Introduction
Aquaporins (AQPs) are a family of hydrophobic
transmembrane proteins that are selectively energized and
impermeable to small molecules, which have been confirmed to exist
in various entities, including bacteria, yeast and plants (1). Recently, various studies have
demonstrated a complex network in the epithelium of the airway,
which regulates water transportation, furthermore, the studies
indicated that six types of AQPs are expressed in the lungs
(2,3).
Numerous studies from the 1990s have identified that
water is involved in the process of cell metabolism and achieves
transmembrane transport via AQPs, which are highly selective for
water molecules. AQPs, within various cells and tissues, are
important in the immediate distribution of intra- and extracellular
water under physiological and pathological conditions (4,5).
Previous studies have shown that following birth, moisture was
rapidly absorbed in the alveoli of mammalian embryos to enable a
transition to spontaneous breathing, whilst AQP1 expression
simultaneously increased in the lung tissue (6). AQP1 expression slowly increased in
the fetal lung tissue and increased significantly as the fetus
approached the pre-production period. Moreover, the expression
exhibited time-phase variation shortly after birth (7). King et al (8) demonstrated that the intravenous
injection of saline lead to peribronchiolar edema in healthy
individuals, but no changes were observed in individuals with
congenital absence of AQP1. A further study identified that the
fluid transportation rate of the alveolar-capillary system
decreased significantly within an AQP1 knock-out animal (9,10).
Previous studies have shown that numerous oxygen
free radicals were produced in high oxygen environments and the
integrity of the cell membrane was destroyed by the interaction of
certain products of oxidative stress and inflammatory cytokines,
which resulted in energy metabolism and cellular function disorders
(11). Alveolar epithelial type II
cells (AEC II) are the stem cells, which are critical for growth,
development and wound repair processes in the lungs. Alveolar
epithelial injury may be significant in the progression of lung
injury in a high-oxygen atmosphere (12).
It has been observed that pulmonary edema is an
early pathological change in lung tissue in a high-oxygen
atmosphere (13); however, the
mechanism of pulmonary edema at the cellular level remains unclear.
Recent studies have demonstrated that AEC II was responsible for
regulating fluid homeostasis in the lungs, however, this did not
include regulation of surfactant secretion (14,15).
It was hypothesized in the present study that problems with AEC II
water permeability may exist in the early stages of hyperoxic lung
injury due to fluid accumulation within the alveoli.
The present study primarily used cultured AEC II as
the experimental model and applied quantitative PCR (qPCR) and
western blot analysis to investigate the expression and functional
changes in AQP1 under hyperoxic conditions. The aim was to explore
the mechanism of pulmonary edema formation during lung injury with
regard to the ability of the lungs to remove water.
Materials and methods
Isolation, culture and verification of
AEC II
Culturing was performed as described by Dobbs et
al (16). The present study
was conducted in strict accordance with the recommendations laid
out by the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health. The animal use protocol was reviewed
and approved by the Institutional Animal Care and Use Committee of
Shengjing Hospital (Shenyang, China). Briefly, neonatal rats (<1
day old) were anesthetized with 10% chloral hydrate and the lung
tissue was removed under sterile conditions. Subsequently, the lung
tissue was washed using pre-cooled D-Hank’s solution and sectioned.
Trypsin (1.5 ml; 0.25%; Merck KGaA, Darmstadt, Germany) was added
and the tissue was digested in a 37°C water bath and agitated for
25–30 min. An equal quantity of Dulbecco’s modified Eagle’s medium
(DMEM; HyClone, Logan, UT, USA) containing 10% fetal bovine serum
(FBS; Clark, Seabrook, MD, USA) was added to terminate digestion,
the tissue was filtered and centrifuged (143 × g) at 4°C for 5 min.
Collagenase I (0.1%; Gibco-BRL, Carlsbad, CA, USA) was added, and
the solution was digested for 15–20 min and centrifuged again (143
× g) at 4°C for 5 min. The cell pellets were resuspended in DMEM
containing 10% FBS, transferred to anti-rat IgG-coated petri dishes
(Santa Cruz Inc., Dallas, TX, USA) and incubated at 37°C for 15 min
to purify the cells. Immunofluorescence of surfactant protein
(SP)-C (a specific marker of AECII cells) and transmission electron
microscopy (TEM; Olympus, Tokyo, Japan) were used to verify the AEC
II cells, in addition, the viability of cells was determined by
trypan blue staining. The purified cells were transferred to a
6-well plate and cultured for 12 h. After washing with PBS, the
cells were fixed by 4% paraformaldehyde for 30 min, and then
blocked by 10% FBS. SP-C antibody was added at 4°C for 12 h
followed by adding FITC-labeled secondary antibody. In addition,
the cellular nuclei were stained with DAPI, and subjected to
fluorescence microscopy for observation.
Groups and methods
The medium was changed following cell attachment.
