Introduction
Severe traumatic wounds are challenging to manage
during surgery and numerous methods of temporary wound closure have
been reported. Presently, wounds are treated by conventional wound
care (CWC) or by a vacuum-assisted closure (VAC) device. VAC may be
applied in the majority of situations involving impaired wound
healing. Firstly, VAC removes stagnant fluid and debris and then
constantly optimizes blood supply and matrix deposition (1). Therefore, the partial oxygen pressure
within the tissue increases and bacterial proliferation is reduced
(2). Secondly, VAC results in
increased local interleukin-8 and vascular endothelial growth
factor (VEGF) concentrations, which may trigger the accumulation of
neutrophils and angiogenesis (3).
With the cyclical application of subatmospheric pressure, VAC
alters the cytoskeleton of cells in the wound bed, triggering a
cascade of intracellular signals that increase the rates of cell
proliferation and division, and subsequent formation of granulation
tissue (4).
With regard to the stimulation of vascularization
and cell growth, VAC has been used in traumatic or non-traumatic
soft tissue defects and postoperative wound infection (5,6).
However, to the best of our knowledge, the effects of VAC on
cytokine levels in severe traumatic wounds have not yet been
investigated.
During wound healing, a robust inflammatory response
starts immediately following any tissue damage. This is followed by
a proliferation response that involves the migration and
proliferation of keratinocytes, fibroblasts and endothelial cells.
Subsequently, re-epithelialization and granulation tissue formation
occurs and a scar finally forms (7). Intercellular adhesion molecule-1
(ICAM-1) is constitutively expressed at a low level by endothelial
cells during these stages, but is rapidly upregulated during
inflammation (8). Basic fibroblast
growth factor (bFGF) is a key factor involved in wound healing
(9,10). Macrophage migration inhibitory
factor (MIF) is a cytokine with multiple functions within and
beyond the immune system (11). In
addition to the main function of inhibiting macrophage migration,
MIF exhibits a broad range of immunostimulatory and proinflammatory
activities (12).
Endothelial cells and fibroblasts play important
roles in the proliferation response of wound healing. VEGF promotes
neovascularization through the extension and growth of existing
arterial and capillary networks. Fibroblasts are the primary source
of collagen, on which wound strength significantly depends.
Collagen I is the major collagen type in soft tissue and represents
~75% of collagens. Wiegand et al reported that protease and
proinflammatory cytokine concentrations are elevated in chronic
wounds compared with those in acute wounds and can be modulated by
collagen I in vitro (13).
Human fibroblast collagenase 1 (MMP-1) is the prototype for all
interstitial collagenases (14).
MMP-1 plays an important role in tissue morphogenesis and wound
repair (14).
In the present study, severe traumatic wounds were
created in a pig model and were treated with VAC or CWC. The
expression levels of cytokines in the wound, including ICAM-1, MIF,
VEGF, bFGF, collagen I and MMP-1, were determined for VAC- and
CWC-treated wounds. These cytokines play essential roles in wound
healing and are valuable for studying the mechanism of VAC in
promoting the healing of severe traumatic wounds.
Materials and methods
Animal model
A total of eight healthy domestic pigs of either
gender were purchased from the Central China Agricultural
University (Wuhan, China). The pigs had an average body weight of
60 kg and were fasted overnight with water ad libitum. The
experimental protocol was approved by the Ethics Committee for
Animal Research at Wuhan University (Wuhan, China). All pigs
received humane care in compliance with the Chinese Convention on
Animal Care.
Anesthesia
An intramuscular injection of 2 mg/kg xylazine
(Bayer AG, Leverkusen, Germany) mixed with 20 mg/kg ketamine
(Farmaceutici Gellini S.p.A., Aprilia, Italy) was used for
premedication. Anesthesia was then induced with intravenous 4 mg/kg
sodium thiopental (Pentothal; Abbott Scandinavia AB, Solna, Sweden)
and 2 μg/kg fentanyl (Leptanal; Lilly France S.A.S., Fegersheim,
France). The pigs were administered a continuous infusion of 3.5
μg/kg/h fentanyl in Ringer’s acetate combined with intermittent
bolus doses of 2.5 mg/kg sodium thiopental. Cuffed endotracheal
tubes were then orally inserted. Mechanical ventilation was
established with a Siemens-Elema ventilator (Siemens-Elema AB,
Solna, Sweden) in a volume-controlled mode (65% N2O, 35%
O2). The ventilatory settings were identical for all
pigs (respiratory rate, 15 breaths per min; minute ventilation, 12
liters per min). A positive end-expiratory pressure of ~5 cm
H2O was applied. A Foley catheter was intubated into the
bladder by suprapubic cystotomy.
Surgical procedure and tissue
collection
A circular wound measuring 3 cm in diameter and 2 cm
in depth, passing through the subcutaneous and muscle tissues, was
created on the back of the pigs. A saline-soaked AMD gauze [Kendall
Healthcare (Covidien), Mansfield, MA, USA] was used as wound
filler. The gauze volume was ~1.5 times larger than the wound
volume to allow for volume reduction during negative-pressure
application. A drainage tube was inserted into the gauze and
connected to a vacuum source (Prospera PRO-III; Prospera
Technologies, LLC., Fort Worth, TX, USA). The wound was then sealed
with a transparent adhesive drape (KCI Medical ApS, Ballerup,
Denmark) that overlapped the wound margins by 10 cm. Following the
completion of the experiments, the pigs were sacrificed with a
lethal dose of intravenous 60 mM potassium chloride (Farmaceutici
Gellini S.p.A.). The VAC group (Wuhan VSD Medical Science and
Technology, Co., Ltd., Wuhan, China) was treated with a continuous
negative pressure of 125 mmHg. The foam and dressings were changed
under clean conditions every 5–7 days depending on the wound volume
(a greater wound volume resulted in fewer days that the VAC held).
