Introduction
Schistosomiasis is a type of zoonotic parasitic
disease that is distributed globally and causes serious harm to
human health (1). Schistosoma
japonicum is mainly endemic to China, Indonesia and the
Philippines and is a significant public health problem in China
(2). The main pathological changes
caused by Schistosoma japonicum infection are the formation
of granulomas and hepatic fibrosis (3). Hepatic fibrosis is the principal
cause of serious complications and mortality due to Schistosoma
japonicum infection (4).
Hepatic fibrosis is a compensatory response that is secondary to
the process of tissue repair following liver inflammation or damage
caused by Schistosoma japonicum infection (5). The lack of an endogenous
anti-inflammatory and pro-resolving mediator leads to persistent
inflammation and causes liver fibrosis (6). Resolvin E1 (RvE1) is a potent
anti-inflammatory and pro-resolving member of the E-series
resolvins produced from eicosapentaenoic acid (EPA) (7). In the present study, the effects of
RvE1 on liver fibrosis in schistosome-infected mice were
investigated.
Materials and methods
Experimental animals
A total of 30 female Kunming mice (pathogen-free),
six-weeks-old and weighing 22±2 g, were purchased from the Jianghan
University Animal House (Wuhan, China). The mice were randomized
into three groups, with 10 mice per group: Control, model and RvE1
intervention. The model and RvE1 intervention groups were infected
with schistosome cercariae through the abdominal skin (20±2
cercariae per mouse). The control mice were not infected. The mice
in the RvE1 intervention group were administered 100 ng RvE1 daily,
from the day of infection. The RvE1 was dissolved in 2 ml normal
saline and administered intragastrically. The mice in the control
and model groups received an equivalent volume of normal saline by
intragastric administration. Treatment occurred every day for 70
days. Subsequently, all mice were sacrificed for analysis. Blood
samples were collected by retro-orbital bleeding. Following opening
up of the abdomen, the whole livers were removed. The hepatic
middle lobules were harvested and the remaining liver tissue was
cryopreserved for the subsequent experiments. Snails infected with
schistosome cercariae were obtained from the Hubei Institute of
Parasitic Diseases (Wuhan, China), and the surgical instruments and
equipment were from the Jianghan University Animal Center. The
present study was approved by the Medical Ethics Committee of
medical college of Jianghan University (Wuhan, China).
Measurement of the serum tumor necrosis
factor (TNF)-α, interferon (IFN)-γ, alanine aminotransferase (ALT)
and aspartate aminotransferase (AST) levels
Serum samples from the individual mice were
collected prior to when the mice were sacrificed. The TNF-α and
IFN-γ levels were measured using ELISA kits (R&D Systems,
Minneapolis, MN, USA). Liver injury was assessed by measuring the
serum levels of the liver-associated enzymes ALT and AST using
commercially available kits (Shanghai Rongsheng Biotech Co. Ltd.,
Shanghai, China).
Liver tissue homogenates
The liver tissue was thawed and rinsed, then cut
into sections. The tissue (0.2 g) was placed into a 10-ml beaker. A
volume of homogenate solution (0.1 mM Tris-HCl, 0.01 mM EDTA-2Na,
0.01 mM sucrose and 0.8% NaCl) nine-fold (w:v = 1:4) the amount of
the tissue was added. The liver was processed with a homogenizer
for 6–8 min in order to fully homogenize the sections. The samples
were centrifuged at 3,000 × g at 4°C for 10–15 min. Appropriate
amounts of clear supernatant liquid were used for the protein
detection.
Laminin (LN), hyaluronic acid (HA),
procollagen type III (PC-III) and type IV collagen (IV-C)
detection
The LN, HA, PC-III and IV-C concentrations in the
serum samples were detected by radioimmunoassays conducted
according to the instructions provided with the assay kits. LN, HA,
PC-III and IV-C assay kits were provided by Tianjin Atomic Energy
Industry (Tianjin, China).
