Introduction
Histiocytic necrotizing lymphadenitis (HNL), also
known as Kikuchi-Fujimoto disease, was first described in Japan in
1972 (1,2). HNL is a benign syndrome most commonly
involving cervical lymphadenopathy, fever and night sweats. The
etiology of HNL is unknown but it is hypothesized to be triggered
by an autoimmune or viral process with an exaggerated T
cell-mediated immune response (3–5).
Definite diagnosis of HNL is made only via histopathological
analysis from an open biopsy of the affected lymph nodes (6). The prognosis of HNL is generally
favorable. The majority of patients with HNL have a self-limited
course of the disease that resolves within several months, with a
low recurrence rate of 3–4% (3).
There is no specific treatment for HNL, but in severe cases, the
use of corticosteroids has been recommended (7). The present study reports on a case of
delayed recurrence of HNL with generalized lymphadenopathy 14 years
after the original episode.
Case report
A 30-year-old Chinese female presented with
progressive fever and multiple lymph node enlargement (cervical,
axillary and inguinal). The patient had daily fevers up to 103°F
and presented with a sore throat, cough and fatigue. On physical
examination, the patient exhibited significant and tender cervical,
axillary and inguinal lymphadenopathy, with the largest node being
3×1.5 cm located at the left cervical region. The complete blood
count revealed leukopenia, with a white blood cell count of 3,100
cells/mm3 and an increase in the lymphocyte ratio with
41% lymphocytes. Additional testing revealed elevated lactate
dehydrogenase (LDH), β2 microglobulin (B2-MG) and erythrocyte
sedimentation rate (ESR), while IgG was minimally elevated.
Thyroid-stimulating hormone (TSH) was significantly decreased. The
antinuclear antibody (ANA) test was negative, while C-reactive
protein and liver function tests were normal, as shown in Table I. Infectious etiologies, including
tuberculosis, human immunodeficiency virus (HIV), Epstein-Barr
virus (EB virus), adenovirus, respiratory syncytial virus,
influenza virus, parainfluenza virus, mycoplasma, cytomegalovirus,
hepatitis A, hepatitis B and hepatitis C were negative.
 | Table ILaboratory data at the initial
evaluation. |
Table I
Laboratory data at the initial
evaluation.
| Variable | Admission value | Reference range |
|---|
| WBC (per
mm3) | 3,100 | 4,000–10,000 |
| ESR (mm/h) | 46 | 0–20 |
| Lymphocytes (%) | 41 | 20–40 |
| CRP (mg/dl) | 0.24 | <0.80 |
| ANA | Negative | Negative |
| B2-MG (mg/l) | 2.75 | 0.91–2.2 |
| LDH (IU/l) | 251 | 91–180 |
| TSH (mIU/l) | 0.0005 | 2–10 |
| IgG (g/l) | 16.6 | 9.5–12.5 |
| AST (IU/l) | 32 | 0–40 |
| ALT (IU/l) | 27 | 0–40 |
Ultrasound examinations revealed enlargement of
multiple lymph nodes at the cervical, axillary and inguinal
regions. In addition, ultrasound revealed homogeneously enhancing
lesions, while the central hilar architecture of the lymph nodes
had disappeared. There were hypoechoic areas on both thyroid lobes.
