levels in the normal cornea (Fig. 3).
Discussion
Ocular trauma and corneal ulceration are significant
causes of corneal blindness. These two causes are often
under-reported but may be responsible for 1.5–2 million new cases
of monocular blindness worldwide each year (14). When the cornea is wounded by
chemicals, infection and hypersensitive immune responses (for
example during the pathogenesis of SJS), the healing process starts
immediately to re-establish corneal homeostasis by restoring the
ocular surface permeability barrier function. Healing is achieved
by the migration of adjacent cells to cover the injured area
(4,15). The migrating cells undergo
substantial phenotypic changes; these cells are then attached to
the substratum as well as to each other. Thus, sufficient
cell-to-cell contacts are retained to make sure the cells migrate
as a cohesive sheet (16). One of
the phenotypical attributes of the healing cornea is its gap
junction-mediated (17) syncytial
arrangement. Syncytial arrangements may be important in the
coordination of physiological processes of tissue healing and
regeneration. Also, it is becoming increasingly clear that Cxs have
profound effects on gene expression during the process of
development. Gap junction-deficient and -mutant mice present a
range of debilitating phenotypes, such as hereditary diseases of
the lens (zonular pulverulent cataract) (18), nervous system (X-linked
Charcot-Marie-Tooth disease) (19), hereditary non-syndromic deafness
(20), skin (palmoplantar
keratoderma) (21), and bone and
teeth (occulodigitoldental dysplasia) (22). These are additional reasons that
altered expressions of gap junction proteins in injured human
corneas require further investigation.
Cxs are the building units of gap junctions. In
forming a gap junction, six Cxs oligomerize to form a hexameric
hemichannel called a connexon. Two connexons join together to form
a functional gap junction and direct cell-cell communication,
namely paracrine signaling, is established. However, it should be
noted that unpaired hemichannels also exist on the cell surface and
play active roles in paracrine intercellular signaling, implicating
the release of signaling molecules, such as ATP, from cells
(23). These hemichannels
constructed of Cxs are distributed around cells where their
channels, when open, complete communication occurring directly
across gap junctions.
Yuan et al (24) detected 10 Cx isoforms (Cx26, Cx30,
Cx30.3, Cx31, Cx31.1, Cx32, Cx43, Cx45, Cx50 and Cx58) in the
central and peripheral primate corneal epithelium by qPCR.
Laux-Fenton et al (25)
demonstrated that eight Cx transcripts (Cx26, Cx30.3, Cx31, Cx31.1,
Cx33, Cx37, Cx43 and Cx50) were present in rat central cornea, and
the peripheral cornea additionally expressed Cx30, Cx40, Cx45 and
Cx46. In the present study, the mRNA expression levels of Cx26,
Cx30.3, Cx31, Cx31.1, Cx32, Cx43, Cx45 and Cx50 of the normal and
diseased human corneas were evaluated by qPCR. The expression of
all eight Cx mRNAs was detected, which supported the results of
Yuan et al and Laux-Fenton et al; however, only Cx26,
Cx31.1 and Cx43 showed significant differences between diseased
corneas and normal corneas. Therefore, the study focused on whether
there were any alterations in the protein expression levels and the
exact topographic distribution of these three Cx proteins in the
diseased corneas.
Flow cytometry was employed to determine the Cx26,
Cx31.1 and Cx43 expression levels. As the purpose of this study was
to explore whether Cx proteins are upregulated or downregulated and
to elucidate the role of antisense Cx treatments in modulating
corneal wound healing, the cell types used in the flow cytometry
experiments were not discriminated. Antisense Cx treatments are
likely to be applied topically to the ocular surface in
vivo; various cell types, such as corneal epithelial cells,
corneal keratocytes and immune cells that have infiltrated during
the diseased state are likely to be affected by the antisense
treatments. The focus of the present study was the variation in
protein expression levels of the specific Cx proteins in the
overall diseased corneas. The results of flow cytometry supported
the findings of the qPCR experiment, which indicated that Cx26,
Cx31.1 and Cx43 mRNA levels as well as protein levels were
upregulated in the chemically burned corneas and infected corneas,
whereas for SJS-affected corneas, only Cx31.1 and Cx43 were
upregulated; Cx26 did not show significant differences in protein
levels from normal values.
