Introduction
Craniopharyngiomas (CPs) are non-glial tumors and
account for 2–5% of all intracranial tumors (1–3),
constituting 6–9% of all childhood intracranial tumors (4–6). The
age of onset presents bimodal distribution, mainly in 5–15 and
45–55-year-old individuals (5,7) and
does not have a significant gender difference. Based on
histopathology, CPs are benign tumors. However, the manifestation
of CPs is malignant in behavior due to the location of the tumors.
CPs are usually located adjacent to the hypothalamus, optical
nerve, pituitary stalk, pituitary gland and Circle of Willis.
Involvement of these structures within the tumor often leads to
visual disorder, endocrine dysfunction and other manifestations
(2,8,9).
The gold standard in the treatment of CPs is
gross-total resection (GTR) (10–23).
Adjuvant radiotherapy is administered in selected cases of residual
or recurrent tumors (10,24–30)
but chemotherapy is rarely used (31–33).
Postoperative curative results and long-term survival rates are
improved when CPs are treated with GTR. The inability to perform
GTR during the first microsurgery may lead to tumor regrowth and
other clinical issues.
CPs mainly originate from the pituitary stalk, which
plays a crucial role in endocrine function and water-electrolyte
equilibrium. Pituitary stalk preservation may result in incomplete
resection as it is often infiltrated or invaded with tumor tissues.
Pituitary stalk resection may induce endocrine dysfunction,
disruption of the water-electrolyte balance, diabetes insipidus
(DI) and other clinical manifestations. The potential benefits of
GTR, regarding pituitary stalk preservation or resection, should
outweigh the possible recurrence and postoperative endocrine
dysfunction for patients. Thus, the problem of pituitary stalk
preservation or resection during CP surgery is a significant issue
for neurosurgeons.
There have been a number of preliminary studies with
regard to pituitary stalk preservation or resection. A previous
study of adult individuals indicated that pituitary stalks should
be preserved as much as possible (34), as the recurrence rate is not
affected but the patients retain complete anterior pituitary
function. By contrast, a previous study of children (35) indicated that total resection of CPs
together with the pituitary stalk should be performed as the
pituitary stalk has no significant role in endocrine functional
recovery. These studies indicate that guidelines for pituitary
stalk preservation should be established in a greater number of
cases, including adults and children.
The aim of the present study was to determine new
guidelines regarding pituitary stalk preservation or resection
during microsurgery of CPs. A total of 15 pituitary stalk samples
from 2010 onwards were analyzed with an ultra-electron microscope
to reveal the degree of infiltration or invasion of tumor cells and
the guidelines on pituitary stalk preservation or resection were
modified accordingly. In total, 203 cases of CPs in adults and
children were simultaneously analyzed on a retrospective basis to
investigate the difference in the recurrence rate, endocrine
function and incidence of DI between patients in Group A
(preservation of the pituitary stalk) and Group B (resection of the
pituitary stalk).
Materials and methods
Patients
Between 1992 and 2012, 203 patients with
histopathologically confirmed cases of CP underwent surgery. The
gender and age of each patient and the origin, size and location of
the tumor, as well as the clinical features, were recorded. All
preoperative or postoperative procedures were conducted in
accordance with the Declaration of Helsinki. Ethical approval was
provided by The Xiangya Hospital, Central South University
(Changsha, China).
Neuro-imaging
All the patients had undergone preoperative computed
tomography (CT) and magnetic resonance imaging (MRI) scans of the
head. Certain patients had also undergone digital subtraction
arteriography. The size and location of the tumor was recorded.
Postoperative CT scans of the head were immediately performed
following surgery, while MRI scans with contrast were conducted
within 24 h (to exclude any neovascularization that may have
mimicked the residual tumor). Repeat MRI scans were performed every
3 months during year 1, every 4 months during year 2, every 6
months during years 3–5 and every year as from year 6. All the data
were collected as soon as possible.
Recommendations for pituitary stalk
preservation or resection
Certain principles were observed with regard to the
management of the pituitary stalk during the microsurgery of the
CPs. Pituitary stalks were preserved completely if the CP had an
intrasellar origin. Of the 165 cases of CPs originating from the
pituitary stalk, the following three criteria were considered for
complete resection. Firstly, the origin of the CP was the pituitary
stalk and all parts were incorporated within the tumor. Secondly,
the pituitary stalk macroscopically showed a significant thickened
sausage-like appearance and the volume had significantly increased.
Finally, the color of the pituitary stalk was grayish-white,
indicating deprivation of the blood supply.
Postoperative analysis of the pituitary
stalk under an ultra-electron microscope
From 2010 onwards, 15 specimens of resected
pituitary stalk together with the tumor were examined using an
ultra-electron microscope (H-7500 transmission electron microscope;
Hitachi Company, Shiga, Japan) to determine whether the tumor was
invasive or if infiltration was present.
Patient grouping and postoperative
outcomes
Detailed case histories and intraoperative
observations of the patients were recorded carefully. Long-term
follow-ups, regarding long-term survival, tumor recurrence,
endocrine status, visual acuity (VA) and visual field (VF), were
carried out between 1992 and 2012. The shortest follow-up time was
3 months while the longest was 20 years, with an average time of
53.4±47.2 months.
According to the conditions of pituitary stalk
preservation, patients were divided into Group A (preservation of
the pituitary stalk) and Group B (resection of the pituitary
stalk). Differences in the recurrence rate and endocrine functions
between the two groups were investigated.
Statistical analysis
The starting point of overall survival (OS) was
defined as the day the patients had surgery and the terminal point
was defined as the day the patients succumbed to their illness. The
starting point of progression-free survival (PFS) was defined as
the day that patients had surgery and the terminal point was
defined as the day that the tumor recurrence or regrowth was
detected. Kaplan-Meier and logrank tests were used to analyze the
OS and PFS. χ2 and Fisher’s exact tests were used to
compare the data between the two groups. P<0.05 was considered
to indicate a statistically significant difference. All statistical
data were calculated with Office 2003 (Microsoft Corporation,
Redmond, WA, USA) and SSPS version 15.0 (SSPS, Inc., Chicago, IL,
USA).