Suction was turned off for 30–60 min prior to changing the VAC
dressing. The contralateral wounds, named as the CWC group, were
treated with a moist gauze. Dressings were changed every other day
and visible debris was cleaned with a cotton-based tissue. A
transparent adhesive drape was then used to overlap the wound
margins by 5 cm. Tissues from the VAC and CWC groups were collected
by removing viable tissue from the center of the wound. Following
tissue collection, the pigs were sacrificed with a lethal dose of
potassium chloride that was injected into the heart.
RNA extraction and cDNA synthesis
For quantitative polymerase chain reaction (qPCR),
~100 mg specimen was washed with phosphate-buffered saline (PBS)
prior to RNA extraction. Total RNA was extracted with TRIzol
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA). RNA
concentration was determined by absorbance measurements at 260 nm,
while the purity was determined by the ratio of the absorbance at
260 and 280 nm (260/280 ratio) with a BioPhotometer (Eppendorf,
Hamburg, Germany). Reverse transcription with ~1 μg RNA was
performed with random primers using a ReverTra Ace kit (Toyobo Co.,
Ltd., Osaka, Japan). The mRNA expression levels of the target genes
(ICAM-1, MIF, VEGF, bFGF, collagen I, and MMP-1) and internal
control gene, β-actin, were quantified with a qPCR detection system
(SLAN Real-Time PCR system; Shanghai Hongshi Medical Technology,
Co., Ltd., Shanghai, China) with SYBR Green I (Toyobo Co., Ltd.).
PCR was performed according to standard procedures following
optimization and was within the exponential range of amplification
(15). The primer sequences were
analyzed by the nucleotide basic local alignment search tool for
specific gene amplification (16).
The process was conducted with omission of a cDNA template as a
negative control. Triplicate measurements were performed for all
genes per subject and the mean data were used. For the relative
quantification of the gene expression level, standard curves were
constructed by considering at least three points of a 10-fold
dilution series of cDNA in water. Relative gene expression data are
expressed as the n-fold change in the transcription of the target
genes normalized against the endogenous control in the same
sample.
Protein extraction and western
blotting
For protein extraction, ~100 mg frozen granulation
tissue was ground in liquid nitrogen, washed twice with ice-cold
PBS, lysed in 1 ml radioimmunoprecipitation assay (RIPA) lysis
buffer (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and
then sonicated on ice.
The lysates were collected and centrifuged at 12,000
× g for 10 min at 4°C. Proteins in the supernatants were collected
and stored at −80°C until the concentration was analyzed with a
bicinchoninic acid (BCA) protein assay kit (Shanghai Sangon
Biological Engineering Technology & Services Co., Ltd.,
Shanghai, China). Following heating at 99°C for 5 min in a loading
buffer, equal volumes of the tissue lysates (40 μg protein) were
loaded for analysis by sodium dodecyl sulfate polyacrylamide gel
electrophoresis and subsequently electrotransferred from the gels
onto polyvinylidene difluoride membranes (Millipore Corporation,
Billerica, MA, USA). The transferred membranes were blocked with 5%
skimmed milk in Tris-buffered saline with 0.05% Tween (TBST) and
washed six times in TBST. Target proteins (ICAM-1, MIF, VEGF, bFGF,
collagen I, and MMP-1) were detected with anti-target protein and
anti-β-actin monoclonal antibodies (mAbs; Santa Cruz Biotechnology,
Inc.), which were diluted according to the manufacturer’s
instructions and incubated overnight at 4°C. This was followed by
incubation with peroxidase-conjugated goat anti-rabbit
immunoglobulin (1:2,000; Santa Cruz Biotechnology, Inc.) in TBST
for 1 h. Signals were developed using an enhanced chemiluminescent
reagent (Pierce Biotechnology, Inc., Rockford, IL, USA) and β-actin
was used as an internal loading control. Band intensity was
analyzed using Quantity One software (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Relative expression was calculated as the
intensity ratio of the target protein to that of β-actin.
Immunohistochemistry
Sections were cut from formalin-fixed,
paraffin-embedded granulation tissue biopsies and hydrated through
graded alcohols. For antigen unmasking, the sections were treated
in trypsin solution for 10 min at 37°C. The sections were then
washed with deionized water and incubated with 3%
H2O2 for 5 min. The sections were incubated
in the primary polyclonal antibody, anti-CD31, at 1:200 (Santa Cruz
Biotechnology, Inc.) or anti-VEGF at 1:200 (Santa Cruz
Biotechnology, Inc.) for 1 h at room temperature. Next the sections
were incubated with secondary antibodies and peroxidase-conjugated
streptavidin-biotin complex (Santa Cruz Biotechnology, Inc.) at
37°C for 30 min. Immunoreactivity was visualized with
diaminobenzidine (Zymed Laboratories, Inc., South San Francisco,
CA, USA). Negative controls were prepared by omitting the primary
antibody.