Cx43 is the most predominant gap junction protein
expressed in the basal layers of the corneal epithelium and in the
anterior stroma (26,27). It contributes crucially to the
regulation of corneal cell growth and differentiation, thus having
a significant impact on the maintenance of corneal homeostasis
(28,29). Ratkay-Traub et al (30) investigated the changes in Cx43
expression following excimer laser photorefractive keratectomy in
rabbits and found that Cx43 expression was upregulated and
relocated to the upper cell layers of the epithelium 24 h following
surgery. Laux-Fenton (31) further
examined the dynamics of Cx43 protein expression during normal
corneal wound healing by using a rat scrape wound model and excimer
laser surgery, and found downregulation of the Cx43 protein in the
migrating epithelium at the wound front, but upregulation in the
dividing epithelium further back from the wound leading edges. Cx43
was also upregulated in the stroma where it was involved in
hypercellularity that ultimately resulted in corneal haze. In the
present study, it was observed that in all the diseased human
corneas the expression of Cx43 was upregulated both in the
epithelium and stroma, which is similar to the results of
Ratkay-Traub et al (30)
and Laux-Fenton (31). The results
of the present study further confirmed that the Cx43 expression
patterns of animal models, which are usually employed to study the
corneal wound healing process, are similar to that of human corneal
diseases.
Recently, it was identified that in the rat corneal
stroma following wounding, Cx43 protein is initially lost below the
injury site as a result of cell dieback, but is subsequently
upregulated in the stroma in the central and the peripheral areas
of the wound site (32). In the
present study, it was not possible to show the dynamic change in
the expression of Cx43 in human corneal samples; however, it was
demonstrated that Cx43 expression increases after corneal wounding.
Patients with corneal chemical burns are not suitable for corneal
transplantation surgery until the inflammatory reaction has ceased.
In the samples collected in the present study, the mean surgery
time was 0.5±0.3 years following the initial chemical burn
accident, which means that the chemical burn samples were collected
0.5±0.3 years after the corneas were wounded, indicating Cx43 may
have additional roles once the wound is healed with scarring.
Further investigation in animal models is required to elucidate the
dynamic changes of Cx43 mRNA and protein levels in the process of
chemical wound healing. As for the patients with corneal infection,
they underwent surgery as soon as they failed the medication
treatments and their diseased corneas showed the strongest
expression of Cx43 protein within all the groups. This result
indicates that Cx43 is closely associated with corneal
inflammation. It has been found that Cx43 is expressed in activated
leukocytes and at leukocyte-leukocyte contact sites during their
extravasations under inflammatory conditions. Additionally,
functional Cx43 channels are involved in the release of cytokines
and immunoglobulins (33).
Therefore, the upregulated expression of Cx43 protein may be partly
attributed to the infiltration of inflammatory cells into the
diseased corneas. SJS is an immunological disease in which skin and
mucous membranes are the primary target. Cx43 protein was
upregulated compared with the normal corneal level by 10.9% in
SJS-affected corneas, possibly as SJS-affected corneas are more
prone to necrosis and have only minimal inflammation (34).
Cx31.1 is a relatively rare gap junction protein and
appears to be unique in its inability to form functional gap
junction channels, either with itself or with other Cx isoforms
(35,36). It has been shown to be expressed in
the middle and outer layers of the corneal epithelium in rat
corneas, in suprabasal and superficial layers of epithelium as well
as the upper parts of the cornea stroma (37). Expression of Cx31.1 in the corneal
epithelium extends from the suprabasal layers of polyhedral wing
cells through the flat squamous cells of superficial layers, which
are shed into the tear film (31).