Results
Patients
Between 1992 and 2012, 203 patients underwent
surgery by the same surgeon and their histopathological
examinations confirmed the diagnosis of CPs. Of these 203 patients,
128 cases (63.1%) were adults (male, 75; female, 53) while 75 cases
(36.9%) were children (male, 45; female, 30). The youngest patient
was 3.5 years old and the oldest was 70 years old, and the average
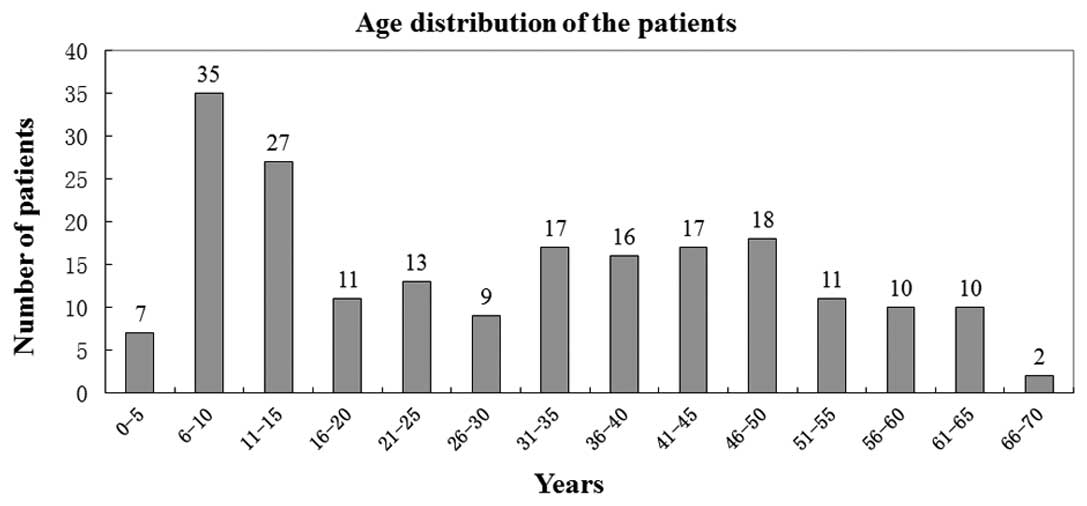
age of the patients was 30.1±18.45 years. The age distribution of
the patients is shown in Fig.
1.
Among the 203 patients, there were 193 cases of
primary tumors and 10 cases of recurrent tumors. Four patients were
required to undergo secondary surgery in view of tumor progression.
A total of 207 surgeries were conducted.
In the study, tumor sizes ranged between 10 and 90
mm and were further subdivided into 4 subgroups: 11 cases, <2.0
cm; 118 cases, 2.0–4.0 cm; 64 cases, 4.0–6.0 cm; and 14 cases,
>6.0 cm. The average tumor size was 38.6±14.0 mm.
Among the 203 patients, 165 cases originated from
the pituitary stalk, 36 cases sourced from the sellar area and 2
cases had an ectopic origin (cerebellopontine angle and optic
chiasm).
The 203 patients were assigned by tumor location
according to Yaşargil’s classification (12), as depicted in Table I. The clinical features of the 203
patients are described in Table
II.
 | Table ITumor location and distribution. |
Table I
Tumor location and distribution.
| Location | Cases, n (%) |
|---|
| Intrasellar | 1 (0.49) |
| Suprasellar | 44 (21.67) |
|
Intra-suprasellar | 45 (22.17) |
|
Intra-suprasellar-3rd ventricular | 29 (14.00) |
| Suprasellar-3rd
ventricular | 82 (40.39) |
| 3rd
ventricular | 1 (0.49) |
| Ectopic
(cerebellopontine angle) | 1 (0.49) |
 | Table IIClinical symptoms. |
Table II
Clinical symptoms.
| Manifestation | Cases, n (%) |
|---|
| VA impairment | 141 (69.5) |
| VF defect | 107 (51.7) |
| Headache | 103 (50.7) |
| Polydipsia and
polyuria | 71 (35.0) |
| Nausea and
vomiting | 59 (29.0) |
| Growth retardation
(children) | 42 (56.0)a |
| Fatigue | 39 (19.2) |
| Decreased libido
(adults) | 34 (26.6)b |
| Irregular
menstruation (female adults) | 27 (50.9)c |
| Epileptic fits | 11 (5.3) |
| Galactorrhea | 5 (2.5) |
Surgical approaches and results
The subfrontal-lamina terminalis approach was the
most frequently used. According to the tumor locations, the
surgical approaches varied. Trans-sphenoidal surgery was only used
once in the study for the case of a sellar CP. The surgical
approaches that were used are listed in Table III.
 | Table IIIVarious surgical approaches employed
during microsurgery of CPs. |
Table III
Various surgical approaches employed
during microsurgery of CPs.
| Surgical
approaches | Surgeries, n
(%) |
|---|
| Subfrontal-lamina
terminalis | 103 (49.76) |
|
Subfrontal-preoptic | 76 (36.71) |
| Subfrontal-optic
nerve-ICA | 16 (7.73) |
|
Pterional-preoptic | 4 (1.93) |
| Pterional-optic
nerve-ICA | 3 (1.45) |
| Transcallosal | 2 (0.97) |
| Retrosigmoid
approach | 2 (0.97) |
|
Trans-sphenoidal | 1 (0.48) |
During the >20 years of the present study, 203
patients underwent microsurgery and 175/203 (86.2%) had GTRs.
Between 1992 and 2002 (first stage) GTR accounted for only 19/36
cases (52.8%); however, there was an abrupt increase in the GTR
rate during the second stage between 2003 and 2012 with 156/167 GTR
cases (93.4%). A typical case is shown in Fig. 2. There was a significant difference
in the GTR rate between the two stages (χ2, 37.78;
P<0.05), indicating that in the hands of a skilled surgeon,
patients suffering from CPs are likely to have an improved surgical
outcome since the senior author has a higher GTR rate.
