Introduction
Avascular necrosis of the femoral head is usually
caused by a lack of osteoblast progenitor cells and blood vessels
(1). The formation of new blood
vessels in the bone necrosis region is a prerequisite for bone
tissue repair. Growth factors have an important role in the
construction of bone by tissue engineering. Previous studies have
focused on the role of single growth factors in bone tissue
engineering (2,3). However, the synergistic effects of
various growth factors have rarely been studied. Furthermore,
growth factors are usually prepared by genetic recombination, which
has certain shortcomings, including difficulty of preparation, high
cost and immunogenicity.
In the present study, platelet-rich plasma (PRP) was
used as a source of growth factors. PRP is an autologous platelet
concentrate and is obtained from fresh whole blood by
centrifugation. The biological activity of PRP depends on the
enrichment in platelets. It has been confirmed that PRP prepared by
appropriate technology contains a variety of growth factors at high
concentrations, including platelet-derived growth factor,
transforming growth factor-β, vascular endothelial growth factor,
insulin-like growth factor and epidermal growth factor. PRP has an
important role in promoting the proliferation of bone precursor
cells in various animals, including rabbits and goats (4,5). In
addition, it promotes the proliferation of bone marrow mesenchymal
stem cells (BMSCs) and their differentiation into osteoblasts
(6). The growth factors contained
in PRP have many functions, including promoting angiogenesis and
accelerating the formation of collagen, thus promoting tissue
healing and bone formation. The composition of growth factors
released by PRP is consistent with that of human growth factors.
Therefore, PRP is considered as an ideal source of growth factors
for clinical application. PRP is primarily used in the repair of
bone defects in the oral cavity and maxillofacial region; however,
few studies have investigated the use of PRP in bone tissue
engineering.
Marx et al (7) first used PRP in combination with
autologous bone to promote new bone formation. Kim et al
(8) combined PRP with β-tricalcium
phosphate (β-TCP) and implanted the composite into skull defects in
athymic mice, which resulted in a significant improvement in bone
formation. The authors hypothesized that the application of
inorganic materials as scaffolds requires the growth factors
released by PRP, which may promote vascularization. In a study by
Yokota et al (9) a model of
rabbit iliac bone necrosis was established using liquid nitrogen,
and a single injection of PRP was observed to accelerate the
vascularization of necrotic bone. Tao et al (10) utilized a composite of TCP and PRP
to repair ischemic necrosis of the femoral head in a rabbit model,
and demonstrated that TCP-PRP composite materials not only provide
a scaffold for osteoblasts, but also promote necrotic bone repair.
Latalski et al (11)
retrospectively studied 19 patients undergoing limb lengthening
with an external fixator. The study showed that the treatment time
for patients who received PRP was significantly shorter compared
with that of the control group, and that the treatment had
satisfactory clinical results. In addition, Curi et al
(12) treated 25 cases of
bisphosphonate-related osteonecrosis of the jaw (BRONJ) by
resecting the necrotic bone and placing autologous PRP in the bone
resection region. It was found that treatment with PRP was an
effective strategy for the treatment of the patients with BRONJ.
Furthermore, Scala et al (13) reported that the injection of PRP
may be used as an effective method for repairing
osteoradionecrosis.
In the present study, tissue-engineered bone was
constructed by co-culturing BMSCs and β-TCP in a three-dimensional
perfusion bioreactor. The BMSC-TCP composite was then implanted
into rabbits and vascularization of the composite was evaluated
in vivo.
Materials and methods
Reagents and instruments
Rabbit BMSCs were purchased from Cyagen Biosciences
Inc. (Santa Clara, CA, USA). β-TCP was obtained from Shanghai
Bio-lu Biomaterials Co., Ltd. (Shanghai, China). Hematoxylin and
eosin (H&E), anti-cluster of differentiation (CD)31 antibody,
anti-von Willebrand factor (vWF) antibody and 3,3′-diaminobenzidine
(DAB) chromogenic reagent for immunohistochemistry were purchased
from Boster Biological Technology Co., Ltd. (Wuhan, China).
Low-glucose Dulbecco’s modified Eagle’s medium (DMEM) was obtained
from HyClone (Rockford, IL, USA).