Cx31.1 has also been shown to have a dynamic expression pattern
during wound healing (38,39). Cx31.1-specific antisense
oligodeoxynucleotides were used to evaluate its roles in a corneal
epithelium model. Following antisense Cx31.1 treatment in rat and
human corneal organotypic culture models, not only was there
evidence for Cx31.1 knockdown, but also the cornea epithelium
appeared significantly thicker within just 24 h and a marked
reduction in epithelial apoptotic cell numbers was observed. The
results indicated that Cx31.1 may play a role in triggering
apoptosis, leading to cell sloughing into the tear film (6). In the current experiment, it was
identified that Cx31.1 expression levels were significantly higher
at the mRNA and protein levels in the chemically burned and
infected corneas. These findings support the results of Chang et
al (6), as following
chemical burns and infection, apoptosis is triggered both in
corneal epithelial cells and corneal keratocytes (40). The present results, together with
those of Chang et al further indicate that antisense Cx31.1
is likely to be a promising candidate for the treatment of corneal
wounds.
Cx26 is normally expressed in the early embryonic
proliferative epidermis. It is upregulated during wound
re-epithelization and downregulated to low levels as terminal
differentiation occurs to achieve the skin’s permeability barrier
(41,42) Persistent inflammatory cell
infiltration has been observed in re-epithelialized skin with high
levels of Cx26, which may be due to impaired skin barrier function,
Cx26 modulation or both (43).
Djalilian et al suggested that decreasing the Cx26 levels of
epithelialized skin lesions may encourage keratinocyte
differentiation and immune response modulation in the skin
(42). There are few publications
about Cx26 in corneas in a pathological state; however, their
distribution in normal corneas has been investigated (24,25).
In the present study, Cx26 expression was shown to be upregulated
at the mRNA and protein levels in chemically burned and infected
corneas, which may have subsequently impaired the barrier function
of these corneas.
In conclusion, the mRNA and protein levels of Cx31.1
and Cx43 were significantly upregulated in the human cornea samples
affected by chemical burns or infection. The results further
demonstrate the pathology of Cxs during the wound healing period
and indicate that antisense Cx43 and Cx31.1 may be potential
candidates for the treatment of chemically burned and infected
corneas.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 30973434 and 30901807), the
Fundamental Research Funds for the Central Universities (grant no.
10YKPY26) and the Development of Important New Drugs from the
Ministry of Health of China (grant no. 2009ZX09303-007).
References
|
1
|
Cao F, Eckert R, Elfgang C, et al: A
quantitative analysis of connexin-specific permeability differences
of gap junctions expressed in HeLa transfectants and Xenopus
oocytes. J Cell Sci. 111:31–43. 1998.PubMed/NCBI
|
|
2
|
Kumar NM and Gilula NB: The gap junction
communication channel. Cell. 84:381–388. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yeager M and Nicholson BJ: Structure of
gap junction intercellular channels. Curr Opin Struct Biol.
6:183–192. 1996. View Article : Google Scholar
|
|
4
|
Chin KY: Connexins, a new target in wound
treatment. J Wound Care. 20:386–390. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ormonde S, Chou CY, Goold L, et al:
Regulation of connexin43 gap junction protein triggers vascular
recovery and healing in human ocular persistent epithelial defect
wounds. J Membr Biol. 245:381–388. 2012. View Article : Google Scholar
|
|
6
|
Chang CY, Laux-Fenton WT, Law LY, et al:
Antisense down regulation of connexin31.1 reduces apoptosis and
increases thickness of human and animal corneal epithelia. Cell
Biol Int. 33:376–385. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hughes WF Jr: Alkali burns of the eye;
review of the literature and summary of present knowledge. Arch
Ophthal. 35:423–449. 1946. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
WF H: Alkali burns of the eye; clinical
and pathologic course. Arch Ophthal. 36:189–214. 1946. View Article : Google Scholar
|
|
9
|
Wagoner MD: Chemical injuries of the eye:
current concepts in pathophysiology and therapy. Surv Ophthalmol.