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR)
The total RNA of the liver tissues was extracted
with TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA). Two micrograms of the total RNA was used for each reverse
transcription reaction for cDNA synthesis. Quantitative PCR was
performed using a sequence detector (ABI-Prism StepOnePlus; Applied
Biosystems, Foster City, CA, USA) and SYBR Premix Ex Taq [Takara
Biotechnology (Dalian) Co., Ltd., Dalian, China] according to the
manufacturer’s instructions. PCR was performed using the following
primers for TNF-α: 5′-TGAGCACTGAAAGCATGATCC-3′ and
5′-ATCACTCCAAAGTGCAGCAG-3′. A ‘housekeeping’ gene encoding
β-non-muscle actin was used as a normalization control. The
sequencing involved thermal cycling at 95°C for 1 min
(denaturation), 50°C for 1 min (annealing) and 72°C for 1 min
(extension). The products were evaluated by agarose gel
electrophoresis and analyzed using the ChemiDoc MP imaging system
(Bio-Rad, Hercules, CA, USA).
Western blot analysis
To detect the levels of TNF-α protein, 20 μg protein
extracted from the each liver was separated by SDS-PAGE and
electroblotted onto a nitrocellulose membrane, which was probed
with anti-TNF-α mouse antibody (1:1,000; Cell Signaling Technology,
Inc., Beverly, MA, USA) and a polyclonal antibody against
glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:1,000; Cell
Signaling Technology, Beverly, MA, USA). HRP-conjugated goat
anti-mouse IgG (1:50,000; Cell Signaling Technology, Beverly, MA,
USA) was used to detect the bound mouse antibody, while
HRP-conjugated goat anti-rabbit IgG (1:50,000; Cell Signaling
Technology, Beverly, MA, USA) was used to detect the anti-GAPDH
polyclonal antibody. The secondary antibodies were detected using
an ECL Immunoblot Detection system (Pierce Biotechnology, Inc.,
