Introduction
Breast cancer is a hormone-dependent tumor that
involves the interaction of estrogen and its specific receptors.
Estrogen receptor (ER)β, as reported by Kuiper et al
(1), was initially identified in
the cDNA library of rat prostate cells and is a subtype of the ER
superfamily. ERβ is known to be widely expressed in normal cells
and tumor tissues of humans and rats. The expression levels of ERβ
in ovarian, liver, prostate, small intestine and colorectal cancers
have been reported to be associated with tumor occurrence,
development and malignancy (2).
Notably, ERβ is of great significance for breast cancer and ERβ
expression levels in breast cancer are closely associated with the
curative effect of postoperative endocrine therapy (3).
Endocrine therapy is an effective method for the
treatment of estrogen-sensitive breast cancer. Esslimani-Sahla
et al (4) hypothesized that
ERβ protein levels in breast cancer are associated with the
efficacy of endocrine therapy. Hopp et al (5) found that ERβ was highly expressed in
endocrine-resistant breast cancer cells. By contrast, Borgquist
et al (6) reported that low
ERβ expression resulted in a poor prognosis of endocrine therapy.
Therefore, the role of ERβ in endocrine resistance remains
controversial.
In the present study, the association between ERβ
expression and the efficacy of endocrine therapy in breast cancer
was systematically investigated. Cancer tissues from 598 patients
with breast cancer were used in the study and the expression levels
of ERβ were determined by immunohistochemistry. Survival analysis
was conducted between patients with ERβ low or high expression and
patients who received or did not receive endocrine therapy. In
addition, the prognostic factors for breast cancer were analyzed by
Cox multivariate analysis.
Materials and methods
Clinical data
In total, 598 patients with pathologically confirmed
invasive breast cancer were enrolled in the study. All individuals
were diagnosed and treated in the First Affiliated Hospital of
Xinjiang Medical University (Ürümqi, China) between January 2000
and December 2010. The clinical features of the patients are shown
in Table I. Patients received
follow-ups for 2–10 years. During the follow-up period, 15 patients
were censored due to the loss of contact during the follow-up
period or prior to the study cut-off point, or due to mortality
from other causes.
 | Table IClinical features of the breast cancer
patients. |
Table I
Clinical features of the breast cancer
patients.
|
Clinical
features | Cases, n (%) |
|---|
| Age, years |
| ≤49 | 296 (50.8) |
| >50 | 287 (49.2) |
| Menses |
| Menostasis | 305 (52.3) |
| Non-menostasis | 278 (47.7) |
| Tumor size, cm |
| ≤2 | 220 (37.7) |
| >2, ≤5 | 289 (49.6) |
| >5 | 74 (12.7) |
| Histological
grade |
| Grade I | 108 (18.5) |
| Grade II | 328 (56.3) |
| Grade III | 147 (25.2) |
| Clinical stage |
| Stage 0 | 193 (33.1) |
| Stage I | 280 (48.0) |
| Stage II | 110 (18.9) |
| Lymph node
metastasis |
| Negative | 322 (55.2) |
| Positive | 261 (44.8) |
| ERβ expression |
| Negative | 460 (78.9) |
| Positive | 123 (21.1) |
| ERα expression |
| Negative | 391 (67.1) |
| Positive | 192 (32.9) |
| HER-2 |
| Negative | 326 (55.9) |
| Positive | 257 (44.1) |
| Chemotherapy |
| Yes | 497 (85.2) |
| No | 86 (14.8) |
| Radiotherapy |
| Yes | 388 (66.6) |
| No | 195 (33.4) |
| Endocrine
therapy |
| Yes | 254 (43.6) |
| No | 329 (56.4) |
Prior written and informed consent was obtained from
every patient and the study was approved by the Ethics Review Board
of Xinjiang Medical University.
Immunohistochemistry
Breast cancer tissue specimens were fixed in 10%
formaldehyde for 24 h and then embedded in paraffin. The specimens
were then sliced into 3-μm sections. Following dewaxing and
rehydrating in graded alcohols, sections were incubated with
anti-ERβ primary antibodies. An ERβ positive sample was used as a
positive control. In the negative control, the primary antibody was
replaced with phosphate-buffered saline. The anti-ERβ antibodies
and the working solution were purchased from Fuzhou Maixin
Biotechnology Development Co., Ltd. (Fuzhou, China).