41:275–313. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin A, Patel N, Yoo D, DeMartelaere S and
Bouchard C: Management of ocular conditions in the burn unit:
thermal and chemical burns and Stevens-Johnson syndrome/toxic
epidermal necrolysis. J Burn Care Res. 32:547–560. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang YS, Huang FC, Tseng SH, et al:
Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal
necrolysis: acute ocular manifestations, causes, and management.
Cornea. 26:123–129. 2007. View Article : Google Scholar
|
|
12
|
Yip LW, Thong BY, Lim J, Tan AW, Wong HB,
Handa S and Heng WJ: Ocular manifestations and complications of
Stevens-Johnson syndrome and toxic epidermal necrolysis: an Asian
series. Allergy. 62:527–531. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hynes AY, Kafkala C, Daoud YJ and Foster
CS: Controversy in the use of high-dose systemic steroids in the
acute care of patients with Stevens-Johnson syndrome. Int
Ophthalmol Clin. 45:25–48. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oliva MS, Schottman T and Gulati M:
Turning the tide of corneal blindness. Indian J Ophthalmol.
60:423–427. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawakita T, Higa K, Shimmura S, et al:
Fate of corneal epithelial cells separated from limbus in vivo.
Invest Ophthalmol Vis Sci. 52:8132–8137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chan KY, Patton DL and Cosgrove YT:
Time-lapse videomicroscopic study of in vitro wound closure in
rabbit corneal cells. Invest Ophthalmol Vis Sci. 30:2488–2498.
1989.PubMed/NCBI
|
|
17
|
Matic M, Petrov IN, Rosenfeld T and
Wolosin JM: Alterations in connexin expression and cell
communication in healing corneal epithelium. Invest Ophthalmol Vis
Sci. 38:600–609. 1997.PubMed/NCBI
|
|
18
|
Schlingmann B, Schadzek P, Busko S,
Heisterkamp A and Ngezahayo A: Cataract-associated D3Y mutation of
human connexin46 (hCx46) increases the dye coupling of gap junction
channels and suppresses the voltage sensitivity of hemichannels. J
Bioenerg Biomembr. 44:607–614. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scherer SS and Kleopa KA: X-linked
Charcot-Marie-Tooth disease. J Peripher Nerv Syst. 17:9–13. 2012.
View Article : Google Scholar
|
|
20
|
Wangemann P: Supporting sensory
transduction: cochlear fluid homeostasis and the endocochlear
potential. J Physiol. 576:11–21. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
de Zwart-Storm EA, Rosa RF, Martin PE, et
al: Molecular analysis of connexin26 asparagine14 mutations
associated with syndromic skin phenotypes. Exp Dermatol.
20:408–412. 2011.PubMed/NCBI
|
|
22
|
Lorentz R, Shao Q, Huang T, Fishman GI and
Laird DW: Characterization of gap junction proteins in the bladder
of Cx43 mutant mouse models of oculodentodigital dysplasia. J Membr
Biol. 245:345–355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gomes P, Srinivas SP, Van Driessche W,
Vereecke J and Himpens B: ATP release through connexin hemichannels
in corneal endothelial cells. Invest Ophthalmol Vis Sci.
46:1208–1218. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yuan X, Chen Z, Yang Z, et al: Expression
pattern of connexins in the corneal and limbal epithelium of a
primate. Cornea. 28:194–199. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Laux-Fenton WT, Donaldson PJ, Kistler J
and Green CR: Connexin expression patterns in the rat cornea:
molecular evidence for communication compartments. Cornea.