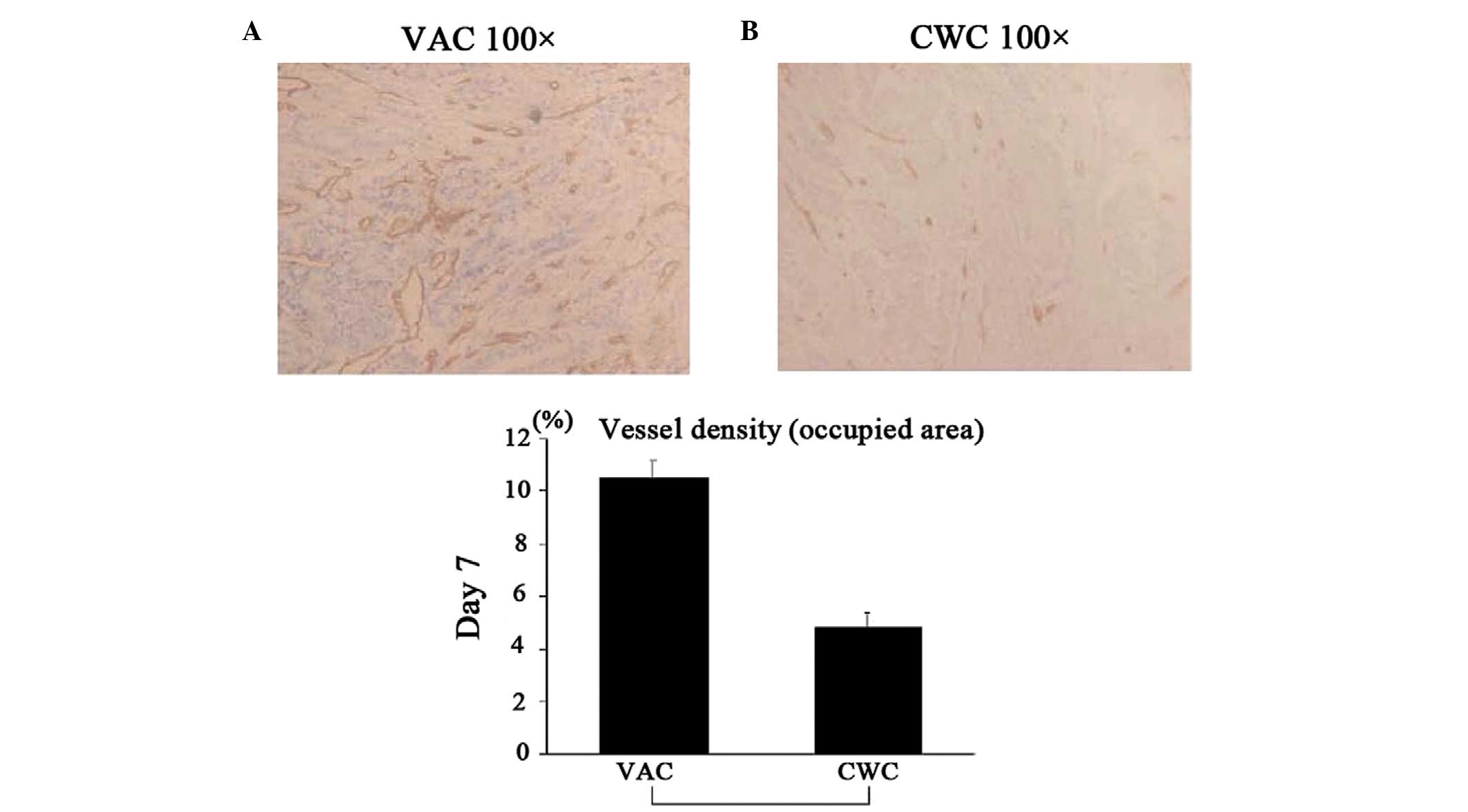
Vessel density following VAC and CWC
VAC and CWC wound biopsies (20 cases each) were
randomly selected and then investigated by an experienced
pathologist who was blinded to the type of wound dressing. The
vessels were highlighted by CD31 that was counted per 1
mm2. The area with the highest vessel density was
selected when the vessel density in the biopsy was heterogeneous.
Experiments were performed twice.
Statistical analysis
All statistical analyses were performed using SPSS
version 13.0 software for Windows (SPSS, Inc., Chicago, IL, USA).
To analyze the target genes and proteins in the VAC or CWC
treatment groups, one-way repeated-measure analysis of variance was
conducted. Statistical analysis was performed using the
Mann-Whitney U test or the Student’s t-test, following Levine’s
test for equality of variances, to compare wound cytokine
expression in granulation tissue samples between the VAC and CWC
treatment groups. The Mann-Whitney U test was used for non-normal
continuous variables. Vessel density and VEGF expression in VAC-
and CWC-treated wounds were analyzed with the Student’s t-test and
the Mann-Whitney U test, respectively. P<0.05 was considered to
indicate a statistically significant difference.
Results
ICAM-1, MIF, VEGF, bFGF and collagen I
mRNA expression levels are higher in the VAC group than in the CWC
group
The mRNA expression levels of ICAM-1, MIF, VEGF,
bFGF and collagen I were significantly higher in the VAC group than
in the CWC group. No significant difference was observed in the
MMP-1 mRNA expression levels between the two groups. The results
are summarized in Fig. 1.