Determination of ERβ expression
levels
Cells with brown staining in the nucleus were
considered ERβ positive cells. Five fields at high-magnification
were randomly selected. The ERβ positive rate was the ratio of the
number of ERβ positive cells to the total number of cells in each
field. An ERβ positive rate <1% was defined as ERβ negative (−).
A positive rate between 1 and 10% was defined as ERβ weak positive
(+) and an ERβ positive rate between 10 and 50% was defined as ERβ
positive (++). Finally, an ERβ positive rate >50% was defined as
ERβ strong positive (+++). Cells defined ERβ (−) and (+) were
considered to be ERβ low expression cells, while cells defined ERβ
(++) and ERβ (+++) were considered to be ERβ high expression
cells.
Statistical analysis
SPSS statistical software, version 17.0 (SPSS, Inc.,
Chicago, IL, USA) was used for statistical analysis. Kaplan-Meier
survival curves were constructed for survival analysis and the
log-rank test was used to determine the differences in survival.
Cox multivariate analysis was also performed to analyze prognostic
factors. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of ERβ in breast cancer
The expression levels of ERβ in the breast cancer
tissue samples were analyzed by immunohistochemical staining.
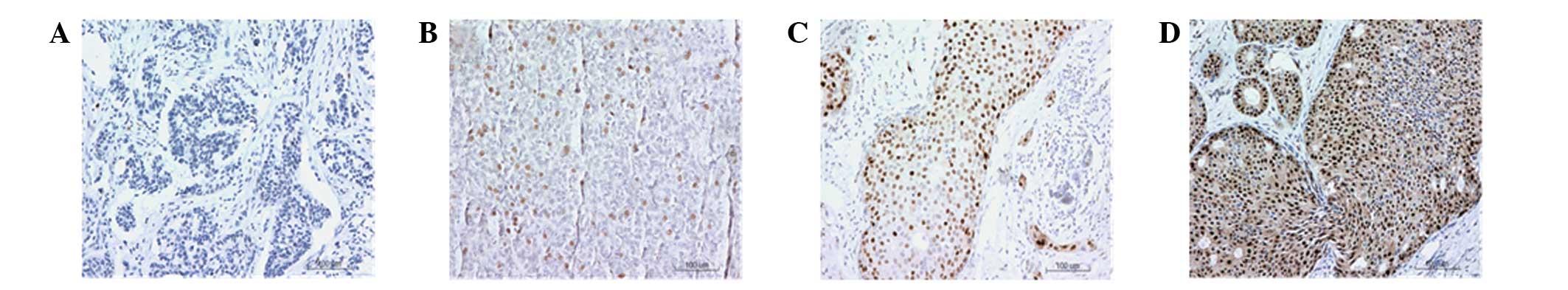
Representative results are shown in Fig. 1. Cells with brown particles in the
nucleus were ERβ positive cells. There were no cells with brown
staining visible in Fig. 1A,
indicating that ERβ expression was negative. However, in Fig. 1B–D, certain cells were positively
stained, indicating a positive expression of ERβ. Cells in which
the expression of ERβ was indicated were counted and the positive
expression rate was calculated. Weak expression of ERβ with a
positive rate of <10% is shown in Fig. 1B. Positive expression of ERβ with a
positive rate between 10 and 50% is demonstrated in Fig. 1C and high expression of ERβ with a
positive rate >50% is shown in Fig.
1D. Cells that were classified as ERβ (−) or (+) were defined
as ERβ low expression cells, while cells that were classified as
ERβ (++) or (+++) were defined as ERβ high expression cells.