Rockford, IL, USA).
Liver pathology
The middle lobule of each liver was fixed in 4%
formaldehyde, embedded in paraffin, sectioned at a thickness of 4
μm and placed onto glass slides. The paraffin-embedded samples were
dewaxed and stained with hematoxylin and eosin. In order to
determine the number and area of the granulomas, five different
visual fields were captured at ×400 magnification under a
microscope (Olympus, Tokyo, Japan). An image analysis system (VIAS,
Ventana Medical Systems, Inc., Tucson, AZ, USA) was applied to
measure the area of the granulomas.
Statistical analysis
The means of triplicate experiments were used for
statistical analysis by one-way analysis of variance with post hoc
Tukey’s test for pairwise group comparisons (SPSS software, version
13; SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference (two-sided).
Results
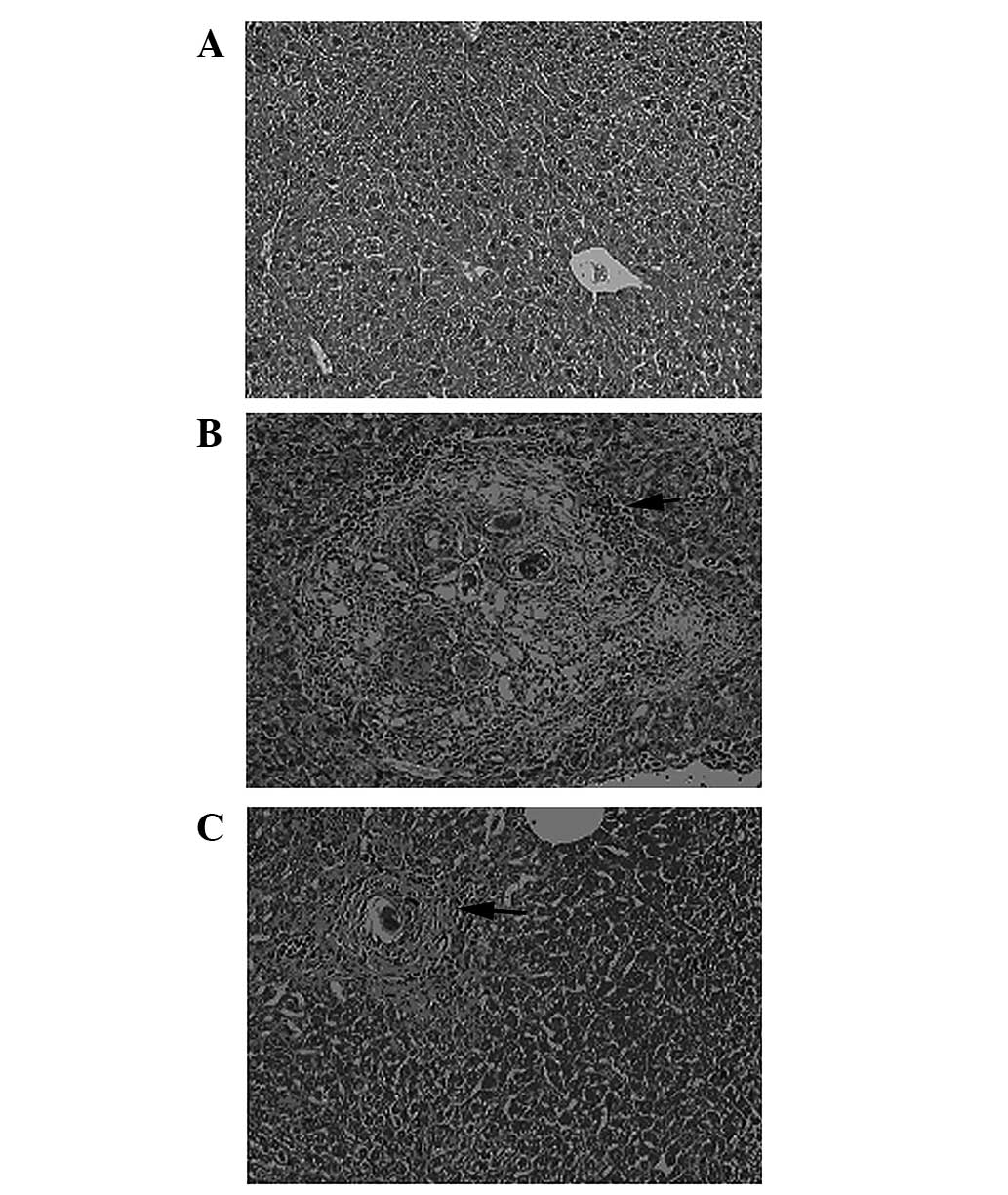
RvE1 treatment improves the liver
pathology in infected mice
The livers from the control mice showed clear
lobules and normal structure under microscopic examination. The
portal and hepatic sinuses appeared normal with uniform
distribution (Fig. 1A). The livers
from model mice showed typical damage in the liver lobules.
Segregation of the liver by collagen fibers, necrosis lesions in
the granulomas and inflammatory cells extensively in the periphery
of the granulomas was observed (Fig.
1B). However, the severity of the hepatocellular necrosis and
fibroplasia was markedly reduced in the livers of the mice treated
with RvE1 compared with that of the model group (Fig. 1C). The mice in the RvE1
intervention group also showed thin fibroseptal attachments and
decreased inflammatory cell infiltrations in the livers,
demonstrating markedly improved or normal architecture of the
hepatic lobules.