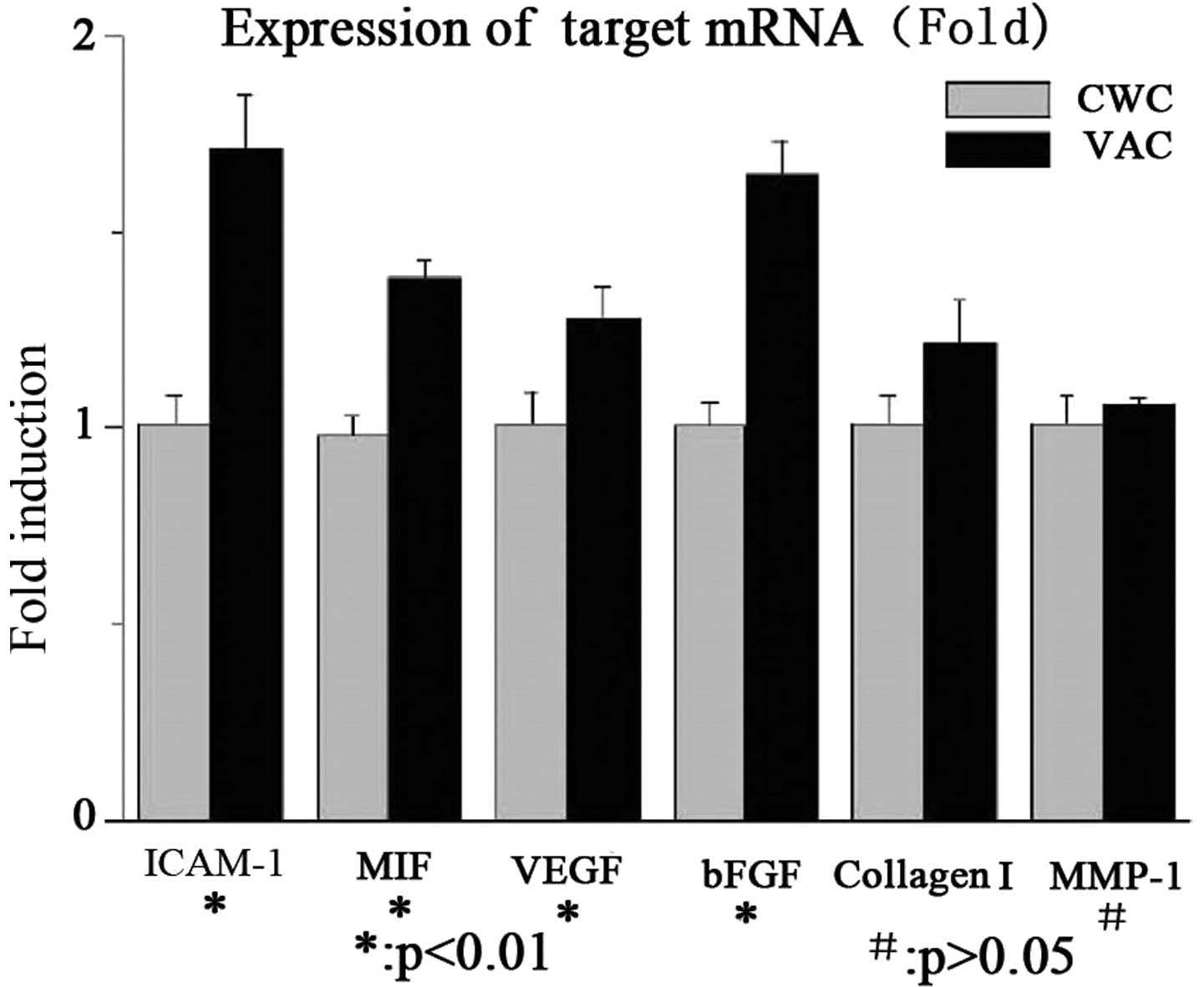 | Figure 1mRNA expression levels of cytokines in
wounds treated with VAC or CWC (fold change). The mRNA expression
levels of ICAM-1, MIF, VEGF, bFGF and collagen I were significantly
higher in the VAC group than in the CWC group (P<0.01). No
significant difference was identified in the MMP-1 mRNA expression
levels between the two groups (P>0.05). VAC, vacuum-assisted
closure; CWC, conventional wound closure; ICAM-1, intercellular
adhesion molecule-1; MIF, migration inhibitory factor; VEGF,
vascular endothelial growth factor; bFGF, basic fibroblast growth
factor; MMP-1, human fibroblast collagenase 1. |
Protein expression levels of ICAM-1, MIF,
VEGF and collagen I are higher in the VAC group than in the CWC
group
The protein expression levels of ICAM-1, MIF, VEGF
and collagen I were observed to be higher in the VAC group than in
the CWC group. However, bFGF was expressed at a very low level
compared with β-actin in the VAC and CWC groups. No significant
difference in the levels of MMP-1 protein expression was observed
between the two groups (Figs. 2
and 3).
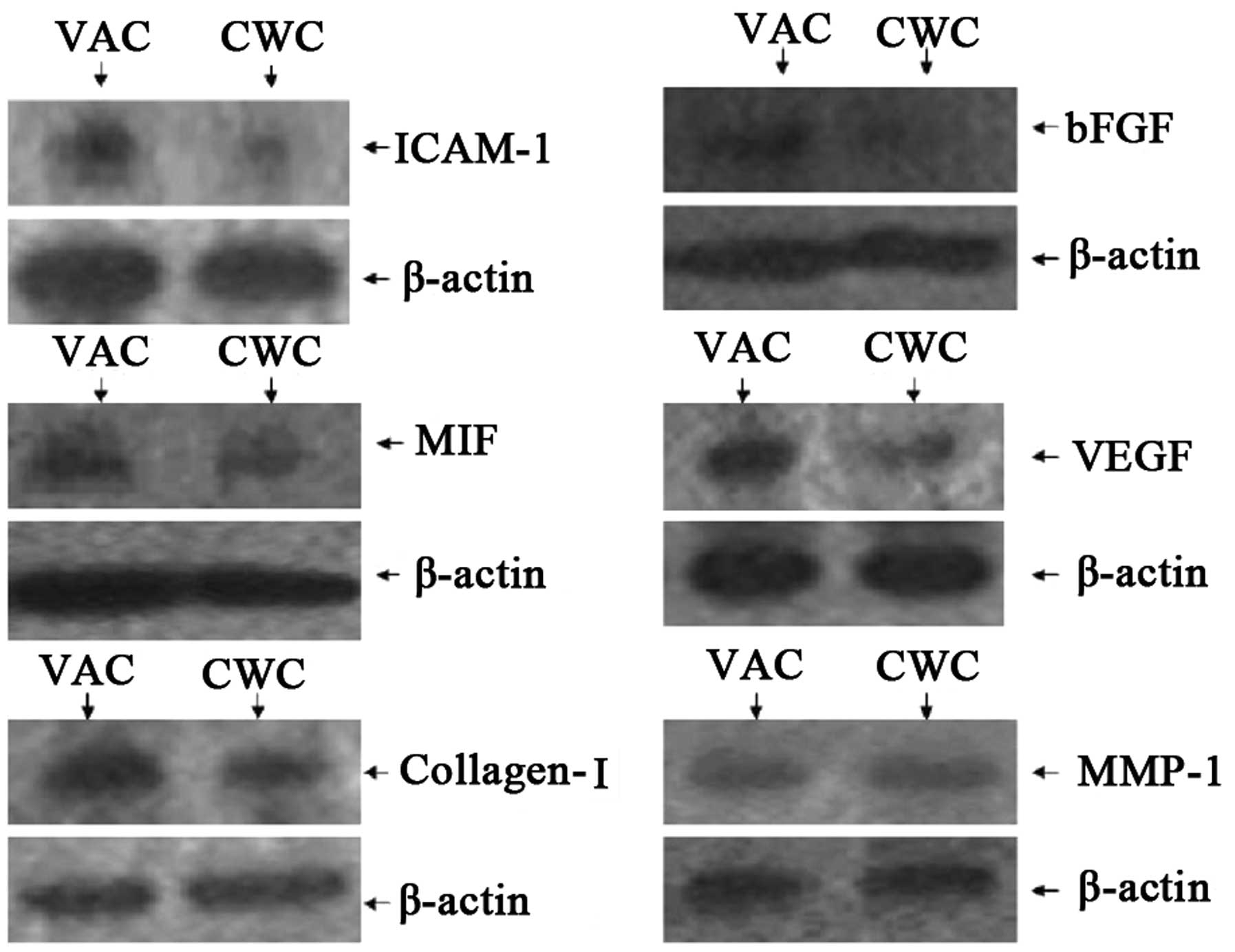 | Figure 2Protein expression levels of cytokines