The perfusion bioreactor used in this study was
jointly designed by our group and the East China University of
Science and Technology (Shanghai, China). The whole system
consisted of a MasterFlex peristaltic pump (Cole-Parmer Company,
Vernon Hills, IL, USA), kcal head, perfusion column (including
seals and sample slots), air filters, two silicone tubes, a
three-way connector and cell culture flasks. Two silicone tubes
were placed into the cell culture flask. One silicone tube was
immersed in the culture medium in order to extract the culture
medium. The tube was then connected to the perfusion column to
transport the medium. The culture medium flowed back into the flask
through another silicon tube. The JSM-T300 scanning microscope was
obtained from JEOL-Technics Co. (Tokyo, Japan) and the light
microscope was from Olympus Optical Co., Ltd. (Tokyo, Japan).
Animals
Ten male New Zealand rabbits, 2 months old, with a
mean weight of 3.50±0.30 kg, were obtained from the Experimental
Animal Center of Shandong Province (Jinan, China). They were kept
under standard conditions with free access to food and water.
All animal experiments were approved by Animal Care
and Use Committee of Shandong University and were conducted
according to the ethical guidelines of Shandong University (Jinan,
China).
Preparation of PRP gel
The rabbits were anesthetized and 5 ml blood was
collected from the ear vein. The blood was mixed with 1 ml sodium
citrate anticoagulant. Then, the mixture was centrifuged at 549 × g
for 6 min. Following centrifugation, the mixture was separated into
a supernatant and a red blood cell layer, with an interface between
them. The supernatant and the red blood cells 1–2 mm below the
interface were transferred to another centrifuge tube and were
centrifuged again (454 × g, 6 min). The supernatant was discarded
and the remaining solution (~0.8 ml) was PRP. A total of 0.8 ml PRP
was then mixed with 0.2 ml anticoagulant (consisting of 1 ml 10%
calcium chloride and l,000 units thrombin) and incubated for 10 sec
in order to form a gel. The prepared PRP gel was then added to 25
ml culture medium to prepare PRP gel culture medium.
Construction of BMSC-TCP composite using
a bioreactor
Rabbit BMSCs were cultured in low-glucose DMEM to
the third generation and then the cells were adjusted to a single
cell suspension of 5xl06 cells/ml. The β-TCP scaffold
was immersed in the cell suspension and the scaffold was then
turned upside down to allow the cells to enter into the pores of
the scaffold. The β-TCP scaffold was then cultured at 37°C in an
incubator at 5% CO2. After 1 day, the BMSC-TCP composite
(β-TCP scaffold covered with cells) was placed into the perfusion
bioreactor column, with all the interfaces sealed by silicone. The
entire system was then placed into an incubator at 37°C containing
5% CO2 and cultured for 21 days. The flow rate was
maintained at 3 ml/min. By measuring the content of glucose in the
culture medium, the culture medium was replaced every 2–3 days. The
BMSC-TCP composite was examined for pathological morphology and
used for further animal experiments.
Experimental grouping
Each rabbit was implanted with β-TCP and four
different combinations of BMSCs and β-TCP, each at a different
site. The five implanted materials were as follows: group A, β-TCP
only; group B, uncultured BMSCs and β-TCP; group C,
bioreactor-cultured BMSCs and β-TCP; group D, PRP-cultured BMSCs
and β-TCP; and group E, bioreactor- and PRP-cultured BMSCs and
β-TCP.
BMSC-TCP composite implantation
The New Zealand white rabbits were anesthetized and
placed in a left lateral position to fix their head and limbs. The
right side was longitudinally incised to expose the superficial
fascia. The BMSC-TCP composite was implanted between the
subcutaneous superficial fascia and deep fascia. The position was
recorded and the skin was sutured. The New Zealand rabbits were
then kept under standard conditions and intramuscularly injected
with 800,000 units penicillin each day, for 3 days following the
surgery. The daily diet and wound healing were observed following
the surgery.
H&E staining
Three months following surgery, the BMSC-TCP
composite was removed, and rinsed with saline. Then, the BMSC-TCP
composite was fixed in 10% formalin, embedded in paraffin and cut
into tissue sections. The tissue sections were dewaxed in xylene
and rehydrated in graded alcohols. Following washing with running
water and distilled water, the sections were stained with
hematoxylin. After washing again, the sections were differentiated.
Then, the sections were stained with eosin after washing with
running water. Following dehydration and differentiation in
alcohol, the sections were mounted and observed under a JSM-T300
scanning electron microscope.
Immunohistochemistry
The expression levels of CD31 and vWF in the
BMSC-TCP composites were detected using immunohistochemistry.
