Introduction
Implantation of the zygote to the endometrium is
dependent on the interaction between the cell and the extracellular
matrix. Integrins are of particular importance as they are known to
bind to the extracellular matrix. In 1989, Hynes et al
(1) identified that integrins are
a family of cell adhesion molecules. Integrins are transmembrane
glycoprotein receptors that are widely distributed on cell surfaces
(1). Individual integrins consist
of an α and a β chain. At present, 18 α and eight β chains have
been discovered and different combinations of these chains produce
24 different integrins in mammals (2). The ανβ3 integrin is an important
member of the integrin family and has a critical role in the
adhesion of vascular endothelial cells and tumor cells during
angiogenesis and tumor metastasis (3–7).
SB-273005,
(4S)-2,3,4,5-tetrahydro-8-[2-[6-(methylamino)-2-pyridinyl]
ethoxy]-3-oxo-2-(2,2,2-trifluoroethyl)-1H-2-benzazepine-4-acetic
acid, is an ανβ3 integrin antagonist (8). SB-273005 is a benzalkonium-derived
compound that was synthesized by Miller et al (9) at SmithKline Beecham.
SB-273005 is a candidate drug for the therapeutic
treatment of patients with osteoporosis (10). It has been found to protect bone
and soft tissue in a mouse model of arthritis (10). Furthermore, SB-273005 has also been
shown to inhibit the adhesion and migration of αvβ3-positive breast
cancer cells, which makes SB-273005 a candidate for a therapeutic
cancer drug for the prevention of tumor-associated angiogenesis
(11). In addition, Wang et
al (12) previously
demonstrated that SB-273005 affects the expression of the ανβ3
integrin in the reproductive system.
Implantation is regarded as a successful allograft
since it relies on the establishment and consolidation of an immune
balance between the mother and fetus (13). Implantation failure may be caused
by multiple factors, including an imbalance in immunological
reactions (14). It has been
previously reported that variations in immune responses and
immunomodulatory mechanisms between the mother and fetus may
influence the dynamic balance between T helper type 1 (Th1) and Th2
cells (15–19).
In a previous study, we demonstrated that SB-273005
has a negative effect on implantation, and that this
anti-implantation activity was due to SB-273005-derived reduction
of ανβ3 integrin levels in the mouse decidual cells (12). SB-273005 may inhibit the
implantation of embryos, promote embryo degeneration and reduce the
blastocyst rate (12), resulting
in significantly lower conception efficiency. However, the
underlying mechanism by which SB-273005 modulates
pregnancy-associated immunoreactions has yet to be elucidated.
To investigate these mechanisms, changes in the
levels of Th1 and Th2 at implantation were investigated in the
present study. The percentages of Th cells and the levels of
cytokines in the peripheral blood and spleen were analyzed using
flow cytometry and enzyme-linked immunosorbent assay (ELISA),
respectively. The hypothesis that implantation is associated with
changes in the spleen regarding Th1 and Th2 cells and cell-derived
interleukin (IL)2 and IL-10 was examined. The potential role of
αvβ3 integrin in these changes was investigated by the inhibition
of αvβ3 with SB-273005.
Materials and methods
Experimental animals
Outbred pathogen-free grade Kunming mice (between 6
and 8 weeks old; weighing 25–30 g) were purchased from the
Laboratory Animal Center of Sun Yat-sen University [Guangzhou,
China; certificate of conformity, 0080353; license no. SCXK (Yue)
2009–0011]. The mice were kept at 25°C with a light cycle of 12 h
on and 12 h off and free access to food and water. All animal
experiments were performed at Sun Yat-sen University according to
experimental protocols approved by the University Animal Research
Ethics Board.
Reagents and instruments
SB-273005 was obtained from GlaxoSmithKline (London,
UK); dimethylsulfoxide (DMSO) was purchased from Sigma (St. Louis,
MO, USA); cluster of differentiation (CD)3-Per, CD8-fluorescein
isothiocyanate, CD8 negative control, interferon
(IFN)-γ-phycoerythrin (PE), CD16+CD56-PE,
mouse-immunoglobulin G (IgG)1-Per, mouse-IgG1-PE, anti-human
IL-4-PE, and permeabilization solution were purchased from BD
Biosciences (Franklin Lakes, NJ, USA); and trypsin and culture
solutions were purchased from Gibco-BRL (Carlsbad, CA, USA). All
other reagents were purchased from Sigma.
Establishment of a pregnancy mouse
model
Female mice were impregnated by one-time co-caging
with males at a 2:1 ratio overnight. Mice were examined for vaginal
plugs the following morning. Mice with clearly visible vaginal
plugs were recorded as being day 1 (D1) pregnant. The pregnant mice
were randomly divided into two groups (n=20 in each group). The
mice in the pregnancy drug group were continuously administered
SB-273005 (dissolved in DMSO) at 3 mg/kg (0.1 ml/mouse) on D3, D4
and D5 by gavage, whilst mice in the normal pregnancy group
received DMSO only. Blood was collected on D8 in the presence of
heparin to prevent coagulation.