Median tumor-free survival time is longer
in patients with low ERβ expression receiving endocrine
therapy
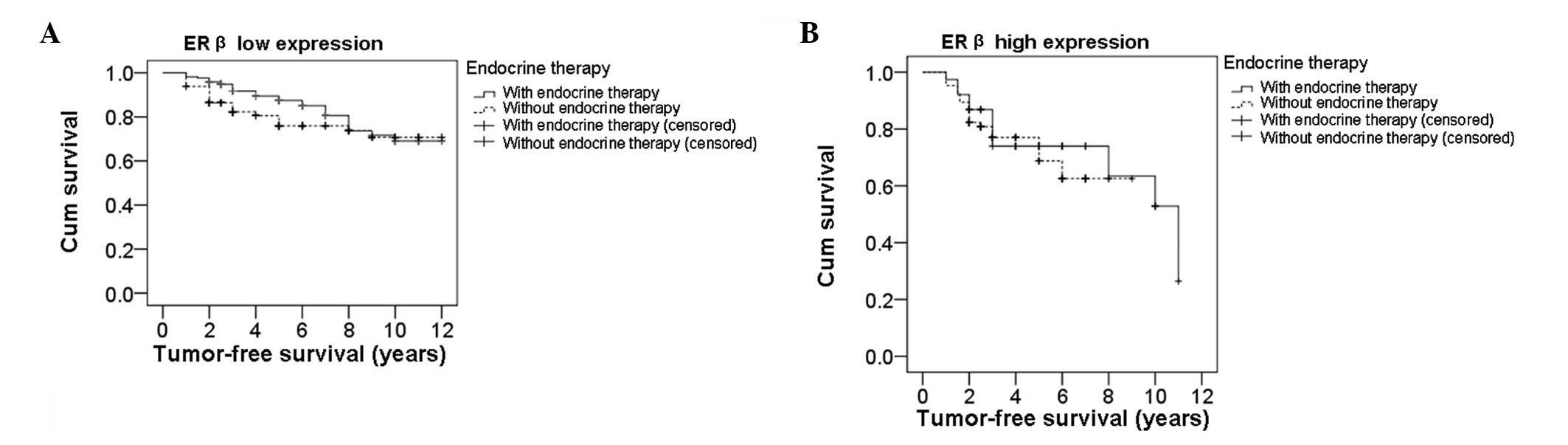
To determine the effect of ERβ expression on the
efficacy of endocrine therapy, survival analysis was performed
using the Kaplan-Meier method. Differences in survival time were
analyzed with the log-rank test. Firstly, the tumor-free survival
times in ERβ low expression patients who received or did not
receive endocrine therapy were analyzed. The survival curves of ERβ
low expression patients are shown in Fig. 2A. The median tumor-free survival
time in patients that received endocrine therapy was 10.11 years,
while in patients that did not receive endocrine therapy, the
median tumor-free survival time was 9.56 years. Statistically, the
difference between these two groups was significant (P=0.038).
Next, tumor-free survival times were analyzed in ERβ high
expression patients who did or did not undergo endocrine therapy.
Fig. 2B shows the survival curves
of ERβ high expression patients. In ERβ high expression patients,
the median tumor-free survival time of patients that received
endocrine therapy was 8.31 years, while the median tumor-free
survival time of patients that did not undergo endocrine therapy
was 6.85 years. However, there was no statistically significant
difference in median tumor-free survival time between these
patients (P=0.583). Therefore, these results indicate that high ERβ
expression levels in breast cancer patients impair the efficacy of
endocrine therapy.