The cells were randomly divided into experimental and control
groups and placed in a high oxygen (hyperoxic) incubator (oxygen
volume fraction, 0.9) or a regular (normoxic) incubator (oxygen
volume fraction, 0.21), respectively. The cells were collected
after 24, 48 and 72 h of culture and stored at −80°C until they
were assayed.
qPCR
Total RNA was extracted from frozen samples and cDNA
was synthesized via a reverse-transcription (RT) reaction. The PCR
primers were designed using Primer Premier 5.0 (Premier Biosoft
Inc., Paolo Alto, CA, USA) according to the rat AQP1 cDNA sequence,
which was obtained from GenBank® and the cDNA was
synthesized by Sun Biotech Co., Ltd. (Beijing, China). The AQP1
primer sequence was as follows: Forward: 5′-ACCCGCAACTTCTCAAAC-3′
and reverse: 5′-CAGGTCATACTCCTCCACTT-3′. PCR was performed
resulting in a final volume of 25 μl cDNA using a SYBR green PCR
master mix reagent kit (USB, Cleveland, OH, USA). A qPCR device
(Exicycler™ 96, Bioneer Company, Daejeon, South Korea)
was used for quantitative analysis of the PCR product and the PCR
reaction conditions were: 95°C for 10 min, 95°C for 10 sec and 60°C
for 40 sec over a total of 40 cycles. The results were derived from
the comparative threshold cycle (Ct) method and normalized by
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal
control. The ΔΔCt method was applied to analyze the relative AQP1
gene expression level observed in the different samples.
Western blot analysis
Protein was extracted from frozen lung tissue
samples and homogenized in radio-immunoprecipitation assay lysis
buffer with phenylmethanesulfonylfluoride. The protein was
quantified and separated via SDS-PAGE gel electrophoresis and
transferred to a cellulose membrane (Biyuntian, Jiangsu, China).
Non-fat milk (5%) was used to block the membrane for 2 h at room
temperature and a mouse anti-rat AQP1 primary antibody (1:500;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was added,
followed by incubation at 37°C for 1 h. Alkaline phosphatase
conjugated goat anti-mouse secondary antibody (1:10,000; Zhongshan
Jinqiao Biotechnology Co., Ltd., Beijing, China) was added,
incubated at 37°C for 40 min and developed using an
electrochemiluminescence kit (Milipore, Billerica, MA, USA). The
film was digitized via an imaging system (Olympus) and the relative
gray values were calculated with the following formula: Relative