Granuloma number and area are reduced in
infected mice receiving RvE1 treatment
The livers from the control mice exhibited no
granulomas; therefore, the mean number and area of granulomas in
each of the infected groups were significantly increased compared
with those of the controls (Table
I). However, the mean area of the granulomas in the RvE1
intervention group was smaller than that in the model group.
 | Table IGranuloma numbers and area in animal
models of Schistosoma japonicum untreated or treated with
RvE1 (n=10 per group). |
Table I
Granuloma numbers and area in animal
models of Schistosoma japonicum untreated or treated with
RvE1 (n=10 per group).
| Group | Mean no. of
granulomas | Mean area of
granuloma (mm2) |
|---|
| Control | 0.0±0.00 | 0.00±0.00 |
| Model | 11.94±2.87a | 17.04±3.51a |
| RvE1 | 10.42±2.47a | 10.26±4.38a,b |
| F-value | 88.44 | 82.99 |
| P-value | 0.00 | 0.00 |
RvE1 lowers the levels of markers of
liver fibrosis in infected mice
To further measure the extent of the liver fibrosis
in the mice, the concentrations of several markers were measured
(Table II). LN, HA, PC-III and
IV-C concentrations were elevated in each of the infected groups
compared with those in the control group. However, in the mice
receiving RvE1 treatment, each of these markers exhibited reduced
levels compared with those in the model group.
 | Table IIConcentrations of LN, HA, PC-III and
IV-C in serum samples from RvE1-treated and untreated mice (n=10
per group). |
Table II
Concentrations of LN, HA, PC-III and
IV-C in serum samples from RvE1-treated and untreated mice (n=10
per group).
| Group | LN (ng/ml) | HA (ng/ml) | PC-III (ng/ml) | IV-C (ng/ml) |
|---|
| Control | 10434.6±67.3 | 34.5±6.3 | 59.2±11.7 | 72.2±17.2 |
| Model | 10741.5±124.2a | 65.8±15.7a | 134.4±31.4a | 139.2±35.2a |
| RvE1 | 10554.8±149.8a,b | 44.4±8.6a,b | 76.7±18.2a,b | 88.2±15.8a,b |