Spiral lung computed tomography revealed multiple mediastinal lymph
node enlargements; the largest was 11×11 mm. An excisional right
cervical lymph node biopsy was performed and the results confirmed
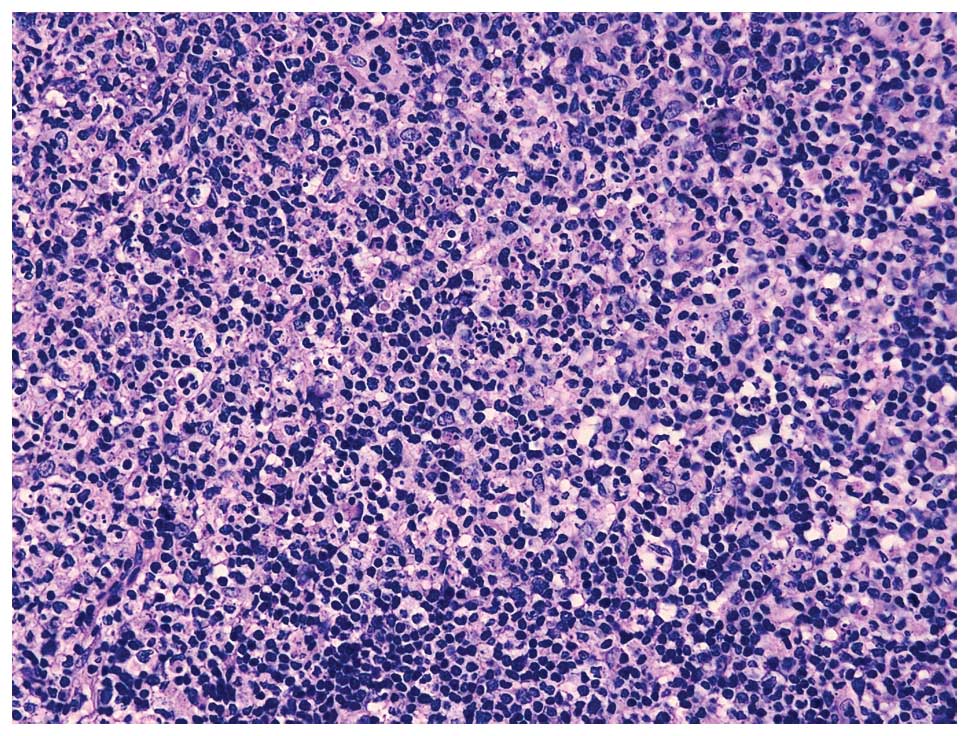
the diagnosis of HNL. Hematoxylin and eosin staining showed the
lymph nodes were replaced by multifocal areas of necrosis and an
abundance of cellular debris was present in the necrotic areas, as
shown in Fig. 1.
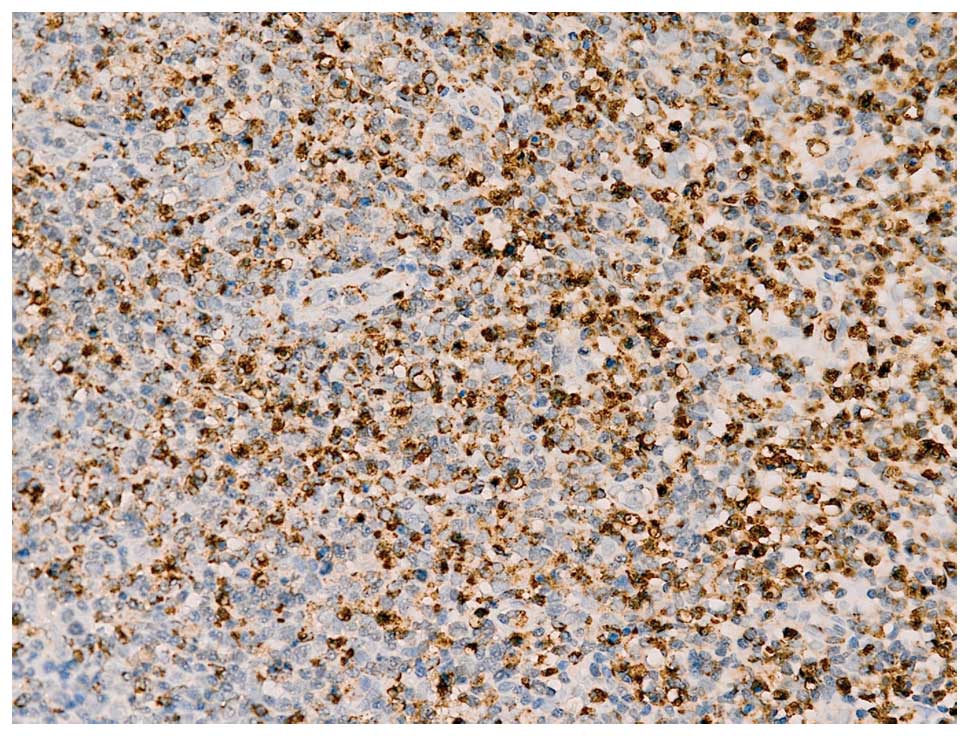
Immunohistochemical staining demonstrated the presence of
CD3+, CD4+, CD8+,
CD20sparsely+, CD21+, Ki-6730%+
and CD68+ (Fig. 2).
Subsequently, the patient received treatment with dexamethasone (5
mg/day via i.v. drip). After one week of therapy, the body
temperature returned to normal and rapid improvement was observed
regarding lymphadenopathy. One month later, ESR, hemogram, IgG,
TSH, LDH and B2-MG returned to normal levels and the enlarged lymph
nodes regressed completely. The dose of dexamethasone was gradually
reduced and stopped after 2 months.