gray value=gray value of target band/gray value of internal control
(GAPDH). Where, a higher relative gray value indicated a higher
protein content.
Measurement of cell volume
Following 24, 48 and 72 h of culture the primary
cultured AEC II cells were washed with phosphate-buffered saline
(PBS) and digested in 0.25% trypsin for 10 min. An equal quantity
of DMEM containing 10% FBS was added to terminate digestion, the
cells were centrifuged at 143 × g for 5 min, washed twice with PBS
and resuspended into a single cell suspension with a final
concentration of 109 cells/ml. The 0.5 ml cell
suspension was loaded into a flow cytometer
(FACSCalibur™, BD Biosciences, Franklin Lakes, NJ, USA),
10,000 cells were detected and the average intensity of the forward
angle light scatter (FALS) was calculated (17). Briefly, when cells are sprayed out
from the nozzle of the flow cytometer in a single line, exposure to
the laser causes forward scattering of the light. The intensity of
the FALS is proportional to cell size, the larger the cell volume,
the higher the intensity of the scattered light. Therefore, the
average intensity of FALS was used as an indicator of cell
volume.
Statistical analysis
SPSS 13.0 software (SPSS Inc., Chicago, IL, USA) was
used for statistical analysis and data were presented as the mean ±
standard deviation. Two-factor analysis of variance was applied and
an L-matrix program (programmed analysis of matrix) enabled
comparisons between the two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Identification of AEC II
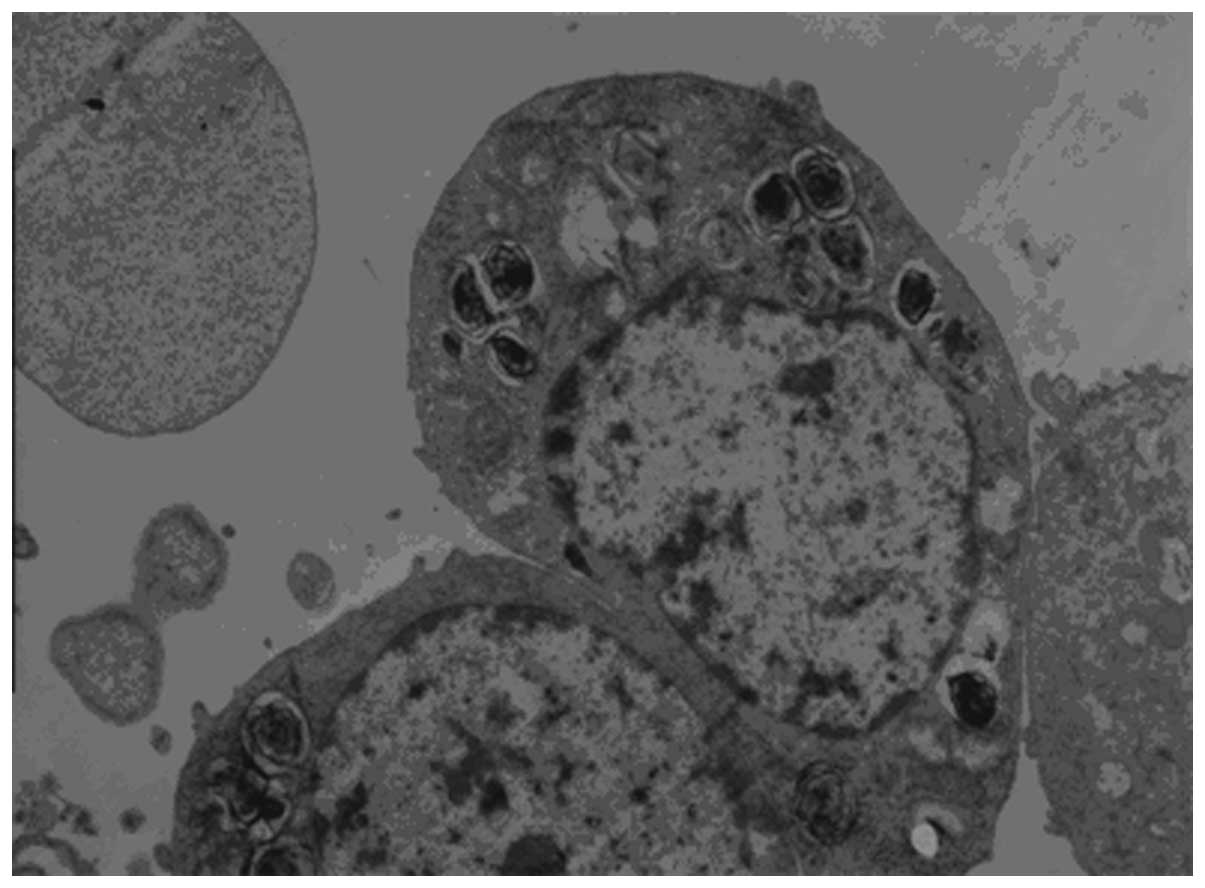
The characteristic lamellar bodies were detected in
the AEC II via TEM and appeared to be arranged as concentric round,
or parallel layered structures. In addition, mitochondria,
endoplasmic reticulum and Golgi apparatus were detected, and
microvilli were observed at the luminal side of the cell (Fig. 1).
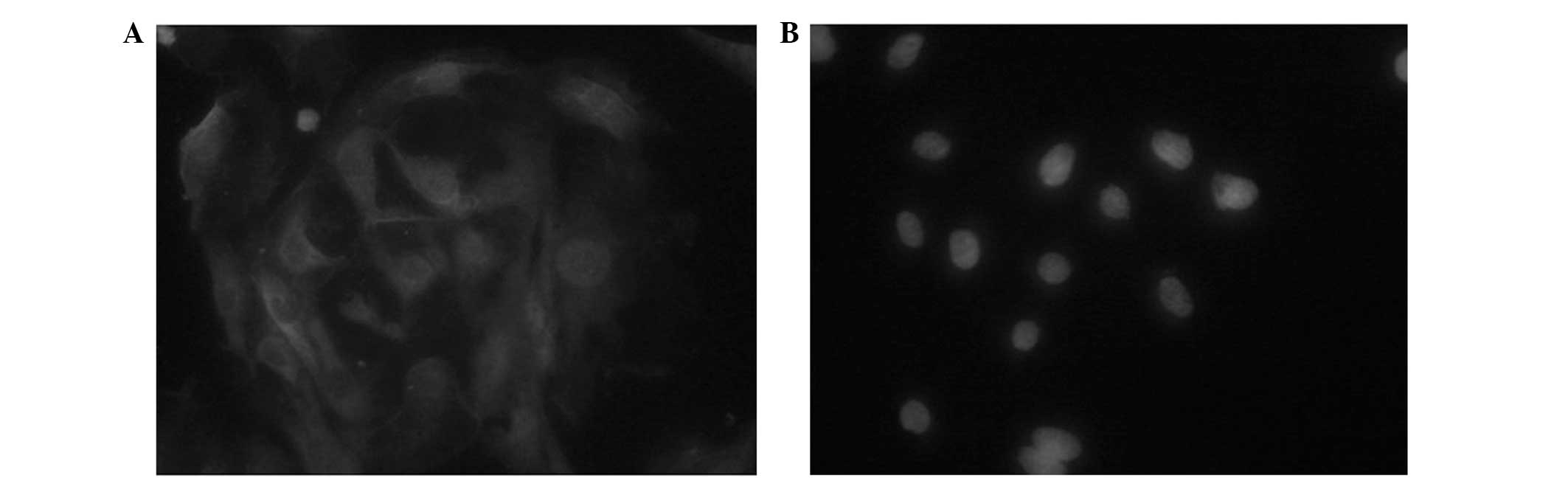
AEC II secretes pulmonary surfactants, such as SP-A,
-B, -C and -D, of which SP-C is the only AEC II-specific
surfactant, therefore, it can be used to identify AEC II (8). SP-C immunofluorescence staining
detected fluorescein isothiocyanate-green fluorescence, which was
located in the cytoplasm and the AEC II cells exhibited positive
expression of SP-C (Fig. 2). The
cell purity was 92.5±3.28% and a trypan blue dye exclusion method
revealed a cell viability of 95±1.06%.