Separation of lymphocytes from peripheral
blood
Lymphocytes in the peripheral blood were separated
by density gradient centrifugation using lymphocyte separation
medium in accordance with the manufacturer’s instructions (Tianjin
Hao Yang Biological Products Co., Ltd., Tianjin, China). A total of
1 ml heparin anti-coagulated whole blood was diluted with 1 ml
Hank’s solution. The solution was then slowly dripped into double
volume of lymphocyte separation medium along the tube wall using a
dropper, followed by centrifugation at 544 × g using a swing bucket
rotor (15 cm radius) for 15 min. The centrifugation force separated
materials into four layers, with the first, second and third layer
being the plasma, circular milky lymphocytes and transparent
separation medium, respectively. The cells in the second layer were
collected, and 5 ml phosphate-buffered saline (PBS) was added to
the cells. The cell solution was thoroughly mixed and centrifuged
at 371 × g for 10 min. Cells were then washed twice and resuspended
with modified RPMI-1640 medium (containing penicillin, 100 U/ml,
and streptomycin, 100 U/ml) supplemented with 10% fetal bovine
serum (FBS) to a density of 1×107/ml for culture.
Preparation of the spleen lymphocyte
suspension
Preparation of lymphocytes from the spleen was
performed as previously described (20). Briefly, the spleen was harvested
from the abdominal cavity under sterile conditions and sectioned
into small pieces in a small volume of PBS. The spleen was then
ground and filtered through a steel mesh (100 mesh) and a nylon
mesh (200 mesh), and rinsed with Hank’s solution. A single-cell
suspension was then prepared by centrifugation at 165 × g for 5
min, followed by the addition of 10 ml erythrocyte lysis buffer to
the cell pellet. The cells were further incubated for 4–5 min to
allow the rupture of red blood cells, and then centrifuged at 165 ×
g for 5 min. The cells were then washed Hank’s solution 2 or 3
times prior to being resuspended in medium (5×106/ml of
culture).
Culture of lymphocytes
Lymphocytes were obtained from the peripheral blood
and spleen and had a viability of >95% according to the results
of trypan blue staining. Cells were seeded into a 24-well culture
plate containing 0.1 μl phorbol ester and incubated in a tissue
culture incubator at 37°C at 5% CO2. After 2 h,
non-adherent cells were removed via a medium change. Adhesive cell
growth was detected after 4–6 h of culture using an inverted
microscope. Cells were then re-seeded at 1×106/ml
following trypsinization for culture until confluence (~3–4 days),
and the medium was changed every other day.
Analysis of cells using flow cytometry
(BD FACSAria™ Fusion, Becton Dickinson and Company, Franklin Lakes,
NJ, USA)
A total of 2.5 μl anti-CD4 was added to a 100-μl
cell solution, and the cells were incubated for 15 min at room
temperature. Cold fixation solution (500 μl) was added and the
cells were further incubated for 10–20 min at room temperature. A
total of 1 ml FBS was then added to the cell solution, and the
cells were centrifuged at 323 × g for 10 min. The cells were washed
with FBS and 1 ml permeabilization solution was added prior to
incubation for a further 15 min at room temperature. Cells were
then collected via centrifugation at 323 × g for 10 min. Then, 1 μl
IFN-γ-FITC and 2.5 μl IL-4 was added and the cells were incubated
at room temperature for 30 min, followed by two washes with 1 ml
FBS via centrifugation at 323 × g for 10 min. A total of 300 μl FBS
was then added prior to the flow cytometric analysis. All
procedures were performed under light-proof conditions.
Flow cytometry procedure
The levels of cytokines in 5,000 lymphocytes were
evaluated by flow cytometric analysis. The data were analyzed using
CellQuest™ software (BD biosciences).