| F-value | 18.01 | 20.41 | 34.05 | 19.75 |
| P-value | 0.00 | 0.00 | 0.00 | 0.00 |
RvE1 lowers the levels of markers of
liver injury in infected mice
To further observe the extent of the liver injury in
the mice, the serum concentrations of ALT and AST were measured
(Table III). The serum ALT and
AST concentrations were increased in each of the infected groups
compared with those of the control group. However, the serum ALT
and AST concentrations were reduced in the mice that received RvE1
treatment compared with those in the model group.
 | Table IIIEffect of treatment with RvE1 on the
levels of serum transaminases (ALT/AST) in a Schistosoma
japonicum-induced liver fibrosis model (n=10 per group). |
Table III
Effect of treatment with RvE1 on the
levels of serum transaminases (ALT/AST) in a Schistosoma
japonicum-induced liver fibrosis model (n=10 per group).
| Group | ALT (U/l) | AST (U/l) |
|---|
| Control | 37.42±7.52 | 41.92±8.30 |
| Model | 358.08±91.57a | 380.89±88.22a |
| RvE1 | 98.70±43.77a,b | 153.85±39.29a,b |
| F-value | 91.92 | 90.21 |
| P-value | 0.00 | 0.00 |
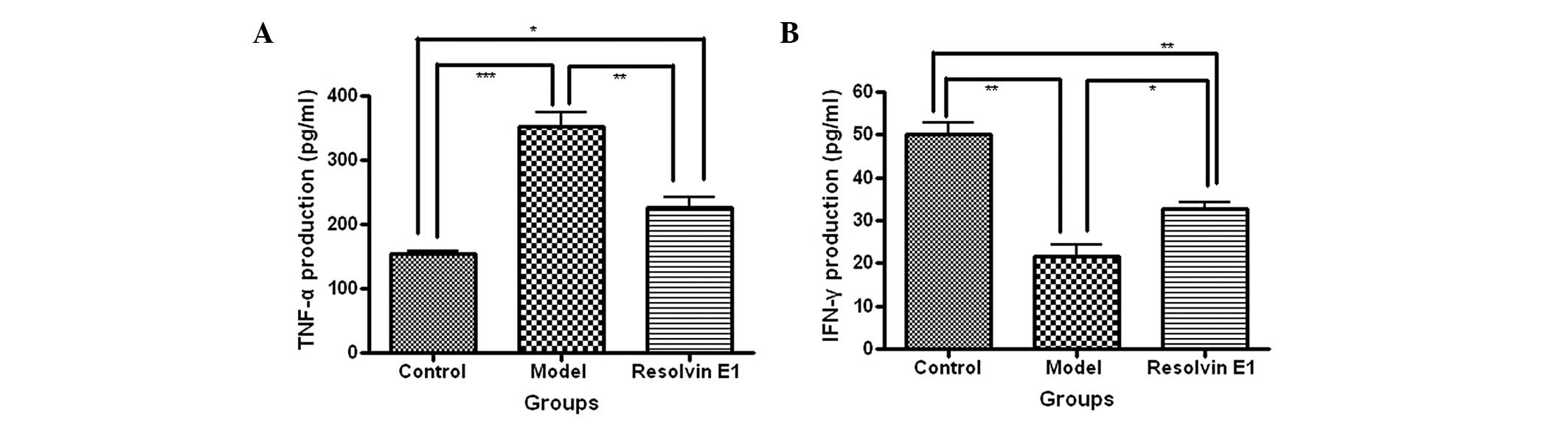
RvE1 affects the TNF-a and IFN-γ
expression levels in infected mice
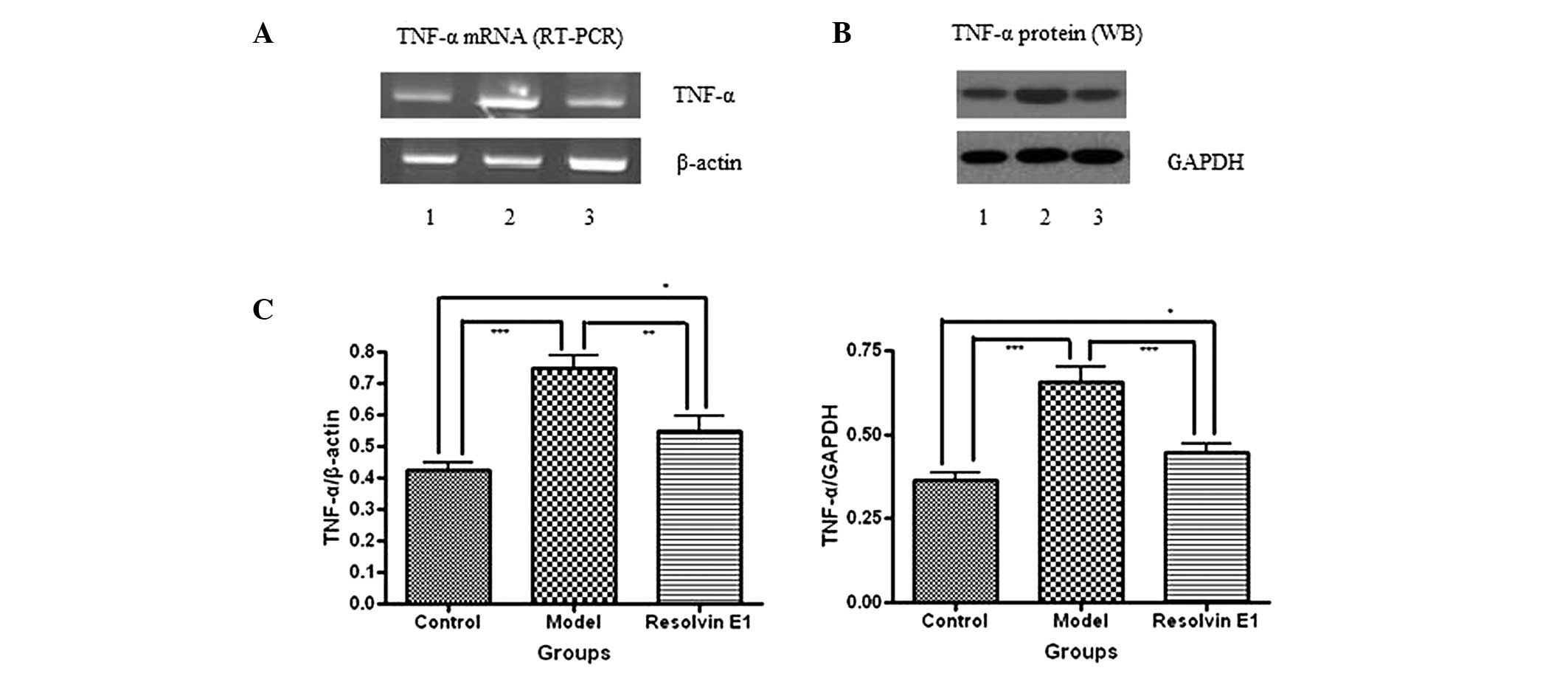
The production and expression levels of TNF-α were
determined by an ELISA, RT-PCR and western blot analysis. The serum
TNF-α production levels were elevated in each of the infected
groups compared with those in the control group. However, the serum
TNF-α production levels were reduced in the mice receiving RvE1
treatment compared with those in the model group (Fig. 2A). In addition, the TNF-α mRNA and
protein levels were also reduced by RvE1 treatment compared with
those in the model group (Fig.
3).
The production and expression levels of IFN-γ were
determined by ELISA. The serum IFN-γ production levels were reduced