in wounds treated with VAC and CWC. ICAM-1, MIF, VEGF and collagen
I were expressed at a higher level in the VAC group than in the CWC
group at the protein level. However, bFGF was expressed at a very
low level compared with β-actin in the VAC and CWC groups
(P<0.01). No significant difference was observed in MMP-1
protein expression levels between the two groups (P>0.05). VAC,
vacuum-assisted closure; CWC, conventional wound closure; ICAM-1,
intercellular adhesion molecule-1; MIF, migration inhibitory
factor; VEGF, vascular endothelial growth factor; bFGF, basic
fibroblast growth factor; MMP-1, human fibroblast collagenase
1. |
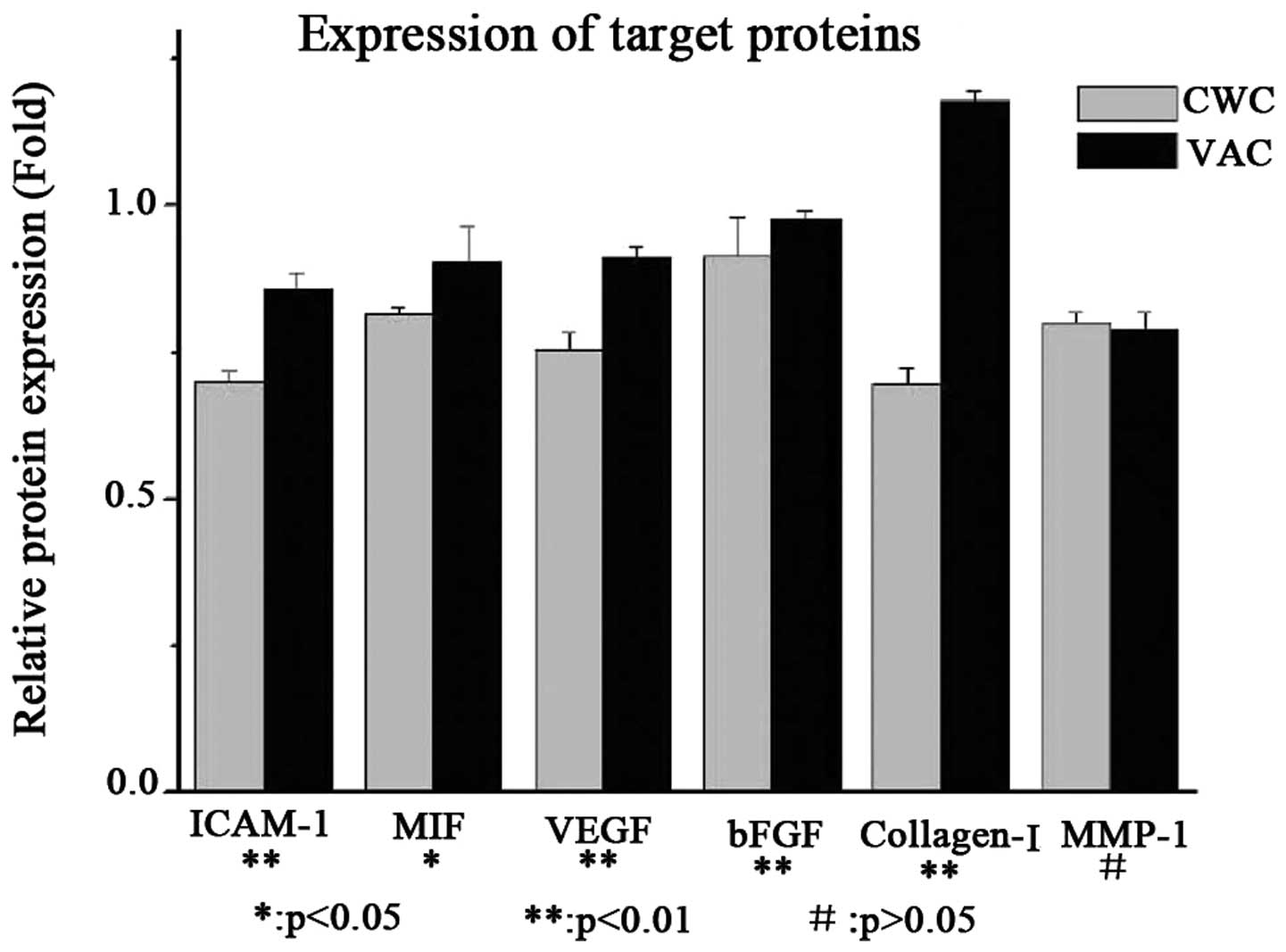 | Figure 3Protein expression levels of cytokines
in wounds treated with VAC and CWC (analysis). ICAM-1, MIF, VEGF
and collagen I were expressed at a higher level in the VAC group
compared with the CWC group at the protein level. However, bFGF was
expressed at a very low level compared with β-actin in the VAC and
CWC groups (P<0.01). No significant difference was observed in
MMP-1 protein expression levels between the two groups (P>0.05).