Detection of cytokines
Lymphocyte cells obtained from the peripheral blood
and spleen were incubated at 37°C in a tissue culture incubator
containing 5% CO2 for 72 h. The cell culture medium was
collected via the removal of cellular debris by centrifugation at
672 × g for 20 min. The levels of the cytokines IL-2 and IL-10 were
determined using a double-antibody sandwich ELISA. The cytokine
activity in the supernatant was determined in accordance with the
manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed using SPSS
software, version 13 (SPSS, Inc., Chicago, IL, USA). Data were
analyzed using a 2-tailed Student’s t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Pregnancy induces alterations in the
levels of Th1 and Th2 cells in the spleen
Immunological reactions are a critical factor that
determines the success of zygote implantation (20). To investigate the changes in the
number of lymphocytes as a result of pregnancy, lymphocytes were
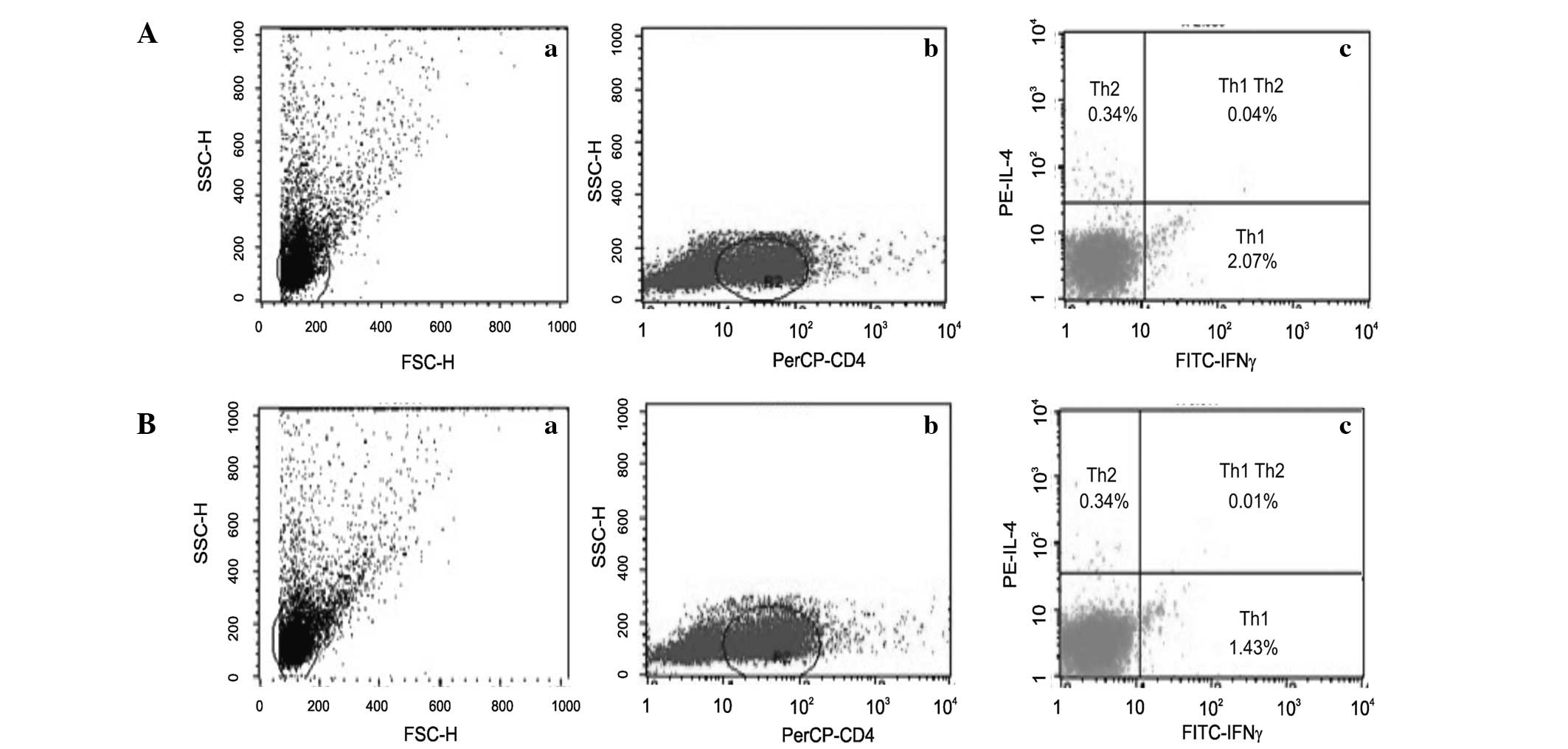
isolated from the peripheral blood (Fig. 1Aa) and the spleen (Fig. 1Ba). Among these lymphocytes, the
Th1 and Th2 populations were identified (Fig. 1Ab and 1Bb). The percentage of Th2
cells remained consistent (0.34%) in lymphocytes isolated from
spleen and peripheral blood; however, the percentage of Th1 cells
was slightly higher in peripheral blood lymphocytes compared with
spleen lymphocytes (Fig. 1Ac and
1Bc).