Patients with low ERβ expression levels
have longer a median tumor-free survival time
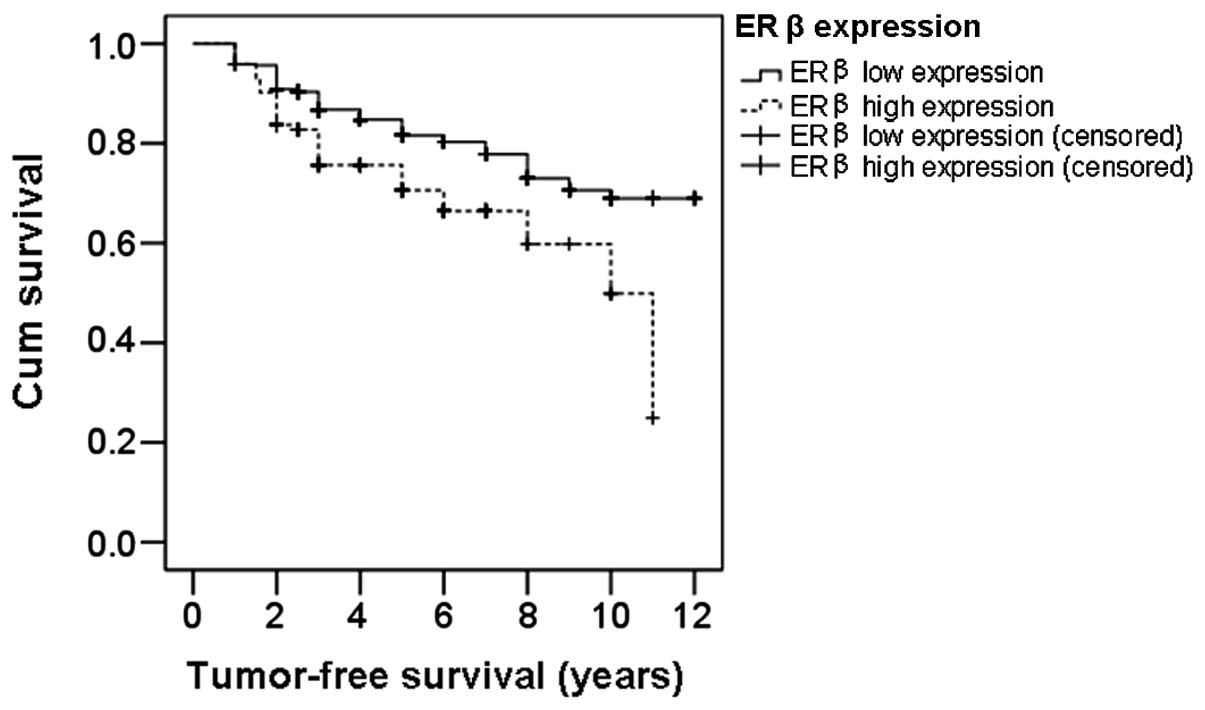
To further investigate the role of ERβ expression in
breast cancer patients, the tumor-free survival times were analyzed
using the Kaplan-Meier method and the differences in survival time
were analyzed with the log-rank test. The survival curves of ERβ
low and high expression patients are shown in Fig. 3. The median tumor-free survival
time in patients with low ERβ expression was 9.79 years, while in
high ERβ expression patients, it was 8.01 years, which was
significantly lower compared with that of the low ERβ expression
patients (P=0.002). This result further indicates that patients
with high ERβ expression levels have shorter tumor-free survival
times and poor prognosis.
Analysis of prognostic factors for breast
cancer
Prognostic factors for breast cancer were analyzed
by Cox multivariate analysis. The analyzed factors were ERβ
expression, tumor size, pathological grade, lymph node metastasis,
chemotherapy, radiotherapy, endocrine therapy, ERα expression and
human epidermal growth factor receptor (HER-2) expression. The
results are shown in Table II.
Independent prognostic factors for breast cancer were identified to
be ERβ expression, tumor size, lymph node metastasis, chemotherapy,
radiotherapy, endocrine therapy and HER-2 expression (P<0.05).
However, pathological grade and ERα expression were not determined
to be prognostic factors (P>0.05).
 | Table IIAnalysis of prognostic factors for
breast cancer by Cox multivariate analysis. |
Table II
Analysis of prognostic factors for
breast cancer by Cox multivariate analysis.