AQP1 gene
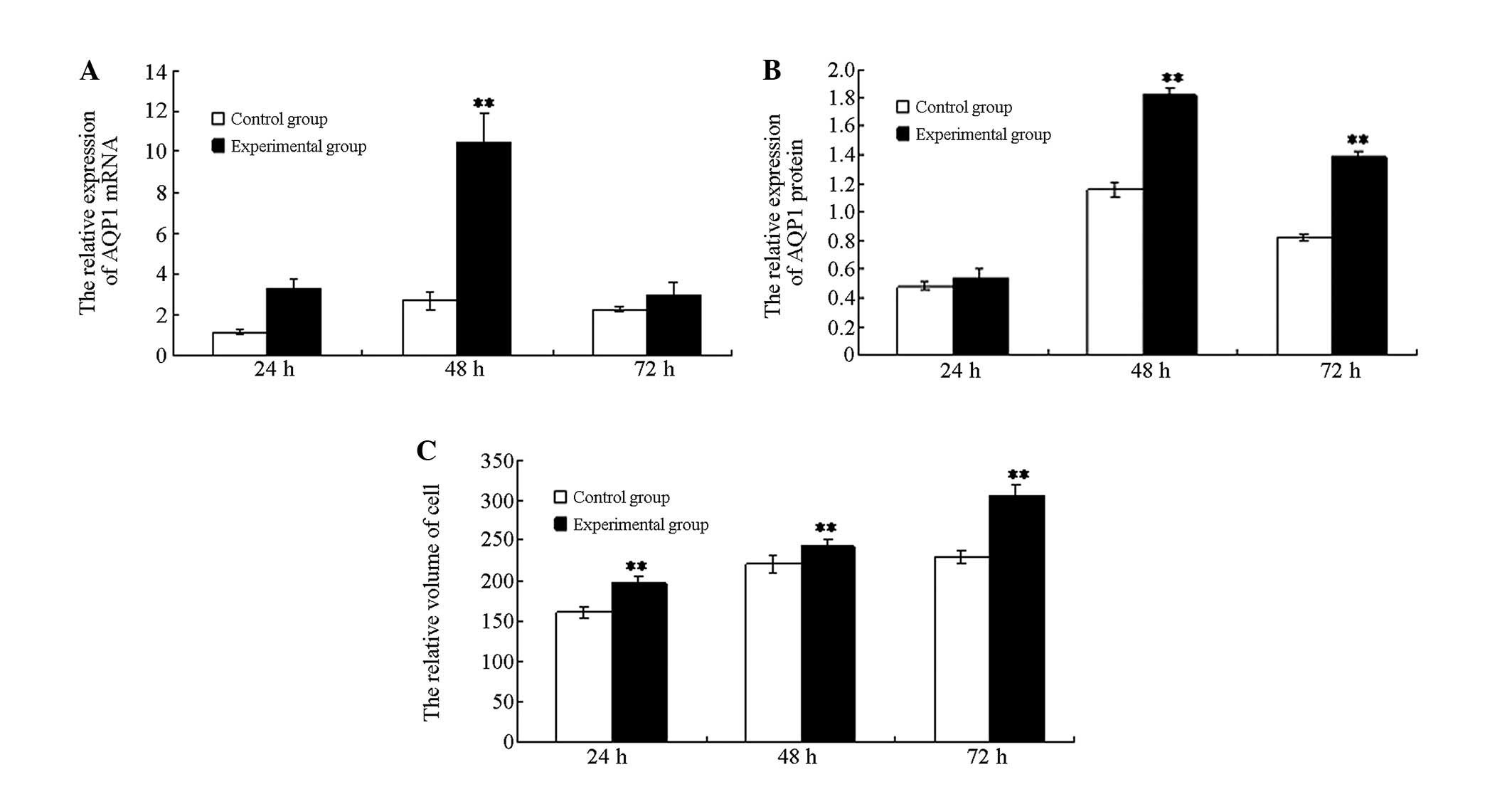
qPCR analysis demonstrated that AQP1 mRNA expression
in the experimental group cells was significantly increased at 48 h
following hyperoxia when compared with the cells of the control
group (P<0.01), while no significant differences were detected
at 24 and 72 h between the two groups (P>0.05). AQP1 mRNA
expression of the experimental group increased initially and
subsequently decreased, which was a statistically significant
change (P<0.01; Fig. 3A).