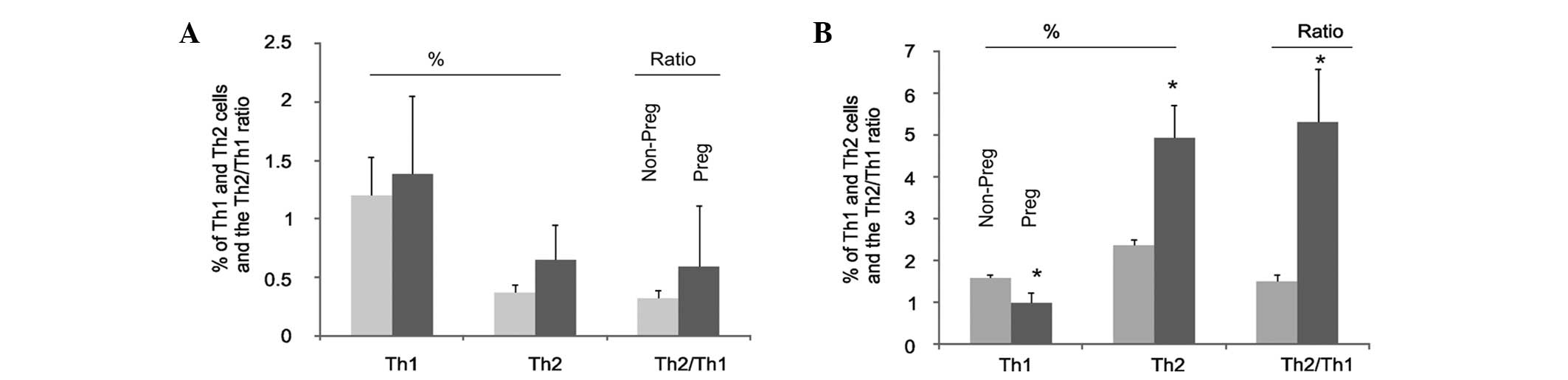
Changes in Th1 and Th2 cells in the spleen and
peripheral blood were then investigated during pregnancy. The
percentages of Th1 and Th2 cells in the peripheral blood increased
in pregnant mice compared with those in non-pregnant mice; however,
the increase was not significant (Fig.
2A). These results are consistent with the finding that the
ratio of Th2:Th1 cells in the peripheral blood in non-pregnant mice
was not significantly different from that in pregnant animals
(Fig. 2A). By contrast, analysis
of spleen lymphocytes revealed that there was a significant
reduction in the percentage of Th1 cells in pregnant mice compared
with that in non-pregnant mice (Fig.
2B), with a concomitant increase of the percentage of spleen
Th2 cells (Fig. 2B). As a result
of these changes, the ratio of Th2:Th1 in the spleen lymphocytes
was markedly increased in pregnant mice compared with that in
non-pregnant mice (Fig. 2B). In
combination, these results demonstrate that there are specific
changes in the levels of Th1 and Th2 cells in the spleens of
pregnant mice.
Pregnancy decreases IL-2 expression and
increases IL-10 expression
To further investigate the changes in Th1 and Th2
cells during pregnancy, the expression of IL2 and IL10, the
specific products of Th1 and Th2 cells, was analyzed (24). The presence of IL-2 and IL-10 in
the peripheral blood and the spleen was analyzed using an ELISA.
The levels of IL-2 in the peripheral blood were significantly
reduced in pregnant mice compared with those in non-pregnant mice,
whereas a significant increase in IL-10 levels was observed in the
pregnant mice (Table I). The
changes in IL-2 and IL-10 levels in the spleen were similar to the
results in the peripheral blood (Table
I). In the pregnant mice compared with the non-pregnant mice,
the level of IL-2 was reduced by 53%, whilst the level of IL-10 was
increased by 51% in the spleen (Table
I). However, the changes in IL-2 and IL-10 levels observed in
the spleen were greater compared with the changes observed in the
peripheral blood (Table I). In
combination, these results not only demonstrate that there is a
pregnancy-associated reduction in IL-2 and an increase in IL-10
levels, but also support the dynamic changes in Th2 versus Th1
cells in the spleens of pregnant mice (Fig. 2).
 | Table IPregnancy causes changes in
interleukin-2 and interleukin-10 levels. |
Table I
Pregnancy causes changes in
interleukin-2 and interleukin-10 levels.
| Parameter | Number of mice | Interleukin-2 | Change (%)a | Interleukin-10 | Change (%)a |
|---|
| Peripheral blood |
| Non-pregnant | 20 | 5.78±0.88 | | 9.29±0.65 | |
| Pregnant | 20 | 3.91±1.63b | −32.3 | 11.61±1.38b | +25.0 |
| Spleen |
| Non-pregnant | 20 | 4.48±3.19 | | 8.84±0.59 | |
| Pregnant | 20 | 2.09±0.74c | −53.3 | 13.36±1.16b | +51.1 |
ανβ3 integrin has an important role in
pregnancy-associated changes in Th1 and Th2 cells in the
spleen
ανβ3 integrin has an important role in the
implantation of the zygote (21),
which suggests that ανβ3 integrin may also have a role in
pregnancy-associated changes in Th1/Th2 cells. To investigate this,
pregnant mice were either mock-treated or treated with an ανβ3
integrin antagonist reagent (SB-273005) and the levels of Th1 and
Th2 cells were then analyzed. Consistent with the findings that
there were no changes in either Th1 or Th2 cells in the peripheral
blood during pregnancy (Fig. 2A),
inhibition of the ανβ3 function by SB-273005 did not significantly
alter either cell population (Fig.