| Risk factors | Regression
coefficient | Standard error | Wald value | P-value | OR value | 95.0% CI |
|---|
| ERβ | 0.581 | 0.212 | 7.519 | 0.006a | 1.787 | 1.18–2.707 |
| Tumor size |
| 2–5 cm | 0.782 | 0.285 | 7.543 | 0.006a | 2.187 | 1.251–3.822 |
| >5 cm | 1.162 | 0.337 | 11.877 | 0.001a | 3.196 | 1.65–6.188 |
| Pathological
grade |
| Grade II | 0.044 | 0.281 | 0.025 | 0.875 | 1.045 | 0.603–1.812 |
| Grade III | 0.192 | 0.309 | 0.385 | 0.535 | 1.212 | 0.661–2.222 |
| Lymph node
metastasis |
| 1–4 pieces | 0.609 | 0.252 | 5.829 | 0.016a | 1.839 | 1.121–3.016 |
| 5–10 pieces | 1.116 | 0.289 | 14.902 | <0.001a | 3.053 | 1.732–5.382 |
| >10 pieces | 1.101 | 0.313 | 12.361 | <0.001a | 3.006 | 1.628–5.553 |
| Chemotherapy | 1.085 | 0.231 | 22.098 | <0.001a | 2.96 | 1.883–4.653 |
| Radiotherapy | 0.556 | 0.208 | 7.135 | 0.008a | 1.744 | 1.16–2.623 |
| Endocrine
therapy | 0.432 | 0.215 | 4.024 | 0.045a | 1.541 | 1.010–2.35 |
| ERα | −0.332 | 0.228 | 2.118 | 0.146 | 0.717 | 0.459–1.122 |
| HER-2 | 0.428 | 0.194 | 4.871 | 0.027a | 1.534 | 1.049–2.243 |
Discussion
In the present study, tumor-free survival times were
compared in breast cancer patients with high and low ERβ expression
levels who received or did not receive endocrine therapy. The
median tumor-free survival time was 10.11 years in ERβ low
expression patients treated with endocrine therapy, while in ERβ
low expression patients who did not undergo endocrine therapy, the
median tumor-free survival time was 9.56 years. In ERβ high
expression patients treated with endocrine therapy, the median
tumor-free survival time was 8.31 years, while in ERβ high
expression patients without endocrine therapy it was 6.85 years.
There was a statistically significant difference (P=0.038) between
patients who did or did not receive endocrine therapy when ERβ
expression levels were low, whereas there was no significant
difference when the ERβ expression levels were high (P=0.583).
These results indicate that in ERβ low expression patients, the
efficacy of endocrine therapy was significant and the prognosis was
better compared with that of the patients who did not receive
endocrine therapy. By contrast, in ERβ high expression patients,
the efficacy of endocrine therapy was not significant and the
prognosis was similar to that of the patients who did not receive
endocrine therapy. These results indicate that the prognosis was
not improved by endocrine therapy in ERβ high expression patients.
In addition, to a certain extent, ERβ high expression may be
associated with endocrine resistance. The reason for resistance may
result from the binding of ER antagonists with ERβ, which activates
the mitogen-activated protein kinase signaling pathway to
facilitate the transcription of genes involved in cell
proliferation and migration (7).
In addition, ERβ has been reported to have a certain
prognostic value (8,9). Chung et al (10) used adenovirus vectors to observe
the effect of ERβ protein expression on gene transcription in MCF-7
cells. The authors found that ERβ regulated downstream genes,
including genes involved in transforming growth factor β signaling,
cell cycle, apoptosis and the inhibition of cell proliferation.
These observations indicated that ERβ was a poor prognosis factor
for carcinogenesis in breast cancer. Jensen et al (11) found that ERβ positively expressed
breast cancer had a higher histological grade than ERβ negatively
expressed breast cancer. In addition, ERβ mRNA expression levels in
cancer tissues were upregulated and the prognosis of ERβ and ERα
double positive breast cancer patients was poorer compared with ERα
single positive patients. In the present study, the median
tumor-free survival time for patients with low ERβ expression (9.79
years) was significantly higher compared with that of patients with
high ERβ expression (8.01 years; P<0.01). This result was in
accordance with previous studies and may be caused by the following
two aspects. Firstly, G protein may be activated by estrogen
through membrane ERβ, rapidly inhibiting the c-Jun N-terminal
kinase pathway and preventing the apoptosis of breast cancer cells
(12). Secondly, ERβ may regulate
the expression of genes in the Wnt signaling pathway (13). Therefore, ERβ may regulate the
proliferation and invasion of breast cancer cells and an imbalance
in its expression acts an important indicator for breast cancer
recurrence and metastasis.