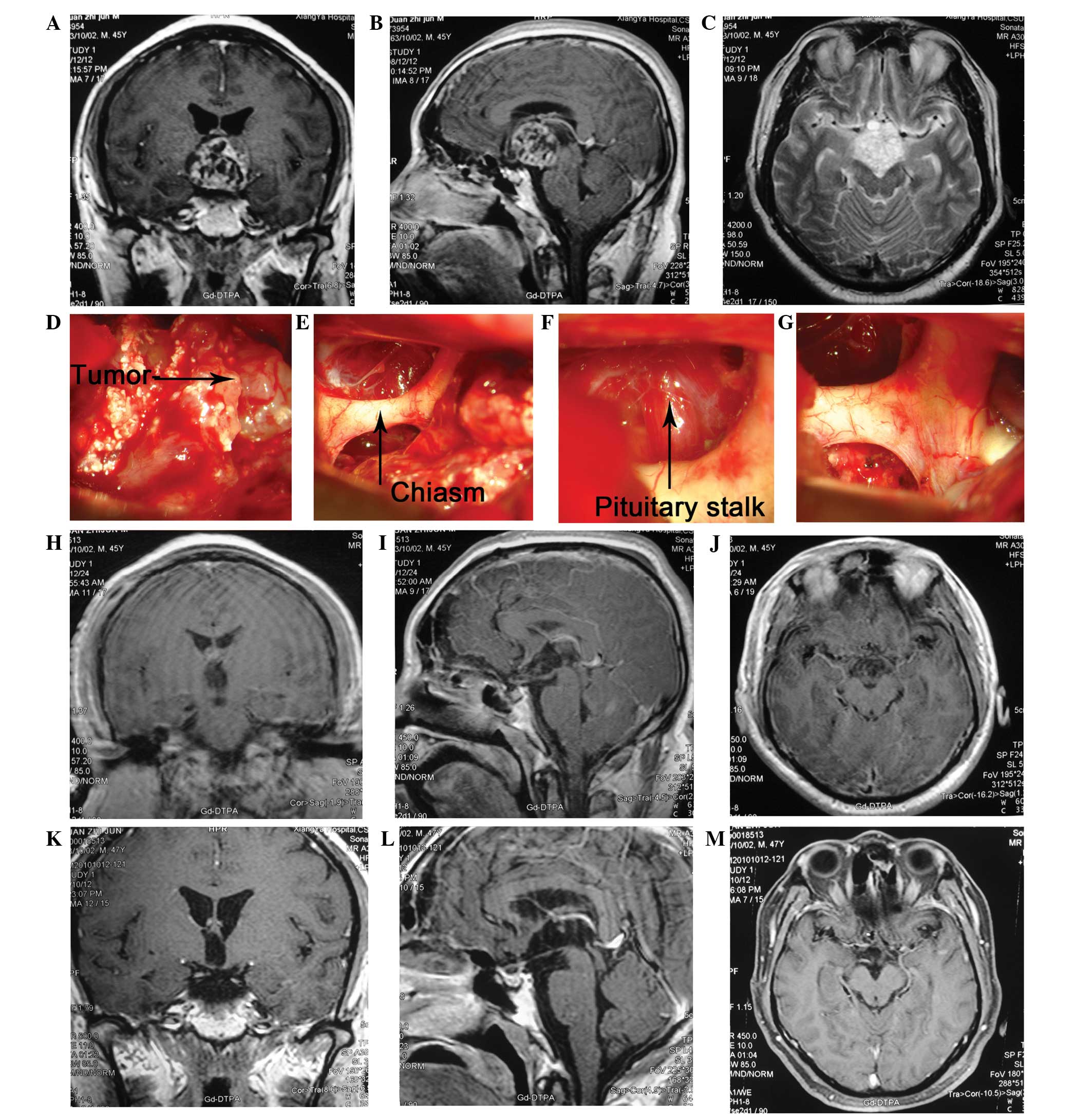 | Figure 2Case of a 45-year-old male who
presented with general fatigue for two months, polyuria with
blurred vision and memory loss for >1 month. The patient was
diagnosed with a CP. During surgery, the tumor was found to
originate from the pituitary stalk. In order to completely remove
the tumor, the pituitary stalk was also removed. Three years
following surgery, the patient resumed work and there was no
evidence of recurrence in MRI scans. (A) The coronary view of CE
MRI showing that the tumor was located in the sellar and parasellar
area. The lesion is mainly solid with contrast enhanced walls. The
borders of the tumor are distinct with absence of peritumoral
edema. Mild compression of the right side of the third ventricle
can also be noted. It also demonstrates the pituitary stalk, the
optic nerve and optic chiasm (see arrow). (B) The sagittal view of
CE MRI and (C) axial view, shows the relationship between the tumor
and the clinoid part of the ICA, optic nerve as well as optic
chiasm. (D, E, F and G) The intra-operative findings of the lesion:
the tumor was located retrochiasmatically and below the clinoid
part of ICA. The pituitary stalk was invaded by the tumor (see
arrow). (H, I and J) Post operative CE MRI depicts complete
excision of tumor together with absence of hemorrhage. (K, L and M)
There was no evidence for tumor recurrence in the 1 year follow up.
CP, craniopharyngioma; MRI, magnetic resonance imaging. |
The perioperative period was defined as the 3 months
following surgery. Perioperative mortality was 4/203 (1.97%). One
patient succumbed to hypothalamus dysfunction, resulting in
hypernatremia, hyperglycemia and coma 35 days following surgery. A
second patient succumbed to cardiopulmonary arrest and bilateral
dilated pupils 15 days following surgery, despite having a ‘clean’
head CT scan (there was not any focal intracranial hemorrhage and
the tumor bed was clear). The third patient succumbed to
uncontrollable hyperpyrexia that developed suddenly 5 days
following surgery. The cause of mortality of the fourth patient was
unknown. The mortalities occurred during the early perioperative
period.
Surgical complications
Postoperative complications were present in the
series, particularly electrolyte disturbances and DI. Detailed
postoperative complications are shown in Table IV.
 | Table IVSurgery-associated complications. |
Table IV
Surgery-associated complications.
| Complications | Cases, n (%) |
|---|
| Electrolyte
imbalance | 142 (70.0) |
| DI | 90 (44.3) |
| Chest
infection | 6 (3.0) |
| Central
hyperthermia | 4 (2.0) |
| Deep vein
thrombosis | 4 (2.0) |
| Disorders of
consciousness | 3 (1.5) |
| Subdural
effusion | 2 (1.0) |
| Intracranial
infection | 2 (1.0) |
| Hypothalamus
dysfunction | 2 (1.0) |
| Intracranial
hematoma | 2 (1.0) |
| Epilepsy | 2 (1.0) |
| Aneurysm of the
right ICA | 1 (0.5)a |
Long-term survival
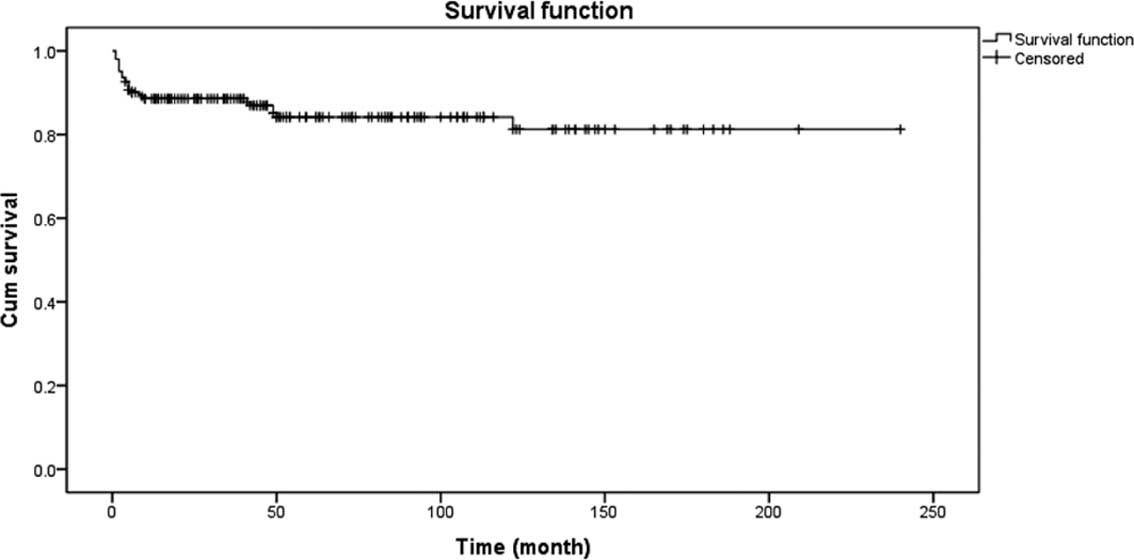
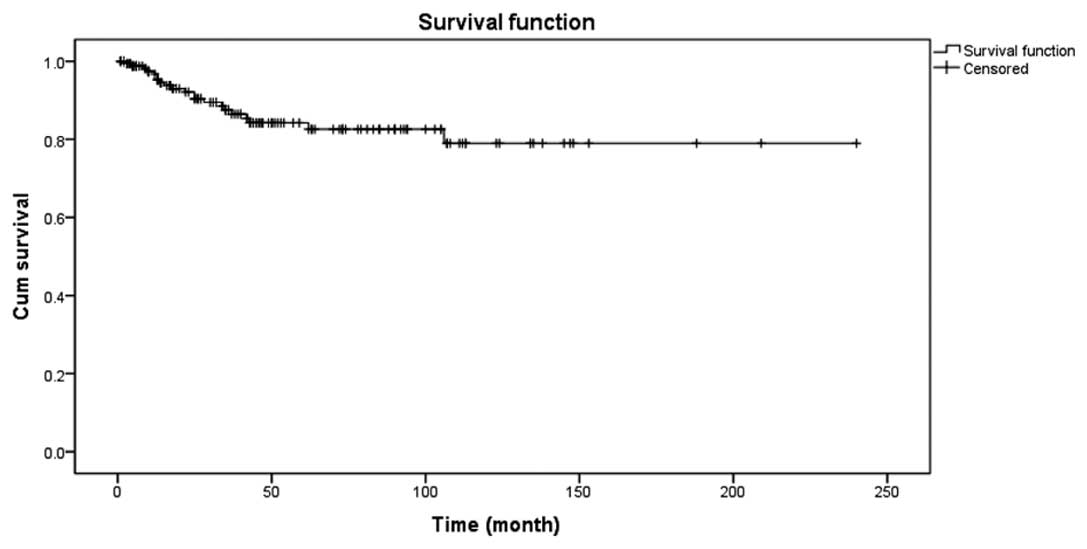
The 5-year survival rate was 84.2% and OS was 81.3%.