VAC, vacuum-assisted closure; CWC, conventional wound closure;
ICAM-1, intercellular adhesion molecule-1; MIF, migration
inhibitory factor; VEGF, vascular endothelial growth factor; bFGF,
basic fibroblast growth factor; MMP-1, human fibroblast collagenase
1. |
Vessel density following VAC and CWC
treatment
To assess the extent of angiogenesis,
immunohistochemical staining with anti-CD31 mAbs was performed. The
vascular density in the wound bed of the VAC group was
significantly higher compared with that in the CWC group (Fig. 4).
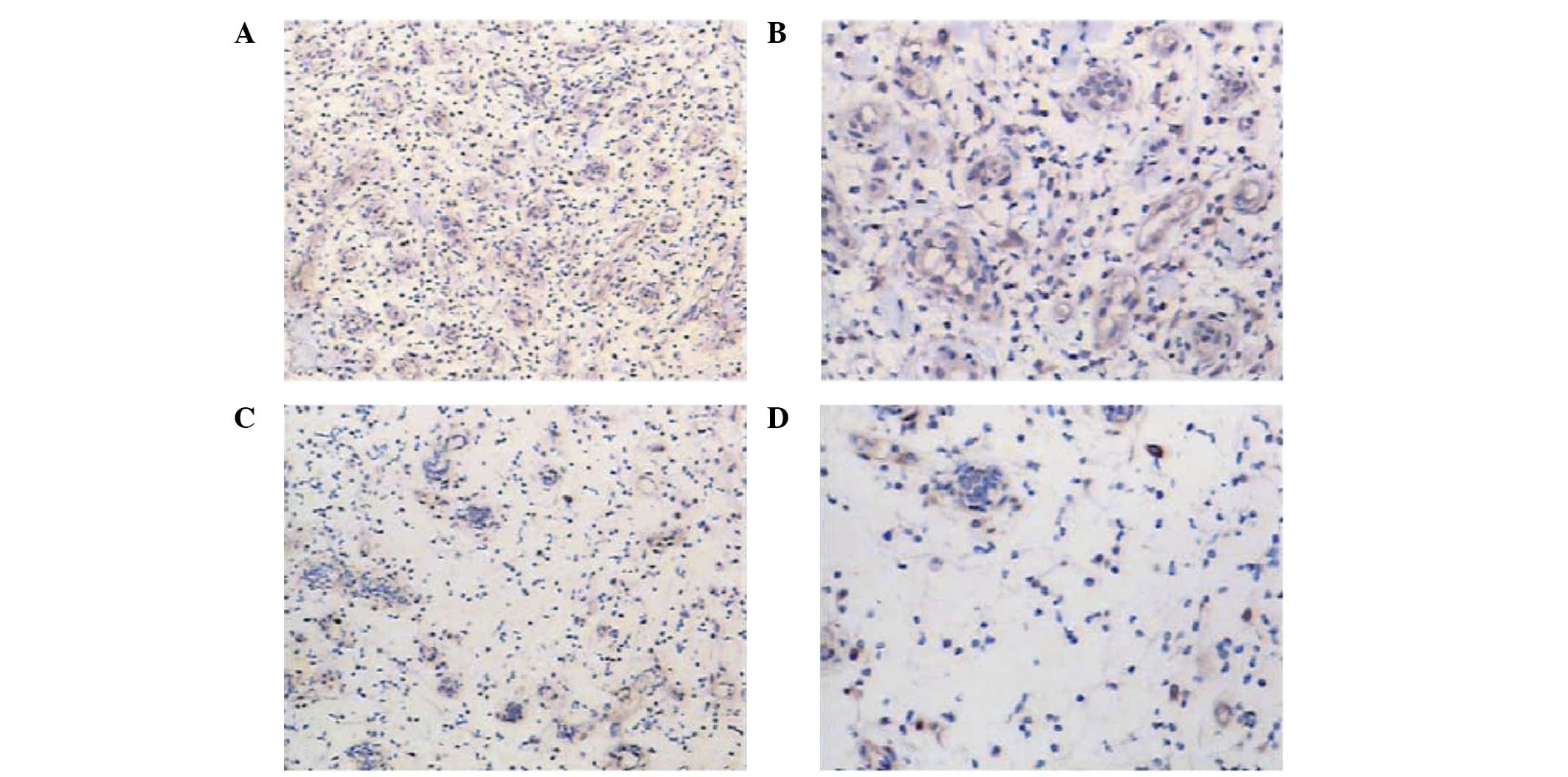
Expression of VEGF in wound biopsies
following VAC and CWC
Immunohistochemical analysis revealed that the
wounds in the VAC group showed visibly higher expression levels of
VEGF (Fig. 5A and B) when compared
with the levels in the CWC group (Fig.