3A). Despite the fact that the spleen levels of Th1 cells were
reduced in pregnant mice (Fig.
2B), SB-273005 did not alter the levels of Th1 cells in
pregnant mice (Fig. 3B). However,
inhibition of ανβ3 by SB-273005 markedly reduced the levels of
pregnancy-upregulated Th2 cells to the level observed in
non-pregnant mice (Fig. 3B). In
combination, these results support the hypothesis that ανβ3
integrin has a critical role in the alteration of Th1 and Th2 cells
in the spleens of pregnant mice.
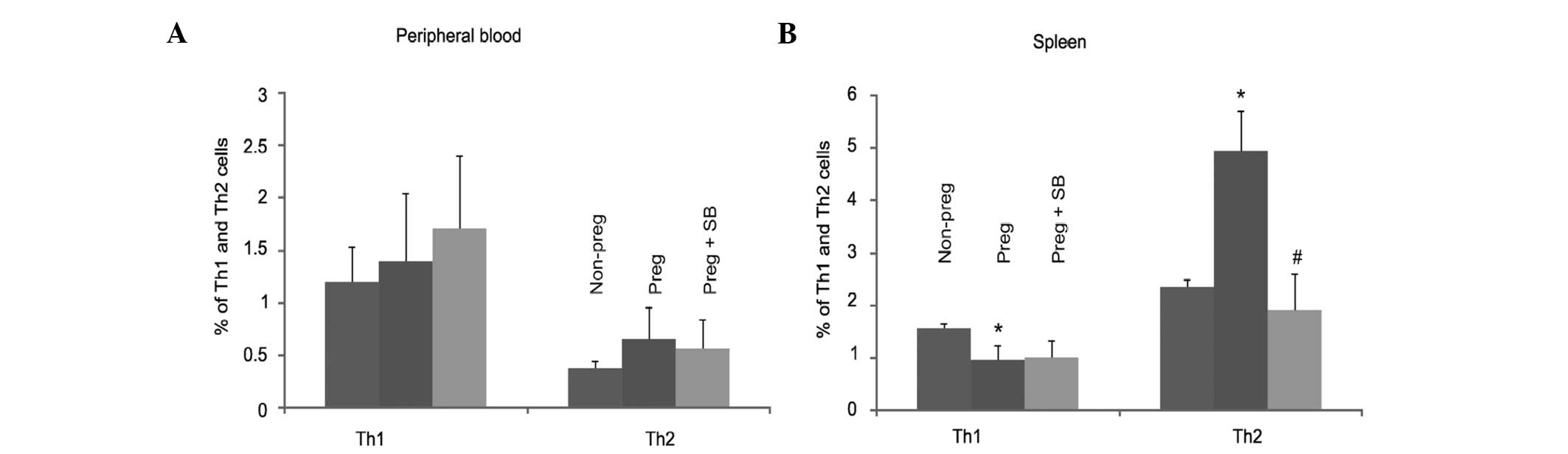 | Figure 3SB-273005 attenuates pregnancy-induced
changes in Th1 and Th2 lymphocytes. Th1 and Th2 cells in (A) the
peripheral blood and (B) the spleen were analyzed in 20
non-pregnant mice and 20 pregnant mice not treated with SB-273005,
and 20 pregnant mice treated with SB-273005. The results are
presented as the mean ± standard deviation. *P<0.05,
compared with non-pregnant mice (2-tailed Student’s t-test);
#P<0.05, compared with pregnant mice (2-tailed
Student’s t-test). Th, T helper; SB-273005,
(4S)-2,3,4,5-tetrahydro-8-[2-[6-(methylamino)-2-pyridinyl]
ethoxy]-3-oxo-2-(2,2,2-trifluoroethyl)-1H-2-benzazepine-4-acetic
acid. |
ανβ3 integrin contributes to changes in
IL-2 and IL-10 levels during pregnancy
Since ανβ3 integrin was found to have a role in
pregnancy-associated changes of Th1/Th2 cells (Fig. 3), the role of ανβ3 integrin in the
alterations of IL-2 and IL-10 in pregnant mice was investigated.
The levels of IL-2 and IL-10 in the peripheral blood and spleen
were measured in pregnant mice in the presence and absence of
SB-273005. A reduction in the level of IL-2 in pregnant mice was
observed in the peripheral blood and the spleen (Table I; Fig.