AQP1 protein
Western blot analysis indicated that at 48 and 72 h,
the AQP1 protein content in the cells of the experimental group was
significantly higher than that of the control group (P<0.01).
However, the AQP1 protein level was not significantly different
between the two groups at 24 h. The AQP1 protein expression in the
experimental group initially increased and subsequently decreased,
which was a statistically significant change (P<0.01; Fig. 3B). These data were consistent with
the fluorescence qPCR results (Fig.
4).
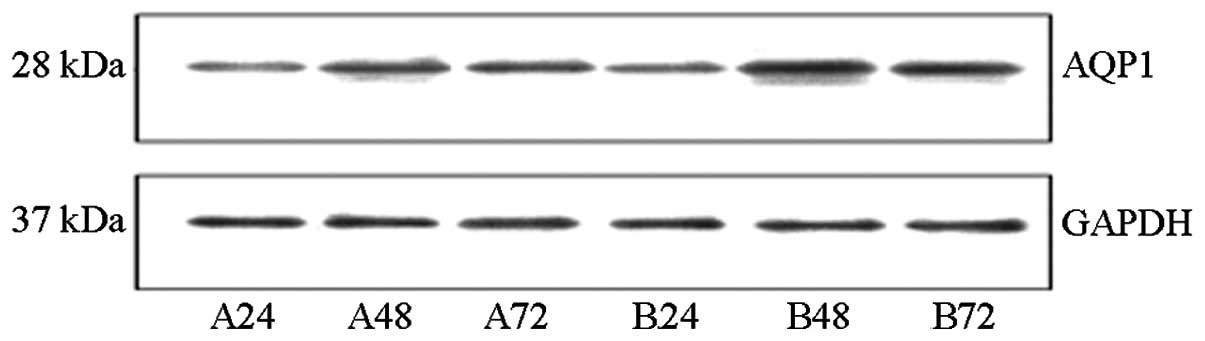 | Figure 4The protein expression of AQP1 in AEC
II. The representative western blot analysis examples of the AQP1
expression band density of each group were normalized to the total
internal control (GAPDH) and expressed as a percentage of the
control. A24, A48, and A72 are control groups for 24, 48 and 72 h,
respectively. B24, B48, and B72 are hyperoxia-exposed group for 24,
48 and 72 h, respectively. AQP1, aquaporin-1; AEC II, alveolar
epithelial type II cells; GAPDH, glyceraldehyde-3-phosphate
dehydrogenase. |
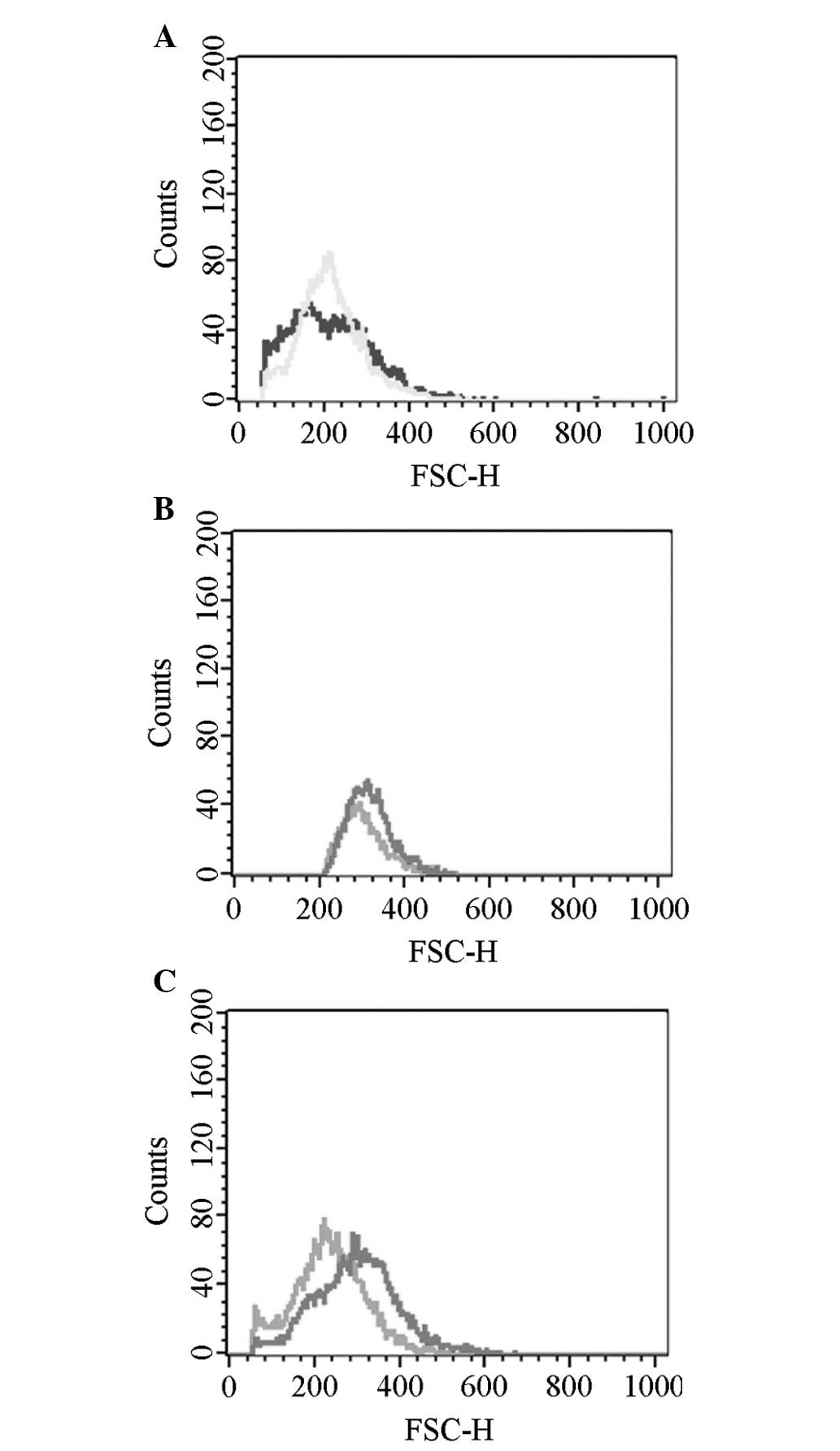
Cell volume
The cell volume of the experimental group
(hyperoxia) was markedly greater than the control group (normoxia;
P<0.01). An intra-group comparison within the experimental group
indicated that the cell volume gradually increased with prolonged
hyperoxia exposure and the differences were identified to be
statistically significant (P<0.01; Figs. 3C and 5).
Discussion
Yue et al (18) demonstrated that the alveolar
membrane intervals were widened and edema were observed in the
hyperoxic group. Furthermore, lanthanum nitrates were shown to be
scattered in the cellular junction. Moreover, a varying quantity of
lanthanum particles appeared in AEC II cells and in the mesenchyma
of the alveolar septum, which indicated that the increased
permeability of the alveolar epithelial cells had contributed to
pulmonary edema (18). However,
the pathogenic mechanisms for hyperoxic pulmonary edema remain
unclear. Studies have shown that AQP1 may be important in lung
fluid transportation, as well as in the pathogenesis of pulmonary
edema (19).
The aim of the present study was to test the
hypothesis that imbalanced AQP1 expression may be one of the
pathogenic mechanisms for hyperoxic lung injury and pulmonary edema
at the cellular level. AQP1 transcription and protein expression in
the cells of the hyperoxia group was observed to be significantly
increased compared with the cells of the control group.
Among the various lung injuries investigated, there
was a reduction in AQP1 expression or activity, and AQP1 and AQP4