Details are shown in Fig. 3. There
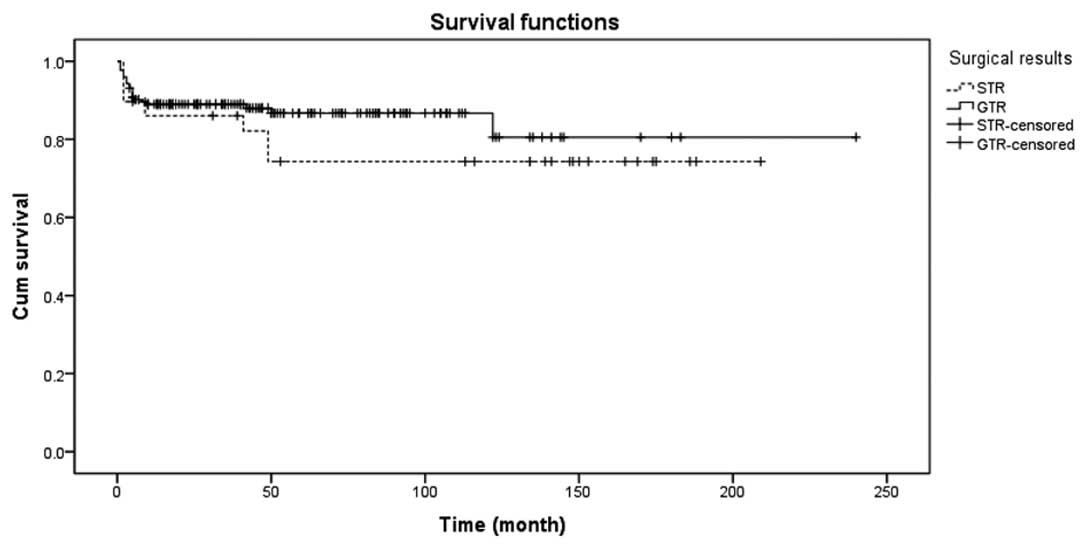
was a significant difference in the long-term survival rate between
the GTR and subtotal resection (STR) groups, as shown in Fig. 4.
Examinations of resected pituitary stalk
specimens with an electron microscope
In total, 15 specimens of resected pituitary stalk,
which satisfied the aforementioned criteria, were examined
postoperatively under an electron microscope to determine whether
the samples were invaded by tumor cells. The results showed that
tumor cells were present in all the 15 resected pituitary stalks
(100%). Fig. 5 shows an electron
microscope image. The pituitary stalk is the unique intracranial
structure that contains longitudinal blood vessels and tissues. The
karyoplasmic ratio in these cells was higher indicating the
presence of tumor cells. The regular morphology indicated the
benign nature of CPs, while the secretory granules located in the
surroundings indicated its endocrine nature. The electron
microscope images clearly indicated that all the pituitary stalk
specimens were invaded with tumor cells. Therefore, it is necessary
to remove the pituitary stalk completely as imminent tumor regrowth
is likely otherwise.
Tumor recurrence
Tumor recurrence was analyzed in the 157 patients
who had undergone GTR and were followed-up on a long-term basis.
The recurrence rate of patients in Group A (4/34, 11.8%) was not
significantly different from that of Group B (10/123, 8.1%;
P>0.05). Of these 157 CP cases, 128 cases originated from the
pituitary stalk. In addition, no significant difference was
identified in the recurrence rate between Group A (1/19, 5.3%) and
Group B (6/109, 5.5%; P>0.05), even when the origin of the CPs
was considered.
Progression rates of tumors following GTR
and STR
In the series, 157 patients received GTR and there
were 14 cases of recurrence with a rate of 14/157 (8.9%). With
regard to the 21 patients who underwent STR, 7 cases of regrowth
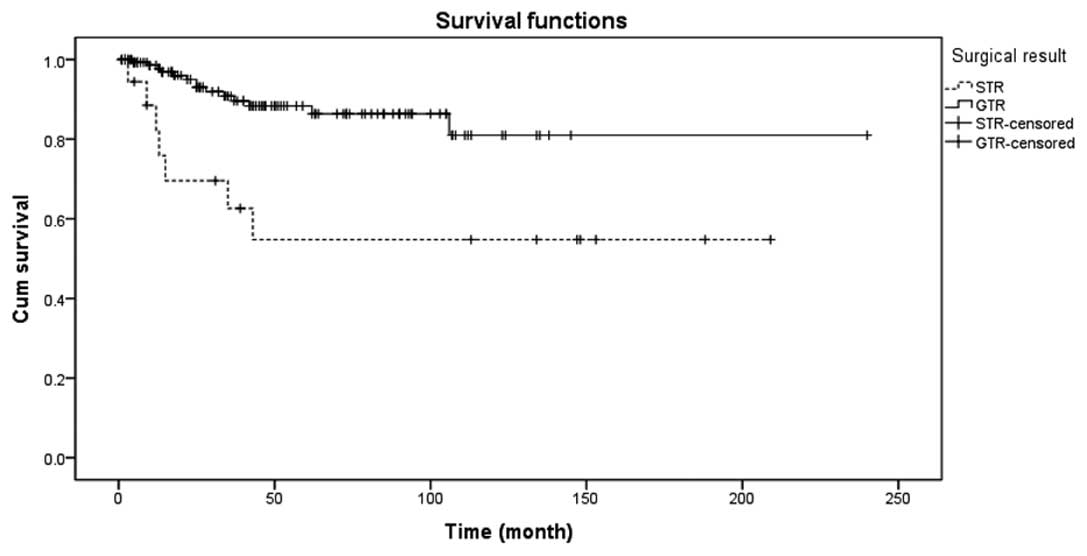
were observed with a rate of 7/21 (33.3%). There was a significant
difference between the two groups, as shown in Fig. 6 (P<0.05). The mean progression
rate of the 178 patients was 21/178 (11.8%), as shown in Fig. 7.