in each of the infected groups compared with those in the control
group. However, the serum IFN-γ production levels were increased in
the mice receiving RvE1 treatment compared with those of the model
group (Fig. 2B).
Discussion
Schistosomiasis is an infectious disease that is
seriously harmful to human health (8). Schistosoma japonicum is one of
the most common public health problem in China (9). Schistosoma japonicum is a
typical chronic infectious disease that induces hepatic
schistosomiasis, and the main pathologic lesions of hepatic
schistosomiasis are granuloma formation and liver fibrosis around
the schistosome eggs (10).
Schistosome eggs are an important source of antigens to which the
host is exposed during Schistosoma japonicum infection and
these cells induce an imbalance in T-cell immunity, which is
considered to be a trigger of liver fibrosis (11). The imbalance of T helper (Th)1/Th2
cell immunity excessively activates Th2 and induces hematopoietic
stem cells to differentiate into fibroblasts (7). These fibroblasts increase the levels
of collagen formation and suppress its decomposition, which
eventually results in matrix protein deposition and fibrosis
(12). Th2-derived cytokines cause
liver damage and proliferation of fibrous tissue, accelerating
fibrosis through the activation of macrophages and the induction of
TNF-α and other inflammatory cytokines (13). There is a correlation between
elevated serum levels of TNF-α and an increased degree of liver
fibrosis, and TNF-α generally promotes liver fibrosis. In addition,
IFN-γ is associated with anti-hepatic fibrosis, which is a very
strong anti-fibrotic factor. These results have been demonstrated
in numerous studies of the induction of liver fibrosis in models
with hepatic schistosomiasis (14–16).
The inflammatory cytokines produced in hepatic schistosomiasis
induce liver fibrosis to protect the liver.