Briefly, samples were fixed in formaldehyde and embedded in
paraffin. Sections were dewaxed, rehydrated in graded alcohols and
processed, prior to incubation with antibodies. After blocking, the
sections were incubated with primary antibodies against CD13 and
vWF. The sections were washed with phosphate-buffered saline,
secondary antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA) were then added and the sections were further incubated.
The sections were then developed using the DAB chromogenic reagent
and sections were counterstained with hematoxylin. Positive
staining was presented as brown granules in cells. Five fields were
randomly selected at high magnification under the light microscope
and the number of positively stained cells was counted. The
percentage of positive cells was the ratio of the number of
positive cells to the number of total cells (≥26 cells were
counted).
The scoring criteria were as follows. Scores based
on the percentage of positive cells: 0, percentage of positive
cells ≤25%; 1, percentage of positive cells between 25 and 50%; 2,
percentage of positive cells between 50 and 75%; and 3, percentage
of positive cells ≥75%. Scores based on intensity of staining: 0,
no positive staining; 1, weak (pale yellow) staining; 2, medium
(brown) staining; and 3, strong (tan) staining. The histological
score was the sum of the above two types of score, and a
histological score of 0 was expressed as negative, a score 2 or 3
as weak positive, a score 4 or 5 as medium positive and score 6 or
7 as strongly positive.
Statistical analysis
The SPSS statistical software package, version 17.0
was used for statistical analyses (SPSS, Inc., Chicago, IL, USA).
Data are presented as the mean ± standard deviation. Analysis of
variance was performed to compare differences between the different
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Morphological analysis of BMSC-TCP
composite
Composite materials were implanted between the
superficial fascia and deep fascia under the skin. For
implantation, each rabbit was implanted at 5 different sites with
β-TCP only (group A), BMSCs and β-TCP (group B),
bioreactor-cultured BMSCs and β-TCP (group C), PRP-cultured BMSCs
and β-TCP (group D), and bioreactor- and PRP-cultured BMSCs and
β-TCP (group E), respectively. Following implantation, the wounds
all healed, without any swelling, effusion and signs of infection.
Three months following implantation, the BMSC-TCP composites were
removed. To observe the morphology of the BMSC-TCP composites-,
scanning electron microscopy and H&E staining was performed.
Representative scanning electron microscopy results are shown in
Fig. 1A. The β-TCP blank scaffold
showed an irregular porous structure, with an average pore size of
500–600 μm, a porosity of 75±10% and a stomatal communication rate