Treatment of recurrent and residual
tumors
In the cases of recurrence in patients that had
undergone GTR, re-GTR, GTR + intracavitary irradiation (RT) with
phosphorus (32P), STR + intracavitary RT with
32P, intracavitary RT with 32P, γ knife
surgery (GKS) and observation were the possible treatment methods.
As for patients treated with STR, the possible treatments were GTR,
re-STR, re-STR + GKS, GKS, RT and observation. The preferred
treatment methods and the number of patients treated with each are
illustrated in Table V.
 | Table VTreatment of residual tumors and
recurrent CPs in 35 patients following resection. |
Table V
Treatment of residual tumors and
recurrent CPs in 35 patients following resection.
| Treatment | Patients, n |
|---|
| Treatment of tumor
recurrence after GTR | 14 |
| Repeat GTR | 2 |
| GTR +
intracavitary RT (32P) | 1 |
| STR +
intracavitary RT (32P) | 1 |
| Intracavitary RT
(32P) | 1 |
| GKS | 2 |
| None | 7 |
| Treatment of
residual tumor after STR | 21 |
| GTR | 1 |
| STR | 1 |
| STR + GKS | 2 |
| GKS | 8 |
| RT | 4 |
| None | 5 |
Endocrine function
Due to incomplete information and loss of follow-up
data, there were 129 cases with a known endocrine status prior to
surgery, 127 cases 1 week following surgery, 109 cases 3 months
postoperatively, 91 cases 1 year following surgery and 91 cases at
the last follow-up, as shown in Table
VI.
 | Table VIEndocrine status at specified
intervals. |
Table VI
Endocrine status at specified
intervals.
| Endocrine
status | Pre-op, n
(%)
n=129 | 1 week, n
(%)
n=127 | 3 months, n
(%)
n=109 | 1 year, n
(%)
n=91 | Last follow-up, n
(%)
n=91 |
|---|
| LH/FSH
deficient | 88 (68) | 92 (72) | 77 (71) | 58 (64) | 59 (65) |
| GH deficient | 26 (20) | 99 (78) | 72 (66) | 53 (58) | 50 (55) |
| TSH deficient | 52 (40) | 100 (79) | 77 (71) | 57 (63) | 54 (59) |
| ACTH deficient | 65 (50) | 101 (80) | 80 (73) | 63 (69) | 64 (70) |
| Hyper PRL | 26 (20) | 51 (40) | 45 (41) | 29 (32) | 28 (31) |
| DI | 45 (35) | 89 (70) | 66 (61) | 39 (43) | 37 (41) |
Endocrine status was defined as normal (entire
endocrine axis was normal), satisfactory (1 or 2 endocrine axes
were abnormal) or poor (≥3 endocrine axes were abnormal). Table VII summarizes the endocrine
statuses of the patients in Group A and B. The endocrine status in
patients who had undergone GTR or STR was evaluated to investigate
any differences with respect to the extent of surgical
decompression. Table VIII
summarizes the endocrine status in the GTR and STR groups.
 | Table VIIAssociation between endocrine status
and groups A and B. |
Table VII
Association between endocrine status
and groups A and B.
| Neuro-endocrine
function |
|---|
|
|
|---|
| Patients | Normal, n (%) | Satisfactory, n
(%) | Poor, n (%) | Total, n |
|---|
| Group A | 5 (21.7) | 18 (78.3) | 0 | 23 |
| Group B | 1 (1.5) | 60 (88.2) | 7 (10.3) | 68 |
| Total | 6 (6.6) | 78 (85.7) | 7 (7.7) | 91 |
 | Table VIIIAssociation between the degree of
resection and endocrine function. |
Table VIII
Association between the degree of
resection and endocrine function.
| Neuro-endocrine
function |
|---|
|
|
|---|
| Extent of
resection | Normal, n (%) | Satisfactory, n
(%) | Poor, n (%) | Total, n |
|---|
| GTR | 6 (7.1) | 73 (85.8) | 6 (7.1) | 85 |
| STR | 0 | 5 (83.3) | 1 (16.7) | 6 |
| Total | 6 (6.6) | 78 (85.7) | 7 (7.7) | 91 |
DI incidence rate
Table IX
summarizes the DI incidence rates in Group A and B.
 | Table IXAssociation between DI and Group
A/B. |
Table IX
Association between DI and Group
A/B.
| DI |
|---|
|
|
|---|
| Patients | Yes, n (%) | No, n (%) |
|---|
| Group A | 5 (16.1) | 26 (83.9) |
| Group B | 44 (37.3) | 74 (62.7) |
| Total | 49 (32.9) | 100 (67.1) |
Outcome of VA and VF
Short- and long-term outcomes of postoperative VA
and VF are summarized in Table X.
In total, 42 patients (55.3%) experienced an improvement in VA and
17 patients (29.3%) had an improved VF status in the short-term
following surgery. However, 5 patients (16.1%) with normal VA and 8
patients (10.5%) with abnormal VA experienced a deterioration. The
deterioration of VF perimetry occurred in 19 patients (38.8%) with
normal VF and 22 patients (37.9%) with abnormal VF.
 | Table XVA and VF recovery for the patients
who received follow-ups. |
Table X
VA and VF recovery for the patients
who received follow-ups.
| | Short-term outcome,
n (%) | Long-term outcome,
n (%) |
|---|
| |
|
|
|---|
| Variable | Patients, n | Improved | Unchanged | Worsened | Improved | Unchanged | Worsened |
|---|
| VA |
| Pre-op normal | 31 | 0 | 26 (83.9) | 5 (16.1) | 0 | 25 (80.6) | 6 (19.4) |
| Pre-op
abnormal | 76 | 42 (55.3) | 25 (32.9) | 8 (10.5) | 46 (60.5) | 20 (26.3) | 10 (13.2) |
| Overall | 107 | 42 (39.3) | 51 (47.7) | 13 (12.1) | 46 (43.0) | 45 (42.1) | 16 (15.0) |
| VF |
| Pre-op normal | 49 | 0 | 30 (61.2) | 19 (38.8) | 0 | 28 (57.1) | 21 (42.9) |
| Pre-op
abnormal | 58 | 17 (29.3) | 19 (32.8) | 22 (37.9) | 19 (32.8) | 14 (24.1) | 25 (43.1) |
| Overall | 107 | 17 (15.9) | 49 (45.8) | 41 (38.3) | 19 (17.8) | 42 (39.3) | 46 (43.0) |
In the long-term follow-up, 46 patients (60.5%) with
abnormal preoperative VA experienced an improvement. In addition,
19 patients (32.8%) with abnormal preoperative VF showed an
improved VF perimetry. However, deterioration in VA was observed in
6 patients (19.4%) with normal VA and 10 patients (13.2%) with
abnormal VA, and deterioration of VF occurred in 21 patients
(42.9%) with normal VF and 25 patients (43.1%) with abnormal
VF.