4), and treatment with SB-273005 significantly inhibited
pregnancy-induced reduction of IL-2 in the spleen (Fig. 4). Similarly, pregnancy-induced
upregulation of IL-10 was significantly reduced by SB-273005 in the
spleen (Fig. 4). In combination,
these results demonstrate the important role of the ανβ3 integrin
in pregnancy-associated alterations of IL-2 and IL-10. These
results also indicate that IL-2 and IL-10 in the spleen are
produced primarily by Th1 and Th2 lymphocytes, respectively.
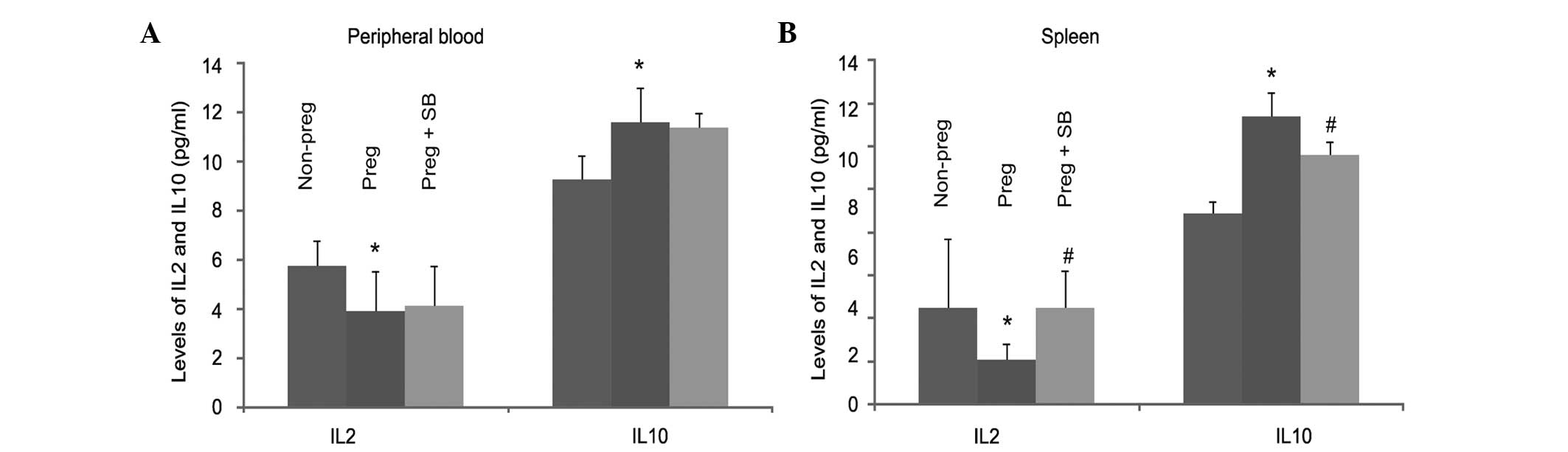 | Figure 4SB-273005 inhibits the
pregnancy-induced changes of IL-2 and IL-10. The levels of IL-2 and
IL-10 in (A) the peripheral blood and (B) the spleen were measured
using an ELISA in 20 non-pregnant mice and 20 pregnant mice not
treated with SB-273005, and 20 pregnant mice treated with
SB-273005. The results are presented as the mean ± standard
deviation. *P<0.05, compared with non-pregnant mice
(2-tailed Student’s t-test); #P<0.05, compared with
pregnant mice (2-tailed Student’s t-test). IL, interleukin;
SB-273005,
(4S)-2,3,4,5-tetrahydro-8-[2-[6-(methylamino)-2-pyridinyl]
ethoxy]-3-oxo-2-(2,2,2-trifluoro ethyl)-1H-2-benzazepine-4-acetic
acid; ELISA, enzyme-linked immunosorbent assay. |
Discussion
SB-273005 has been previously shown to inhibit
embryo implantation, promote embryo degeneration and decrease the
blastocyst formation rate (12).
As a result, SB-273005 significantly reduces the rate of
conception. However, the mechanisms governing SB-273005-derived
inhibition of embryo implantation remain unclear. These were
investigated in the present study and the results indicate that one
of the mechanisms involved is interference in the unique
immunoreactions required for embryo implantation.
Implantation is considered a successful allograft
and it is associated with complex immunological changes. Li et
al (22) previously
demonstrated that overexpression of Th1 cytokines results in
miscarriage. This is consistent with the results from Jin et
al (23), which showed that
IL-2 and IFN-γ were highly expressed in the maternal-fetal
interface during human miscarriage and the expression levels of
IL-4 and IL-10 were concordantly lower in the interface during
miscarriage (23). IL-2/IFN-γ and
IL-4/IL-10 are secreted by Th1 and Th2 lymphocytes, respectively
(24); therefore, the results from
the previous studies support a shift from Th1 to Th2 signaling
events during implantation; and preventing this shift is associated
with miscarriage.