mRNA expression was downregulated in allergen-induced mouse models
of asthma (20). Previous studies
have identified that in hyperoxic lung injury-induced pulmonary
edema mice models, the expression level of AQP1 in lung tissues was
significantly downregulated (21).
Lipopolysaccharide-induced acute lung injury significantly impaired
alveolar fluid clearance; however, following administration of
dopamine, this symptom was relieved (via upregulation of AQP1 and
AQP5 expression), which may have further promoted the reabsorption
of alveolar fluids (22).
Staphylococcus aureus and its major pathogenic factor,
α-hemolysin, have been shown to significantly affect AQP1
expression (23). Furthermore,
previous studies have indicated that viral infections and hypoxia
result in pulmonary edema that is accompanied by the downregulation
of AQP1 expression (24,25). Decreased lung AQP1 and AQP5
expression may be associated with pulmonary edema development and
increased severity of lung injury and pulmonary edema, which may
provide an additional mechanism for pancreatitis-associated lung
injury (26).
However, certain studies indicated that AQP1
expression was increased during lung injuries. For example, Lai
et al (27) demonstrated
that inflammatory factors were able to promote AQP1 expression in a
cell model. In another study, Li et al (28) used results from RT-PCR and western
blot analysis to show that the intratracheal administration of
seawater upregulated the mRNA and protein levels of AQP1 and AQP5
in the lung tissues. Song et al (29) reported that AQPs were not required
for the physiological clearance of lung water in neonatal or adult
lungs or for the accumulation of extravascular lung water in an
injured lung. The results of the present study were consistent with
those of Li et al and Song et al. It was hypothesized
that the widened alveolar intervals, the infiltration of
inflammatory cells into the mesenchyma of the lung, congestion,
hyperplasia and/or reconstruction of the pulmonary vasculature of
the normal lung tissues, may have increased the thickness of the
membrane between the alveolar and vascular cavity, which may have
affected fluid transport. Thus, the osmotic pressures were altered,
which significantly influenced electrolyte metabolism and lung
ventilation. We found that the AQP1 under hyperoxic conditions was
increased at transcription and protein level. This suggests that
the enhanced water transportation is one of the compensatory
mechanisms for improving body internal environment. Furthermore,
over a prolonged time period in a high-oxygen atmosphere, the lung
injury was gradually aggravated, which may have resulted in
decreased expression levels of AQP1 mRNA and protein.