RvE1
(5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoic acid) is
an endogenous anti-inflammatory and pro-resolving mediator derived
from the ω-3 fatty acid EPA during resolution (17). Systemic aspirin treatment enhances
local exudate conversion of EPA to the potent, bioactive RvE1
(18). RvE1 stimulates endogenous
resolution mechanisms in vivo in complex disease models and
in vitro (19,20). In nanogram quantities RvE1 promotes
resolution of acute inflammation by regulating leukocyte
infiltration, increasing the ingestion of apoptotic neutrophils by
macrophages and enhancing the clearance of phagocytes to the lymph
nodes and spleen (21). In the
present study, the effects of administered RvE1 were investigated
and it was demonstrated that RvE1 regulates the levels of
inflammatory factors by an anti-inflammatory and pro-resolving
mechanism, improves the local inflammatory response and effectively
alleviates liver fibrosis in schistosome-infected mice. The serum
levels of TNF-α were reduced in the RvE1-treated mice compared with
those in the untreated infected mice. Similarly, the mRNA and
protein expression levels of TNF-α were reduced in the RvE1-treated
mice compared with those in the untreated infected mice. The serum
levels of IFN-γ were increased in the infected animals treated with
RvE1 compared with those in the untreated infected mice. The
results suggest that the RvE1 treatment changed the cytokine levels
and thereby resolved the inflammation. This anti-inflammatory
response may be responsible for the less severe liver pathology
observed in the infected mice treated with RvE1 compared with that
in the untreated infected mice. The levels of serum ALT and AST
were significantly reduced following the RvE1 treatment compared
with those in the untreated infected mice, which indicated that the
RvE1-treated mice exhibited attenuated liver injury. Thus, it is
hypothesized that interventions in the inflammatory response may
effectively alleviate liver injury.
The effects of administered RvE1 on liver pathology
in schistosome-infected mice were also investigated. Administered
RvE1 treatment reduced the effects of schistosome infection on the
liver. While granuloma formation occurred with the same frequency
as in the untreated infected mice, the area of the granulomas was
significantly reduced in the RvE1-treated mice. In addition, the
concentrations of the serum indicators of liver fibrosis (LN, HA,
PC-III and IV-C) were significantly lower in the infected mice
treated with RvE1 than those in the untreated infected mice. These
results further confirmed the anti-fibrotic effects of the
administration of RvE1.
In conclusion, RvE1 treatment regulates the levels
of cytokines to reduce the inflammatory response within the liver
in order to resist fibrosis following schistosome infection. The
present study suggests that administered RvE1 treatment may slow
the progression of liver fibrosis in individuals affected by
schistosomiasis.
Acknowledgements
This study was supported by the Natural Science
Foundation of Hubei Province (no. 2011CDB173), the Scientific
Research Foundation of Health Department of Hubei Province (no.
XF2010-24 and JX6B34) and the Scientific Research Foundation of
Wuhan City (no. Z2011-9-20\201150699189-20). The research performed
in this study was in compliance with the laws of China and the
authors’ respective institutions.
References
|
1
|
Wilson MS, Mentink-Kane MM, Pesce JT,
Ramalingam TR, Thompson R and Wynn TA: Immunopathology of
schistosomiasis. Immunol Cell Biol. 85:148–154. 2007. View Article : Google Scholar
|
|
2
|
Chitsulo L, Engels D, Montresor A and
Savioli L: The global status of schistosomiasis and its control.
Acta Trop. 2000.77:41–51
|
|
3
|
Pearce EJ and MacDonald AS: The
immunobiology of schistosomiasis. Nat Rev Immunol. 2:499–511. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fabre V, Wu H, PondTor S, Coutinho H,
Acosta L, Jiz M, Olveda R, Cheng L, White ES, Jarilla B, McGarvey
ST, Friedman JF and Kurtis JD: Tissue inhibitor of
matrix-metalloprotease-1 predicts risk of hepatic fibrosis in human
Schistosoma japonicum infection. J Infect Dis. 203:707–714.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zou WL, Yang Z, Zang YJ, Li DJ, Liang ZP
and Shen ZY: Inhibitory effects of prostaglandin E1 on activation
of hepatic stellate cells in rabbits with schistosomiasis.