22:457–464. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dong Y, Roos M, Gruijters T, et al:
Differential expression of two gap junction proteins in corneal
epithelium. Eur J Cell Biol. 64:95–100. 1994.PubMed/NCBI
|
|
27
|
Spanakis SG, Petridou S and Masur SK:
Functional gap junctions in corneal fibroblasts and myofibroblasts.
Invest Ophthalmol Vis Sci. 39:1320–1328. 1998.PubMed/NCBI
|
|
28
|
Matic M, Petrov IN, Chen S, et al: Stem
cells of the corneal epithelium lacks connexins and metabolite
transfer capacity. Differentiation. 61:251–260. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wolosin JM, Budak MT and Akinci MA: Ocular
surface epithelial and stem cell development. Int J Dev Biol.
48:981–991. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ratkay-Traub I, Hopp B, Bor Z, et al:
Regeneration of rabbit cornea following excimer laser
photorefractive keratectomy: a study on gap junctions, epithelial
junctions and epidermal growth factor receptor expression in
correlation with cell proliferation. Exp Eye Res. 73:291–302. 2001.
View Article : Google Scholar
|
|
31
|
Laux-Fenton W: The role of connexins in
corneal homeostasis and repair: mastering the connections to
improve repair (PhD thesis). University of Auckland; Auckland, New
Zealand: 2003
|
|
32
|
Grupcheva CN, Laux WT, Rupenthal ID,
McGhee J, McGhee CN and Green CR: Improved corneal wound healing
through modulation of gap junction communication using
connexin43-specific antisense oligodeoxynucleotides. Invest
Ophthalmol Vis Sci. 53:1130–1138. 2012. View Article : Google Scholar
|
|
33
|
Oviedo-Orta E and Howard Evans W: Gap
junctions and connexin-mediated communication in the immune system.
Biochim Biophys Acta. 1662:102–112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vera LS, Gueudry J, Delcampe A, et al: In
vivo confocal microscopic evaluation of corneal changes in chronic
Stevens-Johnson syndrome and toxic epidermal necrolysis. Cornea.
28:401–407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
No authors listed. Gap junction-mediated
intercellular signalling in health and disease. In: Proceedings of
a meeting; London, United Kingdom. 2–5 March 1998; Novartis Found
Symp. 219. pp. 1–284. 1999
|
|
36
|
Harris AL: Emerging issues of connexin
channels: biophysics fills the gap. Q Rev Biophys. 34:325–472.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Laux-Fenton WT, Donaldson PJ, Kistler J
and Green CR: Connexin expression patterns in the rat cornea:
molecular evidence for communication compartments. Cornea.
22:457–464. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Coutinho P, Qiu C, Frank S, Tamber K and
Becker D: Dynamic changes in connexin expression correlate with key
events in the wound healing process. Cell Biol Int. 27:525–541.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Goliger JA and Paul DL: Wounding alters
epidermal connexin expression and gap junction-mediated
intercellular communication. Mol Biol Cell. 6:1491–1501. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wilson SE: Corneal myofibroblast biology
and pathobiology: generation, persistence, and transparency. Exp
Eye Res. 99:78–88. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Coutinho P, Qiu C, Frank S, et al:
Limiting burn extension by transient inhibition of Connexin43
expression at the site of injury. Br J Plast Surg. 58:658–667.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Djalilian AR, McGaughey D, Patel S, Seo
EY, Yang C, Cheng J, Tomic M, Sinha S, Ishida-Yamamoto A and Segre
JA: Connexin 26 regulates epidermal barrier and wound remodeling
and promotes psoriasiform response. J Clin Invest. 116:1243–1253.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
43
|
de Zwart-Storm EA, Rosa RF, Martin PE,
Foelster-Holst R, Frank J, Bau AE, Zen PR, Graziadio C, Paskulin
GA, Kamps MA, van Geel M and van Steensel MA: Molecular analysis of
connexin26 asparagine14 mutations associated with syndromic skin
phenotypes. Exp Dermatol. 20:408–412. 2011.PubMed/NCBI
|