Discussion
The origin of CPs is quite obscure despite having a
close association with the pituitary gland and stalk. Two
hypotheses have been reported: The embryo-genetic and the
metaplastic theories (36). The
embryo-genetic theory hypothesizes that CPs originate from the
distal portion of the adenohypophysis, in particular the
craniopharyngeal canal or remnant epithelium on Rathke’s cysts. The
second theory stipulates that CPs originate from metaplastic
epithelial squamous cells of the primitive oral cavity; namely from
pars tuberalis of the adenohypophysis. The distal portion of the
adenohypophysis forms part of the pituitary gland, whereas the pars
tuberalis is an integral part of the pituitary stalk. Thus, this is
the theoretical basis for defining intrasellar or pituitary stalk
origin.
The origin of CPs has a close association with the
pituitary stalk. Therefore it is particularly important to identify
this structure during microsurgery. The pituitary stalk is easily
identifiable under normal conditions. However, under a pathological
state, it is difficult to identify due to the distortion of its
shape, displacement or incorporation within the tumor. Based on a
previous study (37), the
following methods were designed to identify the pituitary stalk.
The first relates to the anatomical position of the pituitary
stalk. It is connected upwards by the hypothalamus and courses
downwards through the diaphragma sellae foramen to enter the
intrasellar region and link with the pituitary gland. During
microsurgery, the pituitary stalk is identified as the structure
running through the diaphragma sellae foramen connecting the
hypothalamus and pituitary gland. Secondly, the pituitary stalk may
be identified by its unique microscopic structure. Longitudinal
stria medullaris structures are observed on the surface of the
pituitary stalk. The pituitary stalk is a unique intracranial
structure that contains longitudinal blood vessels and tissues. Any
structure found in the sellar area bearing these characteristics
should be considered as the pituitary stalk.
Since the pituitary stalk has a close association
with CPs, management of the pituitary stalk is a significant issue
during microsurgical excision. However, to date there have only
been a few studies concerning the identification and preservation
of the pituitary stalk. The majority of studies (12,34,38–42)
indicate whether craniotomy or trans-sphenoidal surgery is the most
optimal surgical route to identify as much of the pituitary stalk
as possible (Table XI). In
addition, the studies indicate that whenever the pituitary stalk is
involved within the tumor, it can be resected to achieve GTR.
Moreover, when the pituitary stalk is not invaded or partially
infiltrated by tumor cells, it should be preserved to outweigh the
risk of endocrine dysfunction and DI. Preservation of the distal
end of the pituitary stalk only is likely to alleviate DI and be
beneficial for adenohypophysis recovery. Finally, the studies
indicate that pituitary stalk preservation guarantees the integrity
of the hypothalamus.
 | Table XIKey studies and views associated with
the identification and preservation of the pituitary stalk. |
Table XI
Key studies and views associated with
the identification and preservation of the pituitary stalk.
| Author | Date | Total cases, n | Cases involved in
calculation, n | Pituitary stalk
identification, n (%) | Pituitary stalk
preservation, n (%) | Views |
|---|
| Yaşargil | 1990 | 144 | 115 | 74 (64.3) | 42 (36.5) | Pituitary stalk
preserved as much as possible |
| Honegger | 1999 | 143 | 127 | 74 (58.3) | 69 (54.3) | Pituitary stalk
preserved as much as possible |
| Van Effenterre | 2002 | 122 | 122 | 122 (100.0) | 64 (52.5) | Pituitary stalk
preserved as much as possible |
| Nishizawa | 2006 | 22 | 22 | 11 (50.0) | 2 (9.1) | Tumor resected to
the maximum extent |
| Stamm | 2008 | 7 | 7 | 7 (100.0) | 4 (57.1) | Pituitary stalk
preservation |
| Shi | 2008 | 309 | 309 | 235 (76.1) | 186 (60.2) | Pituitary stalk
preserved as much as possible |
| Jung | 2009 | 41 | 39 | NA | 24 (61.5) | Pituitary stalk
preserved as much as possible |
| Jung | 2010 | 17 | 17 | 17 (100.0) | 7 (41.2) | Tumor resected to
the maximum extent |
| Yamada | 2010 | 90 | 52 | 52 (100.0) | 16 (30.8) | Tumor resected to
the maximum extent |
| Current study | 2012 | 203 | 203 | 152 (74.9) | 34 (16.7) | Tumor resected to
the maximum extent if resection standards are achieved or
preservation. |
Two contradictory views exist regarding the
preservation of the pituitary stalk. One study of 17 children
(35) demonstrated that the
patients who had undergone microsurgery preserving the pituitary
stalk had a higher recurrence rate and poor endocrine functions.
The study strongly indicates that pituitary stalk resection to the
highest degree appears to be a more radical treatment than
pituitary stalk preservation. By contrast, another study considered
that preserving the pituitary stalk around the tumor was important,
as the patients consider this surgery more tolerable (43).
In the present study, guidelines of pituitary stalk
preservation or resection were proposed. In cases where the
pituitary stalk was removed, according to the guidelines, tumor
cells were detected in all the specimens of the resected pituitary
stalk with an ultra-electron microscope (15/15, 100%). These
results support the proposed guidelines as being effective and
reliable.
Postoperative recurrence and regrowth of CPs is
quite common and problematic. The recurrence and regrowth rate
varies between 7 and 30% and is directly proportional to the degree
of resection, histopathological types and other factors. The vast
majority of studies propose GTR for the treatment of CPs as the
recurrence rate is relatively low. In cases where patients did not
undergo GTR, higher regrowth rates and secondary surgery risks were
observed. Currently there are various views regarding the
recurrence and regrowth of CPs. Certain studies hypothesize that
secondary surgery may be performed in order to achieve total
radical cure. Others consider secondary surgery coupled with
adjuvant radiation therapy to be preferable for total cure. With
regard to the first hypothesis, GTR is a difficult procedure since
serious adhesions hinder a clear dissection. The GTR may also
induce hypothalamic dysfunction. Secondary surgery coupled with
adjuvant RT is a procedure that is prone to complications,
including radionecrosis, dementia and radio-induced tumors. In the
current study, 14 cases of recurrence were observed in the 157 GTR
cases (8.9%), whereas 7 cases of regrowth (33.3%) were recorded out
of the 21 cases of STR. Therefore, there is a significant
difference regarding tumor progression between GTR and STR
patients.