The results from the present study provide
additional support for the downregulation of Th1 signaling and the
upregulation of Th2 signaling during embryo implantation. Compared
with non-pregnant mice, a change in the Th1 and Th2 cell
populations was observed in the spleen of pregnant mice on Day 8 of
pregnancy, when zygote implantation takes place. Specifically, a
reduction in the percentage of Th1 cells and a concordant increase
in the percentage of Th2 cells was detected in the spleen of D8
pregnant mice (Fig. 2B). To
further support this shift, the levels of Th1 cell-produced IL-2
decreased, while those of Th2 cell-derived IL-10 increased
(Table I). The results from the
present study support the use of Th2 signaling over Th1-mediated
immunoreactions during zygote implantation, and this is consistent
with the idea that elevation of Th2 signaling with concordant
inhibition of the Th1 pathway induces immune tolerance, a condition
critical for pregnancy (25). The
inability to downregulate Th1 cells and cytokines is likely to
cause a miscarriage (26).
The mechanisms responsible for the unique
alterations of Th1- and Th2-mediated immunoreactions during
pregnancy are complex; however, in the present study it was
demonstrated that the ανβ3 integrin contributes to these changes.
This is based on the observation that SB-273005, a well-established
antagonist of ανβ3 (12),
attenuated the pregnancy-associated changes in the levels of Th1
and Th2 and their derived cytokines. In particular, SB-273005
increased the levels of Th1 cells and Th1 cell-produced IL2, and
decreased the levels of Th2 cells and Th2 cell-derived IL-10 in
pregnant mice (Table I). This
suggests that SB-273005 may inhibit implantation by reversing the
increase in the Th2 to Th1 ratio. Inhibition of Th2 affects the
distribution of the associated cytokines, and as a result, a
disturbance of immune tolerance may occur, leading to immune
reactions and ultimately miscarriage.
The immune balance between the mother and fetus is
critical for implantation (27–29).
It has previously been shown that this immune tolerance is largely
achieved by downregulating Th1 and upregulating Th2-mediated
immunoreactions (30), and this is
supported by the results of the present study. Furthermore, it was
demonstrated in the present study that ανβ3 integrin has a critical
role in achieving immune tolerance. However, the detailed
mechanisms by which the ανβ3 integrin contributes to reduced Th1
and elevated Th2 signaling require further investigation.
Acknowledgements
The authors would like to thank the Laboratory
Animal Center of Sun Yat-sen University for assistance with animal
experiments. This study was supported by grants from the National
Natural Science Foundation of China (grant No. 81201568).
Abbreviations:
|
DMSO
|
dimethylsulfoxide
|
|
FBS
|
fetal bovine serum
|
|
FCM
|
flow cytometer
|
|
IL-2
|
interleukin-2
|
|
IL-10
|
interleukin-10
|
|
PBS
|
phosphate-buffered saline
|
|
Th
|
T helper cells
|
|
SB-273005
|
(4S)-2,3,4,5-tetrahydro-8-[2-[6-(methylamino)-2-pyridinyl]ethoxy]-3-oxo-2-(2,2,2-trifluoroethyl)-1H-2-benzazepine-4-acetic
acid
|
References
|
1
|
Hynes RO, Marcantonio EE, Stepp MA, Urry
LA and Yee GH: Integrin heterodimer and receptor complexity in
avian and mammalian cells. J Cell Biol. 109:409–420. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Labat-Robert J: Cell-Matrix interactions:
the role of fibronectin and integrins. A survey. Pathol Biol
(Paris). 60:15–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang JL, Wang JL, Gao N, Chen ZT, Tian YP
and An J: Up-regulated expression of beta3 integrin induced by
dengue virus serotype 2 infection associated with virus entry into
human dermal microvascular endothelial cells. Biochem Biophys Res
Commun. 356:763–768. 2007. View Article : Google Scholar
|
|
4
|
Huo T, Du X, Zhang S, Liu X and Li X:
Gd-EDDA/HYNIC-RGD as an MR molecular probe imaging integrin
alphanubeta3 receptor-expressed tumor-MR molecular imaging of
angiogenesis. Eur J Radiol. 73:420–427. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reardon DA, Neyns B, Weller M, Tonn JC,
Nabors LB and Stupp R: Cilengitide: an RGD pentapeptide ανβ3 and
ανβ5 integrin inhibitor in development for glioblastoma and other
malignancies. Future Oncol. 7:339–354. 2011.
|
|
6
|
Brooks PC, Clark RF and Cheresh DA:
Requirement of vascular integrin alpha ν beta 3 for angiogenesis.
Science. 264:569–571. 1994.
|
|
7
|
Dalmas Wilk DA, Scicchitano MS and Morel
D: In vitro investigation of integrin-receptor antagonist-induced
vascular toxicity in the mouse. Toxicol In Vitro. 27:272–281.