A review of patient history revealed a diagnosis of
HNL 14 years previously. During the first examination, the patient
presented with high fever and cervical lymphadenopathy of one-week
duration. An excisional cervical lymph node biopsy was performed
and the results confirmed a diagnosis of HNL. The patient received
prednisolone treatment, resulting in rapid remission in
lymphadenopathy and fever. The patient was discharged with a full
recovery.
Discussion
HNL is a benign self-limiting condition that causes
lymphadenopathy, most commonly observed in adults younger than 40
years of age. A female predominance has been reported (8). The majority of patients present with
cervical lymphadenopathy and the next most common clinical
manifestation is fever. Other less commonly reported observations
include leukopenia, atypical lymphocytes on peripheral smear, liver
dysfunction bone marrow involvement, fatigue, hepatosplenomegaly
and skin rash (3,9,10). A
definite diagnosis of HNL may be made reliably only via
histopathological analysis from an open biopsy of the affected
lymph nodes (7). There is no
specific treatment for HNL, although in severe cases, the use of
corticosteroids has been recommended to prevent a fatal outcome
(7). Signs and symptoms associated
with HNL usually resolve after several months (3,6,7).
A low recurrence rate of 3–4% has been reported
(3,6,7) and
recurrence has been recorded over a period of two to 12 years
following initial presentation (11,12).
The patient in the present study recurred 14 years after the
initial onset, which represents the longest delayed recurring case
of HNL. Patients with recurrent episodes were more likely to
present with fever, cough and fatigue with frequent extranodal
involvement at the initial presentation (13). Although the disease is
characterized by regional lymphadenopathy, few patients show
generalized lymphadenopathy. In the present case, there was
simultaneous involvement of the cervical lymph nodes with the
axillary and inguinal lymph nodes, even the mediastinal lymph nodes
were involved at recurrence, indicating a generalized lymphoma that
often leads to a misdiagnosis. In the present case, a diagnosis of
HNL was confirmed according to the results from the pathological
slices.
The etiology of HNL recurrence is unknown, but
certain viral infections, including EB virus, parvovirus B19 or
human herpes virus 8, have been hypothesized to be triggers for the
relapse of HNL (14–16). Stéphan et al (17) observed that the recurrence of HNL
was associated with the persistence of EB viral infection. Atarashi
et al (18) reported a case
of recurrent HNL in a human T lymphotropic virus type I carrier.
For the present case, infectious etiologies, including EB virus,
cytomegalovirus and HIV, were all negative. It is unknown whether
other viral infections were associated with HNL in the present
patient.
An association between recurrent HNL and autoimmune
diseases has been reported. Cheng et al (19) described the clinical manifestations
and outcomes of 195 patients diagnosed with HNL. A total of 14 of
96 patients (14.6%) had clinical recurrence of HNL, five of which
developed an autoimmune disease, such as systemic lupus
erythematosus (SLE). Individuals with HNL have been hypothesized to
be more susceptible to SLE, thus, should be routinely screened for
this disorder (3). HNL may
precede, follow or coincide with the diagnosis of SLE. Londhey
et al (20) reported a case
that was initially diagnosed with HNL and SLE simultaneously. The
patient was presently in remission following treatment for SLE. The
fluorescence ANA test is useful in predicting patient prognosis.
However, there is a possibility that recurrent disease with
positive FANA may reflect the overlap between SLE and HNL (13). Lozano Parras et al (21) presented a case of HNL associated
with subacute lymphocytic thyroiditis. In the present case, the
patient showed a significant decrease in TSH levels and ultrasound
revealed hypoechoic areas on both lobes of the thyroid, indicating
possible concurrent thyroiditis.
In conclusion, the present study reported a case of
HNL with a prolonged relapse of 14 years. The patient exhibited
generalized lymphadenopathy, which included enlarged mediastinal
lymph nodes. The patient responded well to a glucocorticoid regime
and a full recovery was achieved in the initial and recurrent
onsets.
References
|
1
|
Kikuchi M: Lymphadenitis showing focal
reticulum cell hyperplasia with nuclear debris and phagocytes: a
clinicopathological study. Nippon Ketsueki Gakkai Zassho.
35:379–380. 1972.
|
|
2
|
Fujimoto Y, Kojima Y and Yamaguchi K:
Cervical subacute necrotizing lymphadenitis. Naika. 30:920–927.