CPs mainly originate from the pituitary stalk and
the present study was conducted to determine whether pituitary
stalk preservation correlates with tumor recurrence. The study by
Jung et al (35) indicated
that there was no significant difference in the recurrence rate
between patients with preserved or resected pituitary stalks. By
contrast, Yamada et al (43) concluded that a higher GTR rate with
a lower pituitary stalk preservation rate results in a low
recurrence rate. Therefore, the authors hypothesize that the lower
recurrence rate may be relative to the lower pituitary stalk
preservation rate. The results of the present study showed that of
the 157 GTR patients, there was no significant difference in the
recurrence rate between patients with preserved pituitary stalks
(4/34, 11.8%) and those with resected pituitary stalks (10/123,
8.1%). Among the 157 patients, 128 cases of CPs originated from the
pituitary stalk. There was no statistical difference in the
recurrence rate between the preservation (1/19, 5.3%) and the
resection groups (6/109, 5.5%). Therefore, we hypothesize that
pituitary stalk resection or preservation, based on the
aforementioned principles, are not likely to increase the
recurrence risk in patients.
Almost all CP patients develop postoperative
endocrine dysfunction (44).
Endocrine function has a close association with the degree of
resection, histopathological subtypes and involvement of the
hypothalamus within the tumor. Replacement therapy is generally
indicated for patients with endocrine dysfunction. The drugs
commonly used are hydrocortisone, thyroxine, sex hormones and
vasopressin tannate. In the current study, postoperative endocrine
dysfunction was more common than preoperative endocrine
dysfunction, as shown by a marked decrease of growth hormone (GH).
Prior to surgery, there were 26 cases (20%) of GH decrease, while
it increased to 99 cases (78%) postoperatively. The sexual gland
axis (LH/FSH, as shown in Table
VI) showed no significant reduction, but it was kept at a
higher ratio. The sexual gland axis is most easily damaged due to
its invasion by CPs or during microdissection by the surgeon.
The pituitary stalk plays a vital role in
postoperative endocrine function. Thus, patients with preserved
pituitary stalks exhibit less endocrine dysfunction
postoperatively. The study by Jung et al (34) indicated that pituitary stalk
preservation benefits adenohypophysis function recovery. In the
present study, endocrine functions in patients with preserved
pituitary stalks were superior to those of patients with resected
pituitary stalks. Thus, pituitary stalk preservation had a positive
significance in the recovery of endocrine function.
Pituitary stalk preservation has a close association
with DI. Therefore, it is important to preserve the pituitary
stalk. Even partial preservation of the pituitary stalk is
important for the production of antidiuretic hormone
postoperatively. Honegger et al (38) reported that the incidence of DI in
patients with resected pituitary stalks was higher compared with
that in patients with total preservation of the pituitary stalk,
which was also observed in the present study. The incidence rate of
DI revealed no significant difference according to pituitary stalk
treatment during the early postoperative stage. However, the
long-term incidence of DI in patients with preserved pituitary
stalks was significantly lower than that of patients with resected
pituitary stalks. Therefore, the pituitary stalk should be actively
identified and preserved during sellar region surgery. Partial
pituitary stalk preservation may also reduce the long-term
incidence of DI. The early recovery conditions for DI in cases with
preserved pituitary stalks were significantly better compared with
those of cases with seriously involved or partially preserved
pituitary stalks, indicating that the protection of the pituitary
stalk is extremely important for promoting the function of the
hypothalamus-pituitary-endocrine axis. These results were also
concordant with the hypothesis by Honegger et al (39) that the postoperative probability of
DI in patients with preserved pituitary stalks was lower compared
with that of patients with resected pituitary stalks.
Postoperative VA was found to improve in more cases
than it deteriorated. However, postoperatively there were more
patients with decreased VF than improved VF, indicating that CPs
have an inevitable effect on VF. The reduction of VF may be
explained by the blood vessels supplying visual organs being
invaded by the tumor or being sacrificed during resection (45). Thus, a greater number of patients
presented with VF defects.
In conclusion, the total resection of CPs together
with the pituitary stalk is recommended if intraoperatively the
pituitary stalk is found to be invaded. Postoperatively, with the
aid of an ultra-electron microscope, all the specimens were
observed to be invaded by tumor cells. In cases where the pituitary
stalk is not involved, microsurgical excision preserving the
pituitary stalk is preferred as there is no significant increase in
the recurrence rate and patients exhibit less endocrine
dysfunction.
Acknowledgements
The study was supported by grants from the Planned
Science and Technology Project of Hunan Province (no. 2012SK2020)
and the Postgraduate Degree Thesis Innovation Projects of Central
South University (no. 2011ssxt207).
References
|
1
|
John-Kalarickal JM, Carlson HE and Davis
RP: Rupture of a craniopharyngioma cyst following trauma: a case
report. Pituitary. 10:103–106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gautier A, Godbout A, Grosheny C, et al;
Craniopharyngioma Study Group. Markers of recurrence and long-term
morbidity in craniopharyngioma: a systematic analysis of 171
patients. J Clin Endocrinol Metab. 97:1258–1267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ondruch A, Maryniak A, Kropiwnicki T,
Roszkowski M and Daszkiewicz P: Cognitive and social functioning in
children and adolescents after the removal of craniopharyngioma.
Childs Nerv Syst. 27:391–397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Elliott RE and Wisoff JH: Fusiform
dilation of the carotid artery following radical resection of
pediatric craniopharyngiomas: natural history and management.
Neurosurg Focus. 28:E142010. View Article : Google Scholar
|
|
5
|
Ohmori K, Collins J and Fukushima T:
Craniopharyngiomas in children. Pediatr Neurosurg. 43:265–278.
2007. View Article : Google Scholar
|
|
6
|
Elliott RE and Wisoff JH: Surgical
management of giant pediatric craniopharyngiomas. J Neurosurg
Pediatr. 6:403–416. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tomita T and Bowman RM: Craniopharyngiomas
in children: surgical experience at Children’s Memorial Hospital.
Childs Nerv Syst. 21:729–746. 2005.
|
|
8
|
Sahakitrungruang T, Klomchan T,
Supornsilchai V and Wacharasindhu S: Obesity, metabolic syndrome,
and insulin dynamics in children after craniopharyngioma surgery.
Eur J Pediatr. 170:763–769. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Puget S, Garnett M, Wray A, et al:
Pediatric craniopharyngiomas: classification and treatment
according to the degree of hypothalamic involvement. J Neurosurg.
106(1 Suppl): 3–12. 2007.PubMed/NCBI
|
|
10
|
Zuccaro G: Radical resection of
craniopharyngioma. Childs Nerv Syst. 21:679–690. 2005. View Article : Google Scholar
|
|
11
|
Caldarelli M, Massimi L, Tamburrini G,
Cappa M and Di Rocco C: Long-term results of the surgical treatment
of craniopharyngioma: the experience at the Policlinico Gemelli,
Catholic University, Rome. Childs Nerv Syst. 21:747–757. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yaşargil MG, Curcic M, Kis M, Siegenthaler
G, Teddy PJ and Roth P: Total removal of craniopharyngiomas.
Approaches and long-term results in 144 patients. J Neurosurg.