5C and D). This was statistically verified by semi-quantitative
analysis (P<0.005).
Macroscopic images of the wounds were captured by
photography under the same conditions and the sizes of the wounds
were digitally measured. Photographs of the wounds were taken with
a digital camera (Canon, Beijing, China) and we measured the length
and width of the wounds with a digital vernier caliper (NSCING Co.,
Ltd., Nanjing, China) every time the dressing were changed.
Similarly to the study by Morykwas et al (17), compared with the CWC-treated
wounds, the VAC-treated wounds in the present study were
significantly drier and were larger on day 7, but were
significantly smaller and closed faster on day 14 (data not shown).
Re-epithelialization started immediately following injury from the
wound edge to cover the denuded site.
Discussion
Wounds are a major public health issue worldwide.
One novel therapeutic approach to treating wounds is VAC therapy,
which has become the most common treatment for severe traumatic
wounds in Zhongnan Hospital.
Numerous theories have been put forward to explain
the marked improvement in clinical outcomes achieved by VAC.
Firstly, the application of suction in the VAC device removes
interstitial fluid and cellular debris, reduces local edema and
decreases the probability of wound infection (18). Secondly, the resulting hypobaric
pressure and increase in blood flow to the wound bed accelerates
vascularization and granulation-tissue formation (19,20).
However, whether the cytokines present in the wound are involved in
the VAC-assisted wound therapy remains unknown.
ICAM-1 is constitutively expressed at a low level by
endothelial cells, but is rapidly upregulated during inflammation
in wound healing (8,21). Nagaoka et al (22) reported that a lack of ICAM-1 delays
wound healing, which is associated with the decreased infiltration
of neutrophils and macrophages. In the present study, ICAM-1 was
expressed in greater amounts at the mRNA and protein levels on day
7 of wound healing following VAC, when compared with those
following CWC. VAC treatment increased ICAM-1 expression in 7 days,
which indicates that VAC promotes the inflammation of wound
healing, based on the higher ICAM-1 expression levels. bFGF has
been shown to induce leukocytes to infiltrate wounds in significant
numbers, although the mechanism remains unclear (10). In addition, bFGF promotes
fibroblast proliferation, neovascularization and keratinocyte
migration, which accelerates wound healing (23). In the present study, the mRNA
expression level of bFGF was higher following VAC treatment
compared with that following CWC. However, bFGF protein was
expressed at low levels in the two groups and exhibited a
significant difference between the groups.
MIF is a potent cytokine with multiple functions
within and beyond the immune system (11). In addition to the main function of
inhibiting macrophage migration, MIF has a broad range of
immunostimulatory and proinflammatory activities (12). MIF also exhibits proangiogenic
activity through the direct induction of VEGF, which is a
representative angiogenic factor in wound healing (24,25).
The results of the present study revealed the upregulation of MIF
in VAC-treated wounds, when compared with the MIF level in
CWC-treated wounds. In addition, MIF positively correlated with
VEGF expression at the mRNA and protein levels. In accordance with
a previous study, the basal expression of VEGF increased on day 7
of wound healing (26).
Accordingly, the vessel density that was signified by
immunochemistry with CD31 confirmed the hypothesis regarding the
effect of VAC on neovascularization.
Collagens play an important role in wound repair and
their deposition primarily determines the quantity of fibronectin
and the quality of wound (27).
Collagen type I represents 75% of collagens (13,28).
MMP-1 is considered to be the prototype of all the interstitial
collagenases and has been shown to play an important role in tissue
morphogenesis and the formation of hypertrophic scars in wound
repair (29,30). MMP-1 cleaves collagen I, II and III
at specific sites on their α chains (31). It has been reported that bFGF
increases collagen degradation and reduces scar formation by
upregulating MMP-1 expression (4).
Considering that MMP-1 degrades collagens and bFGF reduces collagen
deposition and consequently suppresses scar formation, bFGF and
MMP-1 may play an anti-scarring role in wound repair (1–4,18–20,30).
In the present study, the expression level of collagen I was higher
in the VAC group than in the CWC group, which is consistent with
the clinical observations. MMP-1 expression also exhibited no
significant difference between the two groups on day 7 of wound
healing.
The results of the present study indicate that the
time of secondary surgical procedures was significantly shorter in
the VAC group than in the CWC group. In addition, the duration of
treatment until complete closure of the wound was achieved was
significantly shorter. Whelan et al (4) demonstrated that VAC removes excessive
fluids containing bacteria equally throughout the wound, which
enhances neovascularization and accelerates the formation of
granulation tissue. This finding confirmed the observation of the
present study that improved wound healing care of severe traumatic
wounds was achieved following VAC therapy compared with CWC
application.
In addition, it was identified that changes in VAC
dressings were more costly than changes in CWC and that VAC
required more intensive care compared with CWC. However, VAC
required less overall attention than CWC due to the significantly
lower number of repeat debridements and shorter duration of
in-patient care.
In summary, the present study indicated that VAC
significantly increased the expression of ICAM-1, MIF, VEGF and
collagen I compared with the levels induced by CWC treatment. VAC
was found to accelerate inflammation and neovascularization and to
promote collagen deposition. The duration of treatment until
complete wound closure was achieved was significantly shorter in
the VAC group. Furthermore, VAC appears to be a safe procedure
since no major complications occurred. Therefore, VAC provides
critical assistance for the treatment of severe traumatic
wounds.
Acknowledgements
The authors thank Wuhan VSD Medical Science &
Technology, Co., Ltd. (Wuhan, China) for supplying the vacuum pump.
The study was financed by a grant from the National Natural Science
Funds of China (no. 81171713).