A previous study (14) found that ERβ expression was
associated with axillary lymph node metastasis. Axillary lymph node
metastasis is an independent indicator for the treatment and
prognosis of breast cancer. Prognosis is relatively poor for breast
cancer patients with axillary lymph node metastasis. Multivariate
analysis conducted in the present study indicated that ERβ, HER-2,
tumor size, lymph node metastasis, postoperative chemotherapy,
radiotherapy and endocrine therapy are independent prognostic
factors (P<0.05). Positive expression of ERβ and HER-2, larger
tumor size, lymph node metastasis, postoperative chemotherapy,
radiotherapy and endocrine therapy were risk prognosis factors.
This is consistent with previous studies, indicating the positive
value of ERβ in prognosis evaluation.
In summary, for the diagnosis and treatment of
breast cancer, ERα is measured as a routine pathology test. The
2012 Breast Cancer National Comprehensive Cancer Network treatment
guidelines emphasized that adjuvant systemic treatment should be
provided according to the expression of ERs. Based on the
observations of the present study, it may be hypothesized that ERβ
is important for the assessment of postoperative treatment options
and prognosis. Combined detection of ERα and ERβ is likely to guide
endocrine treatment and prognosis assessment and provide more
detailed information for individualized clinical treatment.
Acknowledgements
The study was supported by a grant from the Natural
Science Foundation of Xinjiang Uygur Autonomous Region (no.
2011211A069).
References
|
1
|
Kuiper GG, Enmark E, Pelto-Huikko M, et
al: Cloning of a novel receptor expressed in rat prostate and
ovary. Proc Natl Acad Sci USA. 93:5925–5930. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yuan MQ, Ping GF and Nan KJ: Progress in
research of estrogen receptor beta and primary liver cancer. Xian
Dai Zhong Liu Yi Xue. 16:1826–1829. 2008.(In Chinese).
|
|
3
|
Yager JD and Davidson NE: Estrogen
carcinogenesis in breast cancer. New Engl J Med. 354:270–282. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Esslimani-Sahla M, Simony-Lafontaine J,
Kramar A, et al: Estrogen receptor beta (ERbeta) level not its ER
beta cx variant helps to predict tamoxifen resistance in breast
cancer. Clin Cancer Res. 10:5769–5776. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hopp TA, Weiss HL, Parra IS, et al: Low
levels of estrogen receptor beta protein predict resistance to
tomoxifen therapy in breast cancer. Clin Cancer Res. 10:7490–7499.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Borgquist S, Holm C, Stendahl M, et al:
Oestrogen receptors alpha and beta show different associations to
clinicopathological parameters and their co-expression might
predict a better response to endocrine treatment in breast cancer.
J Clin Pathol. 61:197–203. 2008. View Article : Google Scholar
|
|
7
|
Lee H and Bai W: Regulation of estrogen
receptor nuclear export by ligand-induced and p38-mediated receptor
phosphorylation. Mol Cell Biol. 22:5835–5845. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pettersson K and Gustafsson JA: Role of
estrogen receptor beta in estrogen action. Annu Rev Physiol.
63:165–192. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Osborne CK and Schiff R: Estrogen-receptor
biology: continuing progress and therapeutic implications. J Clin
Oncol. 23:1616–1622. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chung YL, Sheu ML, Yang SC, et al:
Resistance to tamoxifen-induced apoptosis is associated with direct
interaction between Her-2/neu and cell membrane estrogen receptor
in breast cancer. Int J Cancer. 97:306–312. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jensen EV, Cheng G, Palmieri C, et al:
Estrogen receptors and proliferation markers in primary and
recurrent breast cancer. Proc Natl Acad Sci USA. 98:15197–15202.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Razandi M, Pedram A and Levin ER: Plasma
membrane estrogen receptors signal to antiapoptosis in breast
cancer. Mol Endocrinol. 14:1434–1447. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun H, Zhang J, Zhan ZG and Hao XS:
Estrogen receptor β and related genes of its signaling pathway in
development of different models of breast cancer. Zhonghua Shi Yan
Wai Ke Za Zhi. 23:1422–1423. 2006.(In Chinese).
|
|
14
|
Yang SE and Li X: Expression and
significance of ERβ and HER2 in breast cancer. Zhonghua Zhong Liu
Za Zhi. 29:767–768. 2007.(In Chinese).
|