Hepatobiliary Pancreat Dis Int. 6:176–181. 2007.PubMed/NCBI
|
|
6
|
Iredale JP: Models of liver fibrosis:
exploring the dynamic nature of inflammation and repair in a solid
organ. J Clin Invest. 117:539–548. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohira T, Arita M, Omori K, Recchiuti A,
Van Dyke TE and Serhan CN: Resolvin E1 receptor activation signals
phosphorylation and phagocytosis. J Biol Chem. 285:3451–3461. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
WHO Expert Committee. Prevention and
control of schistosomiasis and soil-transmitted helminthiasis.
World Health Organ Tech Rep Ser. 912:2002.PubMed/NCBI
|
|
9
|
Collins C, Xu J and Tang S:
Schistosomiasis control and the health system in P.R. China. Infect
Dis Poverty. 1:82012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gryseels B, Polman K, Clerinx J and
Kestens L: Human schistosomiasis. Lancet. 368:1106–1118. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Coutinho HM, Acosta LP, Wu HW, McGarvey
ST, Su L, Langdon GC, Jiz MA, Jarilla B, Olveda RM, Friedman JF and
Kurtis JD: Th2 cytokines are associated with persistent hepatic
fibrosis in human Schistosoma japonicum infection. J Infect
Dis. 195:288–295. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
MacDonald TT: Decoy receptor springs to
life and eases fibrosis. Nat Med. 12:13–14. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fichtner-Feigl S, Strober W, Kawakami K,
Puri PK and Kitani A: IL-13 signaling through the IL-13alpha2
receptor is involved in induction of TGF-beta1 production and
fibrosis. Nat Med. 12:99–106. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bonnard P, Remoué F, Schacht AM, Pialoux G
and Riveau G: Association between serum cytokine profiles and
schistosomiasisrelated hepatic fibrosis: infection by
Schistosoma japonicum versus S. mansoni. J Infect
Dis. 193:748–750. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Henri S, Chevillard C, Mergani A, Paris P,
Gaudart J, Camilla C, Dessein H, Montero F, Elwali NE, Saeed OK,
Magzoub M and Dessein AJ: Cytokine regulation of periportal
fibrosis in humans infected with Schistosoma mansoni:
IFN-gamma is associated with protection against fibrosis and
TNF-alpha with aggravation of disease. J Immunol. 169:929–936.
2002.PubMed/NCBI
|
|
16
|
Booth M, Mwatha JK, Joseph S, Jones FM,
Kadzo H, Ireri E, Kazibwe F, Kemijumbi J, Kariuki C, Kimani G, Ouma
JH, Kabatereine NB, Vennervald BJ and Dunne DW: Periportal fibrosis
in human Schistosoma mansoni infection is associated with low
IL-10, low IFN-gamma, high TNF-alpha, or low RANTES, depending on
age and gender. J Immunol. 172:1295–1303. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Serhan CN, Clish CB, Brannon J, Colgan SP,
Chiang N and Gronert K: Novel functional sets of lipid-derived
mediators with antiinflammatory actions generated from omega-3
fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory
drugs and transcellular processing. J Exp Med. 192:1197–1204. 2000.
View Article : Google Scholar
|
|
18
|
Serhan CN, Hong S, Gronert K, Colgan SP,
Devchand PR, Mirick G and Moussignac RL: Resolvins: a family of
bioactive products of omega-3 fatty acid transformation circuits
initiated by aspirin treatment that counter proinflammation
signals. J Exp Med. 196:1025–1037. 2002. View Article : Google Scholar
|
|
19
|
Serhan CN: Resolution phase of
inflammation: novel endogenous anti-inflammatory and proresolving
lipid mediators and pathways. Annu Rev Immunol. 25:101–137. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Connor KM, SanGiovanni JP, Lofqvist C,
Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S,
Carper D, Hellstrom A, Kang JX, Chew EY, Salem N Jr, Serhan CN and
Smith LE: Increased dietary intake of omega-3-polyunsaturated fatty
acids reduces pathological retinal angiogenesis. Nat Med.
13:868–873. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schwab JM, Chiang N, Arita M and Serhan
CN: Nature. 447:869–874. 2007. View Article : Google Scholar
|