References
|
1
|
Morykwas MJ, Simpson J, Punger K, et al:
Vacuum-assisted closure: state of basic research and physiologic
foundation. Plast Reconstr Surg. 117(7 Suppl): 121S–126S. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Venturi ML, Attinger CE, Mesbahi AN, et
al: Mechanisms and clinical applications of the vacuum-assisted
closure (VAC) device: a review. Am J Clin Dermatol. 6:185–194.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Labler L, Rancan M, Mica L, et al:
Vacuum-assisted closure therapy increases local interleukin-8 and
vascular endothelial growth factor levels in traumatic wounds. J
Trauma. 66:749–757. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Whelan C, Stewart J and Schwartz BF:
Mechanics of wound healing and importance of vacuum assisted
closure in urology. J Urol. 173:1463–1470. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zannis J, Angobaldo J, Marks M, DeFranzo
A, David L, Molnar J and Argenta L: Comparison of fasciotomy wound
closures using traditional dressing changes and the vacuum-assisted
closure device. Ann Plast Surg. 62:407–409. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Labler L, Keel M, Trentz O and Heinzelmann
M: Wound conditioning by vacuum assisted closure (V.A.C.) in
postoperative infections after dorsal spine surgery. Eur Spine J.
15:1388–1396. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grose R and Werner S: Wound-healing
studies in transgenic and knockout mice. Mol Biotechnol.
28:147–166. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dustin ML, Rothlein R, Bhan AK, et al:
Induction by IL 1 and interferon-gamma: tissue distribution,
biochemistry, and function of a natural adherence molecule
(ICAM-1). J Immunol. 137:245–254. 1986.
|
|
9
|
Buntrock P, Jentzsch KD and Heder G:
Stimulation of wound healing, using brain extract with fibroblast
growth factor (FGF) activity. I Quantitative and biochemical
studies into formation of granulation tissue. Exp Pathol. 21:46–53.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McGee GS, Davidson JM, Buckley A, et al:
Recombinant basic fibroblast growth factor accelerates wound
healing. J Surg Res. 45:145–153. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Calandra T and Roger T: Macrophage
migration inhibitory factor: a regulator of innate immunity. Nat
Rev Immunol. 3:791–800. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leng L and Bucala R: Macrophage migration
inhibitory factor. Crit Care Med. 33(12 Suppl): S475–S477. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wiegand C, Schönfelder U, Abel M, et al:
Protease and pro-inflammatory cytokine concentrations are elevated
in chronic compared to acute wounds and can be modulated by
collagen type I in vitro. Arch Dermatol Res. 302:419–428. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stechmiller J, Cowan L and Schultz G: The
role of doxycycline as a matrix metalloproteinase inhibitor for the
treatment of chronic wounds. Biol Res Nurs. 11:336–344. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kramer MF and Coen DM: Enzymatic
amplification of DNA by PCR: standard procedures and optimization.
Curr Protoc Mol Biol. Chapter 15(Unit 15)2001.
|
|
16
|
Saiki RK, Gelfand DH, Stoffel S, et al:
Primer-directed enzymatic amplification of DNA with a thermostable
DNA polymerase. Science. 239:487–491. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morykwas MJ, Argenta LC, Shelton-Brown EI
and McGuirt W: Vacuum-assisted closure: a new method for wound
control and treatment: animal studies and basic foundation. Ann
Plast Surg. 38:553–562. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Webb LX: New techniques in wound
management: vacuum-assisted wound closure. J Am Acad Orthop Surg.
10:303–311. 2002.PubMed/NCBI
|
|
19
|
Salazard B, Niddam J, Ghez O, et al:
Vacuum-assisted closure in the treatment of poststernotomy
mediastinitis in the paediatric patient. J Plast Reconstr Aesthet
Surg. 61:302–305. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yukami T, Hasegawa M, Matsushita Y, et al:
Endothelial selectins regulate skin wound healing in cooperation
with L-selectin and ICAM-1. J Leukoc Biol. 82:519–531. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nagaoka T, Kaburagi Y, Hamaguchi Y, et al:
Delayed wound healing in the absence of intercellular adhesion
molecule-1 or L-selectin expression. Am J Pathol. 157:237–247.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tanaka E, Ase K, Okuda T, et al: Mechanism
of acceleration of wound healing by basic fibroblast growth factor
in genetically diabetic mice. Biol Pharm Bull. 19:1141–1148. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ozawa K, Kondo T, Hori O, et al:
Expression of the oxygen-regulated protein ORP150 accelerates wound
healing by modulating intracellular VEGF transport. J Clin Invest.
108:41–50. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Raghow R: The role of extracellular matrix
in postinflammatory wound healing and fibrosis. FASEB J. 8:823–831.
1994.PubMed/NCBI
|
|
26
|
Zhou M, Yu A, Wu G, et al: Role of
different negative pressure values in the process of infected
wounds healing treated by vacuum-assisted closure: an experimental
study. Int Wound J. 10:508–515. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gomez DE, Alonso DF, Yoshiji H and
Thorgeirsson UP: Tissue inhibitors of metalloproteinases:
structure, regulation and biological functions. Eur J Cell Biol.
74:111–122. 1997.PubMed/NCBI
|
|
28
|
Reynolds JJ: Collagenases and tissue
inhibitors of metalloproteinase: a functional balance in tissue
degradation. Oral Dis. 2:70–76. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Spyrou GE and Naylor IL: The effect of
basic fibroblast growth factor on scarring. Br J Plast Surg.
55:275–282. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Birkedal-Hansen H, Moore WG, Bodden MK, et
al: Matrix metalloproteinases: a review. Crit Rev Oral Biol Med.
4:197–250. 1993.PubMed/NCBI
|
|
31
|
Schneider AM, Morykwas MJ and Argenta LC:
A new and reliable method of securing skin grafts to the difficult
recipient bed. Plast Reconstr Surg. 102:1195–1198. 1998. View Article : Google Scholar : PubMed/NCBI
|