Tsai et al (30) hypothesized that inducible NO
synthase was enhanced by the high oxygen environment, either
directly or via signaling of the NF-κB transduction pathway, which
may have induced excessive inflammatory mediator expression, such
as tumor necrosis factor (TNF)-α, interleukin (IL)-β, prostaglandin
E2 (PGE2) and matrix metalloproteinases
(MMPs). These inflammatory mediators may have led to an increase in
endothelial permeability, emergence of edema and enhanced levels of
transcription. This was consistent with a previous study where
excessive inflammatory mediator expression (TNF-α, IL-β,
PGE2 and MMPs) were induced, which resulted in the
increased permeability of the endothelial cells and edema (31). A large number of oxygen free
radicals may activate the signaling pathways of mitogen-activated
protein kinases, including the three subunits, extracellular
signal-regulated kinase (ERK), P38 and c-Jun amino-terminal kinase
(JNK). It has been identified that astrocytes in vitro
inhibited the expression of AQP1 via the JNK pathway (32). However, the ERK pathway activated
the expression of AQP1 and AQP5 in hypertonic conditions (33). Furthermore, activation of the P38
signaling pathway upregulated the expression of AQP5 in lung cancer
tissue (34).
Thus, it was hypothesized that, as a result of
varied cell volume during water transportation, plasmalemma
expansion or contraction occurs, which may activate the
mechanically gated ion channels or mechanical receptors on the
plasmalemma. Subsequently, specific signal transductors were
activated that adjusted the transportation of cellular fluids
(35), which may lead to lung
edema during acute hyperoxic lung injury.
In our study, the cell volume was gradually
increased under hyperoxic conditions, but the AQP1 expression was
not induced at 72 h when compared with that in control group. This,
therefore, indicated that AQP1 may not be the only mechanism of
fluid transport in the cell membrane of AEC II, with other
fluid-balance transport systems present, such as sodium channels
and sodium-potassium ATPase enzymes.
In conclusion, there are few studies regarding the
mechanisms of fluid clearance in hyperoxic lung injury and lung
edema, thus, there is currently no definitive conclusion. The
present study indicated that AQP1 water transport dysfunction may
be a predominant cause of pulmonary edema in acute lung injury,
however, further studies are required to provide further
confirmation.
Acknowledgements
The present study was supported by the National
Natural Science Foundation (grant no. 30872781). Guidance and
assistance was received from the Experimental Center of Shengjing
Hospital, China Medical University (Shenyang, China).
References
|
1
|
Itoh T, Rai T, Kuwahara M, et al:
Identification of a novel aquaporin, AQP12, expressed in pancreatic
acinar cells. Biochem Biophys Res Commun. 330:832–838. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Borok Z and Verkman AS: Lung edema
clearance: 20 years of progress: invited review: role of aquaporin
water channls in fluid transport in lung and airways. J Appl
Physiol (1985). 93:2199–2206. 2002.PubMed/NCBI
|
|
3
|
Kreda SM, Gynn MC, Fenstermacher DA, et
al: Expression and localization of epithelial aquaporins in the
adult human lung. Am J Respir Cell Mol Biol. 24:224–234. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Agre P and Kozono D: Aquaporin water
channels: molecular mechanisms for human diseases. FEBS Lett.
555:72–78. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma T, Fukuda N, Song Y, et al: Lung fluid
transport in aquaporin-5 knockout mice. J Clin Invest. 105:93–100.
2000. View
Article : Google Scholar
|
|
6
|
Ruddy MK, Drazen JM, Pitkanen OM, Rafii B,
O’Brodovich HM and Harris HW: Modulation of aquaporin 4 and the
amiloride-inhibitable sodium channel in perinatal rat lung
epithelial cells. Am J Physiol. 274:L1066–L1072. 1998.PubMed/NCBI
|
|
7
|
Zelenina M, Zelenin S and Aperia A: Water
channels (aquaporins) and their role for postnatal adaptation.
Pediatr Res. 57:47R–53R. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
King LS, Nielsen S, Agre P and Brown RH:
Decreased pulmonary vascular permeability in aquaporin-1-null
humans. Proc Natl Acad Sci USA. 99:1059–1063. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song Y, Ma T, Matthay MA and Verkman AS:
Role of aquaporin-4 in airspace-to-capillary water permeability in
intact mouse lung measured by a novel gravimetric method. J Gen
Physiol. 115:17–27. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bai C, Fukuda N, Song Y, Ma T, Matthay MA
and Verkman AS: Lung fluid transport in aquaporin-1 and aquaporin-4
knockout mice. J Clin Invest. 103:555–561. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang L, Maher TJ and Wurtman RJ: Oral
L-glutamine increases GABA levels in striatal tissue and
extracellular fluid. FASEB J. 21:1227–1232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Franco-Montoya ML, Bourbon JR, Durrmeyer
X, Lorotte S, Jarreau PH and Delacourt C: Pulmonary effects of
keratinocyte growth factor in newborn rats exposed to hyperoxia. Am
J Physiol Lung Cell Mol Physiol. 297:L965–L976. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Modi N: Clinical implications of postnatal
alterations in body water distribution. Semin Neonatol. 8:301–306.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Verkman AS: Role of aquaporins in lung
liquid physiology. Respir Physiol Neurobiol. 159:324–330. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lecuona E, Trejo HE and Sznajder JI:
Regulation of Na, K-ATPase during acute lung injury. J Bioenerg
Biomembr. 39:391–395. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dobbs LG, Gonzalez R and Williams MC: An
improved method for isolating type II cells in high yield and
purity. Am Rev Respir Dis. 134:141–145. 1986.PubMed/NCBI
|
|
17
|
Jordan CT, Yamasaki G and Minamoto D:
High-resolution cell cycle analysis of defined phenotypic subsets
within primitive human hematopoietic cell populations. Exp Hematol.