73:3–11. 1990.PubMed/NCBI
|
|
13
|
Elliott RE, Hsieh K, Hochm T,
Belitskaya-Levy I, Wisoff J and Wisoff JH: Efficacy and safety of
radical resection of primary and recurrent craniopharyngiomas in 86
children. J Neurosurg Pediatr. 5:30–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sosa IJ, Krieger MD and McComb JG:
Craniopharyngiomas of childhood: the CHLA experience. Childs Nerv
Syst. 21:785–789. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hoffman HJ, De Silva M, Humphreys RP,
Drake JM, Smith ML and Blaser SI: Aggressive surgical management of
craniopharyngiomas in children. J Neurosurg. 76:47–52. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lapras C, Patet JD, Mottolese C, Gharbi S
and Lapras C Jr: Craniopharyngiomas in childhood: analysis of 42
cases. Prog Exp Tumor Res. 30:350–358. 1987.PubMed/NCBI
|
|
17
|
Laws ER Jr: Conservative surgery and
radiation for childhood craniopharyngiomas. J Neurosurg.
74:1025–1026. 1991.PubMed/NCBI
|
|
18
|
Pierre-Kahn A, Recassens C, Pinto G, et
al: Social and psycho-intellectual outcome following radical
removal of craniopharyngiomas in childhood. A prospective series.
Childs Nerv Syst. 21:817–824. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Erşahin Y, Yurtseven T, Ozgiray E and
Mutluer S: Craniopharyngiomas in children: Turkey experience.
Childs Nerv Syst. 21:766–772. 2005.
|
|
20
|
Kim SK, Wang KC, Shin SH, Choe G, Chi JG
and Cho BK: Radical excision of pediatric craniopharyngioma:
recurrence pattern and prognostic factors. Childs Nerv Syst.
17:531–537. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fahlbusch R, Honegger J, Paulus W, Huk W
and Buchfelder M: Surgical treatment of craniopharyngiomas:
experience with 168 patients. J Neurosurg. 90:237–250. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
De Vile CJ, Grant DB, Kendall BE, et al:
Management of childhood craniopharyngioma: can the morbidity of
radical surgery be predicted? J Neurosurg. 85:73–81.
1996.PubMed/NCBI
|
|
23
|
Matson DD and Crigler JF Jr: Radical
treatment of craniopharyngioma. Ann Surg. 152:699–704. 1960.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kiehna EN and Merchant TE: Radiation
therapy for pediatric craniopharyngioma. Neurosurg Focus.
28:E102010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Habrand JL, Saran F, Alapetite C, Noel G,
El Boustany R and Grill J: Radiation therapy in the management of
craniopharyngioma: current concepts and future developments. J
Pediatr Endocrinol Metab. 19(Suppl 1): 389–394. 2006.PubMed/NCBI
|
|
26
|
Karavitaki N, Brufani C, Warner JT, et al:
Craniopharyngiomas in children and adults: systematic analysis of
121 cases with long-term follow-up. Clin Endocrinol (Oxf).
62:397–409. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stripp DC, Maity A, Janss AJ, et al:
Surgery with or without radiation therapy in the management of
craniopharyngiomas in children and young adults. Int J Radiat Oncol
Biol Phys. 58:714–720. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kalapurakal JA, Goldman S, Hsieh YC,
Tomita T and Marymont MH: Clinical outcome in children with
craniopharyngioma treated with primary surgery and radiotherapy
deferred until relapse. Med Pediatr Oncol. 40:214–218. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thomsett MJ, Conte FA, Kaplan SL and
Grumbach MM: Endocrine and neurologic outcome in childhood
craniopharyngioma: Review of effect of treatment in 42 patients. J
Pediatr. 97:728–735. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang I, Sughrue ME, Rutkowski MJ, et al:
Craniopharyngioma: a comparison of tumor control with various
treatment strategies. Neurosurg Focus. 28:E52010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takahashi H, Yamaguchi F and Teramoto A:
Long-term outcome and reconsideration of intracystic chemotherapy
with bleomycin for craniopharyngioma in children. Childs Nerv Syst.
21:701–704. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cavalheiro S, Dastoli PA, Silva NS, Toledo
S, Lederman H and da Silva MC: Use of interferon alpha in
intratumoral chemotherapy for cystic craniopharyngioma. Childs Nerv
Syst. 21:719–724. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mottolese C, Szathmari A, Berlier P and
Hermier M: Craniopharyngiomas: our experience in Lyon. Childs Nerv
Syst. 21:790–798. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jung TY, Jung S, Choi JE, Moon KS, Kim IY
and Kang SS: Adult craniopharyngiomas: surgical results with a
special focus on endocrinological outcomes and recurrence according
to pituitary stalk preservation. J Neurosurg. 111:572–577. 2009.
View Article : Google Scholar
|
|
35
|
Jung TY, Jung S, Moon KS, Kim IY, Kang SS
and Kim JH: Endocrinological outcomes of pediatric
craniopharyngiomas with anatomical pituitary stalk preservation:
preliminary study. Pediatr Neurosurg. 46:205–212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Prabhu VC and Brown HG: The pathogenesis
of craniopharyngiomas. Childs Nerv Syst. 21:622–627. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qi S, Lu Y, Pan J, Zhang X, Long H and Fan
J: Anatomic relations of the arachnoidea around the pituitary
stalk: relevance for surgical removal of craniopharyngiomas. Acta
Neurochir (Wien). 153:785–796. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Honegger J, Buchfelder M and Fahlbusch R:
Surgical treatment of craniopharyngiomas: endocrinological results.
J Neurosurg. 90:251–257. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Van Effenterre R and Boch AL:
Craniopharyngioma in adults and children: a study of 122 surgical
cases. J Neurosurg. 97:3–11. 2002.PubMed/NCBI
|
|
40
|
Nishizawa S, Ohta S and Oki Y: Spontaneous
resolution of diabetes insipidus after pituitary stalk sectioning
during surgery for large craniopharyngioma. Endocrinological
evaluation and clinical implications for surgical strategy. Neurol
Med Chir (Tokyo). 46:126–135. 2006. View Article : Google Scholar
|
|
41
|
Shi XE, Wu B, Fan T, Zhou ZQ and Zhang YL:
Craniopharyngioma: surgical experience of 309 cases in China. Clin
Neurol Neurosurg. 110:151–159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yamada S, Fukuhara N, Oyama K, et al:
Surgical outcome in 90 patients with craniopharyngioma: an
evaluation of transsphenoidal surgery. World Neurosurg. 74:320–330.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Stamm AC, Vellutini E, Harvey RJ, Nogeira
JF Jr and Herman DR: Endoscopic transnasal craniotomy and the
resection of craniopharyngioma. Laryngoscope. 118:1142–1148. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hoffmann K, Kretschmar B, Buller V and
Kermer P: Craniopharyngioma resulting in pituitary gland
insufficiency and coma in an adult with intellectual disability and
severe challenging behavior. J Neuropsychiatry Clin Neurosci.
22:451-j e418–451 e419. 2010.PubMed/NCBI
|
|
45
|
Mutlukan E and Cullen JF: Visual outcome
after craniopharyngioma. Ophthalmology. 97:539–540. 1990.
View Article : Google Scholar : PubMed/NCBI
|