2013.PubMed/NCBI
|
|
8
|
Lark MW, Stroup GB, Dodds RA, et al:
Antagonism of the osteoclast vitronectin receptor with an orally
active nonpeptide inhibitor prevents cancellous bone loss in the
ovariectomized rat. J Bone Miner Res. 16:319–327. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miller WH, Alberts DP, Bhatnagar PK, et
al: The discovery of orally active nonpeptide vitronectin receptor
antagonists based on a 2-benzazepine Gly-Asp mimetic. J Med Chem.
43:22–26. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Badger AM, Blake S, Kapadia R, et al:
Disease-modifying activity of SB-273005, an orally active,
nonpeptide alphaνbeta3 (vitronectin receptor) antagonist, in rat
adjuvant-induced arthritis. Arthritis Rheum. 44:128–137.
2001.PubMed/NCBI
|
|
11
|
Gomes N, Vassy J, Lebos C, et al: Breast
adenocarcinoma cell adhesion to the vascular subendothelium in
whole blood and under flow conditions: effects of alphavbeta3 and
alphaIIbbeta3 antagonists. Clin Exp Metastasis. 21:553–561. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang S and Liu G: Effects of ανβ3 integrin
antagonist SB-273005 resisting to embryo implantation in mouse.
Journal of Sun Yat-sen University (Medical Sciences). 29:439–443.
2008.(In Chinese).
|
|
13
|
van Mourik MS, Macklon NS and Heijnen CJ:
Embryonic implantation: cytokines, adhesion molecules, and immune
cells in establishing an implantation environment. J Leukoc Biol.
85:4–19. 2009.PubMed/NCBI
|
|
14
|
Bridges GA, Day ML, Geary TW and Cruppe
LH: Triennial Reproduction Symposium: deficiencies in the uterine
environment and failure to support embryonic development. J Anim
Sci. 91:3002–3013. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu C, Wang J, Zhou S, Wang B and Ma X:
Association between -238 but not -308 polymorphism of Tumor
necrosis factor alpha (TNF-alpha)v and unexplained recurrent
spontaneous abortion (URSA) in Chinese population. Reprod Biol
Endocrinol. 8:1142010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cerkiene Z, Eidukaite A and Usoniene A:
Immune factors in human embryo culture and their significance.
Medicina (Kaunas). 46:233–239. 2010.PubMed/NCBI
|
|
17
|
Yang KM, Ntrivalas E, Cho HJ, et al: Women
with multiple implantation failures and recurrent pregnancy losses
have increased peripheral blood T cell activation. Am J Reprod
Immunol. 63:370–378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Strober W: Immunology: The expanding T(H)2
universe. Nature. 463:434–435. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Raghupathy R: Pregnancy: success and
failure within the Th1/Th2/Th3 paradigm. Semin Immunol. 13:219–227.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saito S, Shima T, Inada K and Nakashima A:
Which types of regulatory T cells play important roles in
implantation and pregnancy maintenance? Am J Reprod Immunol.
69:340–345. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fukuda MN and Sugihara K: Cell adhesion
molecules in human embryo implantation. Sheng Li Xue Bao.
64:247–258. 2012.PubMed/NCBI
|
|
22
|
Li TC, Makris M, Tomsu M, Tuckerman E and
Laird S: Recurrent miscarriage: aetiology, management and
prognosis. Hum Reprod Update. 8:463–481. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jin LP, Fan DX, Zhang T, et al: The
costimulatory signal upregulation is associated with Th1 bias at
the maternal-fetal interface in human miscarriage. Am J Reprod
Immunol. 66:270–278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bix M, Kim S and Rao A: Immunology.
Opposites attract in differentiating T cells. Science.
308:1563–1565. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luppi P: How immune mechanisms are
affected by pregnancy. Vaccine. 21:3352–3357. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lunghi L, Ferretti ME, Medici S, Biondi C
and Vesce F: Control of human trophoblast function. Reprod Biol and
Endocrinol. 5:62007. View Article : Google Scholar
|
|
27
|
Saito S, Nakashima A, Shima T and Ito M:
Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J
Reprod Immunol. 63:601–610. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saini V, Arora S, Yadav A and
Bhattacharjee J: Cytokines in recurrent pregnancy loss. Clin Chim
Acta. 412:702–708. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aluvihare VR, Kallikourdis M and Betz AG:
Tolerance, suppression and the fetal allograft. J Mol Med (Berl).
83:88–96. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Biedermann T, Röcken M and Carballido JM:
TH1 and TH2 lymphocyte development and regulation of TH
cell-mediated immune responses of the skin. J Investig Dermatol
Symp Proc. 9:5–14. 2004. View Article : Google Scholar : PubMed/NCBI
|