24:1347–1355. 1996.PubMed/NCBI
|
|
18
|
Yue DM, Tong YJ and Xue XD: Ultrastructure
changes of alveolar epithelial type II cells in newborn rats with
chronic lung disease induced by hyperoxia. J Appl Clin Pediatr.
26:691–693. 2011.(In Chinese).
|
|
19
|
Tsushima K, King LS, Aggarwal NR, De
Gorordo A, D’Alessio FR and Kubo K: Acute lung injury review.
Intern Med. 48:621–630. 2009. View Article : Google Scholar
|
|
20
|
Krane CM, Deng B, Mutyam V, et al: Altered
Regulation of aquaporin gene expression in allergen and
IL-13-induced mouse models of asthma. Cytokine. 46:111–118. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
King LS and Agre P: Pathophysiology of the
aquaporin water channels. Annu Rev Physiol. 58:619–648. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu XM, Wang HY, Li GF, Zang B and Chen WM:
Dobutamine enhances alveolar fluid clearance in a rat model of
acute lung injury. Lung. 187:225–231. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schweitzer K, Li E, Sidhaye V, Leitch V,
Kuznetsov S and King LS: Accumulation of aquaporin-1 during
hemolysin-induced necrotic cell death. Cell Mol Biol Lett.
13:195–211. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Towne JE, Harrod KS, Krane CM and Menon
AG: Decreased expression of aquaporin (AQP)1 and AQP5 in mouse lung
after acute viral infection. Am J Respir Cell Mol Biol. 22:34–44.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Botto L, Beretta E, Daffara R, Miserocchi
G and Palestini P: Biochemical and morphological changes in
endothelial cells in response to hypoxic interstitial edema. Respir
Res. 7:72006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang F, Huang H, Lu F and Chen Y: Acute
lung injury and change in expression of aquaporins 1 and 5 in a rat
model of acute pancreatitis. Hepatogastroenterology. 57:1553–1562.
2010.PubMed/NCBI
|
|
27
|
Lai KN, Leung JC, Metz CN, Lai FM, Bucala
R and Lan HY: Role for macrophage migration inhibitory factor in
acute respiratory distress syndrome. J Pathol. 199:496–508. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li J, Xu M, Fan Q, et al: Tanshinone IIA
ameliorates seawater exposure-induced lung injury by inhibiting
aquaporins (AQP) 1 and AQP5 expression in lung. Respir Physiol
Neurobiol. 176:39–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song Y, Fukuda N, Bai C, Ma T, Matthay MA
and Verkman AS: Role of aquaporins in alveolar fluid clearance in
neonatal and adult lung, and in oedema formation following acute
lung injury: studies in transgenic aquaporin null mice. J Physiol.
525:771–779. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsai CL, Lin YC, Wang HM and Chou TC:
Baicalein, an active component of Scutellaria baicalensis, protects
against lipopolysaccharide-induced acute lung injury in rats. J
Ethnopharmacol. View Article : Google Scholar
|
|
31
|
Huang CS, Kawamura T, Peng X, et al:
Hydrogen inhalation reduced epithelial apoptosis in
ventilator-induced lung injury via a mechanism involving nuclear
factor-kappa B activation. Biochem Biophys Res Commun. 408:253–258.
2011. View Article : Google Scholar
|
|
32
|
McCoy E and Sontheimer H: MAPK induces
AQP1 expression in astrocytes following injury. Glia. 58:209–217.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maruyama T, Kadowaki H, Okamoto N, et al:
CHIP-dependent termination of MEKK2 regulates temporal ERK
activation required for proper hyperosmotic response. EMBO J.
29:2501–2514. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Z, Chen Z, Song Y, Zhang P, Hu J and
Bai C: Expression of aquaporin 5 increases proliferation and
metastasis potential of lung cancer. J Pathol. 221:210–220. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee MD, King LS, Nielsen S and Agre P:
Genomic organization and developmental expression of aquaporin-5 in
lung. Chest. 111(6 Suppl): 111S–113S. 1997. View Article : Google Scholar : PubMed/NCBI
|