1972.
|
|
3
|
Rezai K, Kuchipudi S, Chundi V, et al:
Kikuchi-Fujimoto disease: hydroxychloroquine as a treatment. Clin
Infect Dis. 39:124–126. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu HL, Lee SS, Tsai HC, et al: Clinical
manifestations of Kikuchi’s disease in southern Taiwan. J Microbiol
Immunol Infect. 38:35–40. 2005.
|
|
5
|
Yen A, Fearneyhough P, Raimer SS and
Hudnall SD: EBV-associated Kikuchi’s histiocytic necrotizing
lymphadenitis with cutaneous manifestations. J Am Acad Dermatol.
36:342–346. 1997.
|
|
6
|
Bosch X, Guilabert A, Miquel R and Campo
E: Enigmatic Kikuchi-Fujimoto disease: a comprehensive review. Am J
Clin Pathol. 122:141–152. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hutchinson CB and Wang E: Kikuchi-fujimoto
disease. Arch Pathol Lab Med. 134:289–293. 2010.
|
|
8
|
Tsang WY, Chan JK and Ng CS: Kikuchi’s
lymphadenitis. A morphologic analysis of 75 cases with special
reference to unusual features. Am J Surg Pathol. 18:219–231.
1994.
|
|
9
|
Mugnaini EN, Watson T, Guccion J and
Benator D: Kikuchi disease presenting as a flu-like illness with
rash and lymphadenopathy. Am J Med Sci. 325:34–37. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seno A, Torigoe R, Shimoe K, et al:
Kikuchi’s disease (histiocytic necrotizing lymphadenitis) with
cutaneous involvement. J Am Acad Derm. 30:504–506. 1994.
|
|
11
|
Cho KJ, Lee SS and Khang SK: Histiocytic
necrotizing lymphadenitis. A clinicopathologic study of 45 cases
with in situ hybridization for Epstein-Barr virus and hepatitis B
virus. J Korean Med Sci. 11:409–414. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Blewitt RW, Kumar SN and Abraham JS:
Recurrence of Kikuchi’s lymphadenitis after 12 years. J Clin
Pathol. 53:157–158. 2000.
|
|
13
|
Song JY, Lee J, Park DW, et al: Clinical
outcome and predictive factors of recurrence among patients with
Kikuchi’s disease. Int J Infect Dis. 13:322–326. 2009.
|
|
14
|
Lee HY, Huang YC, Lin TY, et al: Primary
Epstein-Barr virus infection associated with Kikuchi’s disease and
hemophagocytic lymphohistiocytosis: a case report and review of the
literature. J Microbiol Immunol Infect. 43:253–257. 2010.
|
|
15
|
Yufu Y, Matsumoto M, Miyamura T, et al:
Parvovirus B19-associated haemophagocytic syndrome with
lymphadenopathy resembling histiocytic necrotizing lymphadenitis
(Kikuchi’s disease). Br J Haematol. 96:868–871. 1997.PubMed/NCBI
|
|
16
|
Huh J, Kang GH, Gong G, et al: Kaposi’s
sarcoma-associated herpesvirus in Kikuchi’s disease. Hum Pathol.
29:1091–1096. 1998.
|
|
17
|
Stéphan JL, Jeannoël P, Chanoz J and
Gentil-Përret A: Epstein-Barr virus-associated Kikuchi disease in
two children. J Pediatr Hematol Oncol. 23:240–243. 2001.PubMed/NCBI
|
|
18
|
Atarashi K, Yoshimura N, Nodera H, et al:
Recurrent histiocytic necrotizing lymphadenitis (Kikuchi’s disease)
in an human T lymphotropic virus type I carrier. Intern Med.
35:821–825. 1996.
|
|
19
|
Cheng CY, Sheng WH, Lo YC, et al: Clinical
presentations, laboratory results and outcomes of patients with
Kikuchi’s disease: emphasis on the association between recurrent
Kikuchi’s disease and autoimmune diseases. J Microbiol Immunol
Infect. 43:366–371. 2010.PubMed/NCBI
|
|
20
|
Londhey VA, Buche AS, Kini SH and
Rajadhyaksha GC: Kikuchi fujimoto disease and systemic lupus
erythematosus - a rare association. J Assoc Physicians India.
58:642–643. 2010.PubMed/NCBI
|
|
21
|
Lozano Parras MA, Anguita Alonso P,
Cigüenza Gabriel R, et al: Kikuchi’s disease: a case report and
literature review. An Med Interna. 20:247–250. 2003.(In
Spanish).
|