of 100%. Observation of the BMSC-TCP composite under the scanning
electron microscope revealed that BMSCs adhered on the β-TCP
ceramic scaffold and the secretion of extracellular matrix was also
observed. The spreading and proliferation of cells was good on the
scaffold, indicating that the affinity of BMSCs to the β-TCP
scaffold was high. Statistically, the number of adherent BMSCs in
group E was significantly higher compared with that in the other
groups (data not shown).
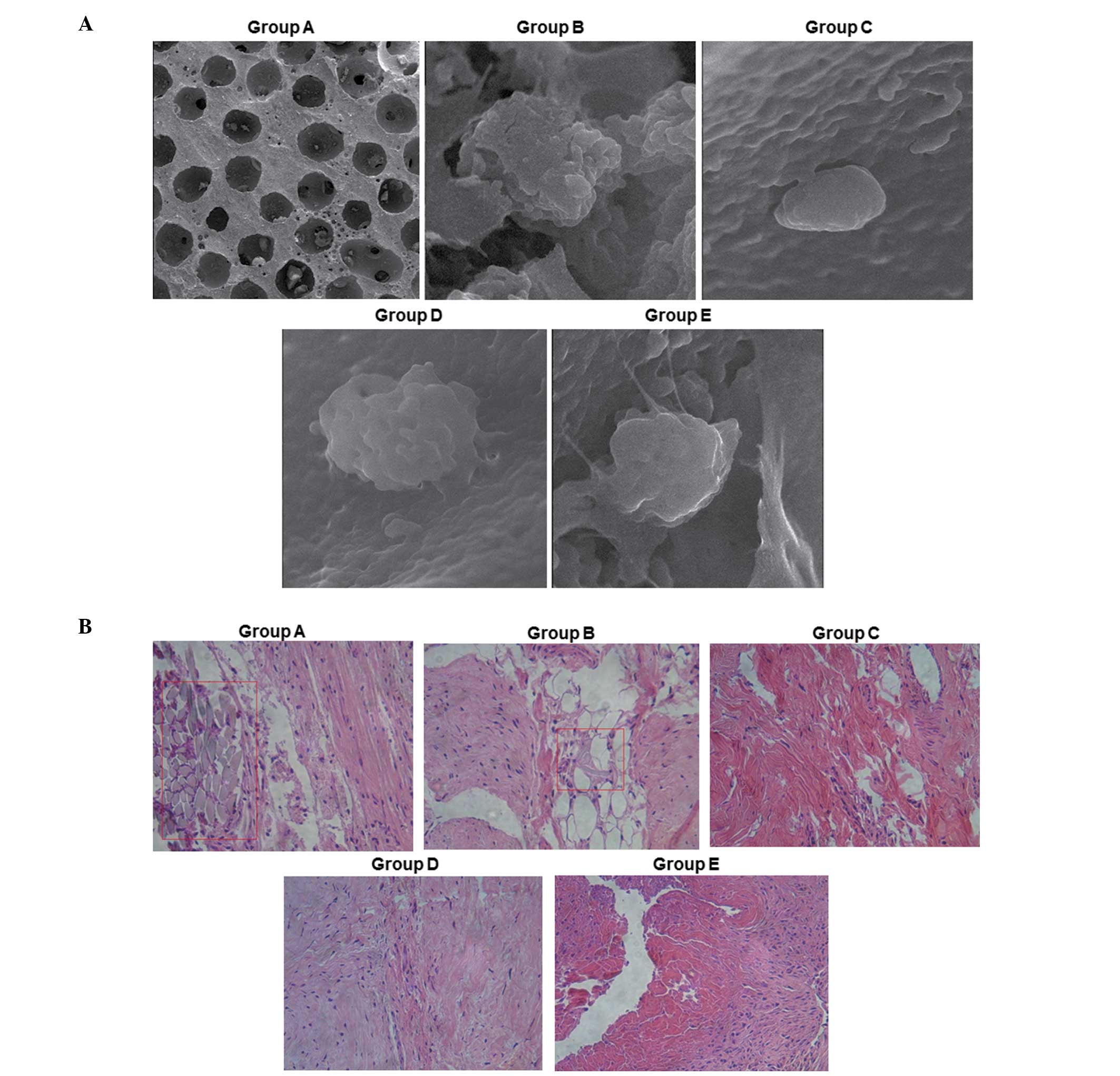 | Figure 1Morphological analysis of β-TCP
scaffold and BMSC-TCP composite. Each rabbit was implanted at 5
different sites with β-TCP only (group A), BMSCs and β-TCP (group
B), bioreactor-cultured BMSCs and β-TCP (group C), PRP-cultured
BMSCs and β-TCP (group D), and bioreactor- and PRP-cultured BMSCs
and β-TCP (group E), respectively. Three months following
implantation, samples were removed from the rabbits (n=10). The
morphology was observed using a JSM-T300 scanning electron
microscope following H&E staining. (A) Representative scanning
electron microscopy results for groups A–E. Magnification, 75kv ×
5k. (B) Representative H&E staining results for groups A–E.
Magnification, ×25. TCP, tricalcium phosphate; BMSCs, bone marrow
mesenchymal stem cells; PRP, platelet-rich plasma; H&E,
hematoxylin and eosin. |
Representative H&E staining results for the
various groups are shown in Fig.
1B. The results of the H&E staining demonstrate that there
was more hyperblastosis in group E than in the other groups.
Additionally, dense connective tissues were identified in group E,
which are suggestive of bone formation. The βTCP scaffold and the
tissue were tightly bound and vascular-like tissue was observed.
However, in groups B, C, D and E, vascular-like tissue was rarely
observed.
Vascularization analysis by
immunohistochemistry
CD31 and vWF are expressed in vascular endothelial
cells. Therefore, to evaluate the vascularization in the BMSC-TCP
composite, immunohistochemical staining of CD31 and vWF was
performed. Representative immunohistochemical staining results for
CD31 and vWF are shown in Figs. 2
and 3, respectively. Cells with
brown granules were positively stained cells. As shown in Figs. 2A and 3A, no positively stained cells were
visible in group A. In groups B (Figs.
2B and 3B), C (Figs. 2C and 3C) and D (Figs. 2D and 3D), there were smaller brown particles
observed within the cells, and these brown particles were unevenly
distributed. However, in group E (Figs. 2E and 3E), a large number of brown-stained
granules were observed under the microscope, with larger particles
also observed. Histological scoring was conducted as described in
Materials and methods and the scores for CD31 in each group are
presented in Table I.
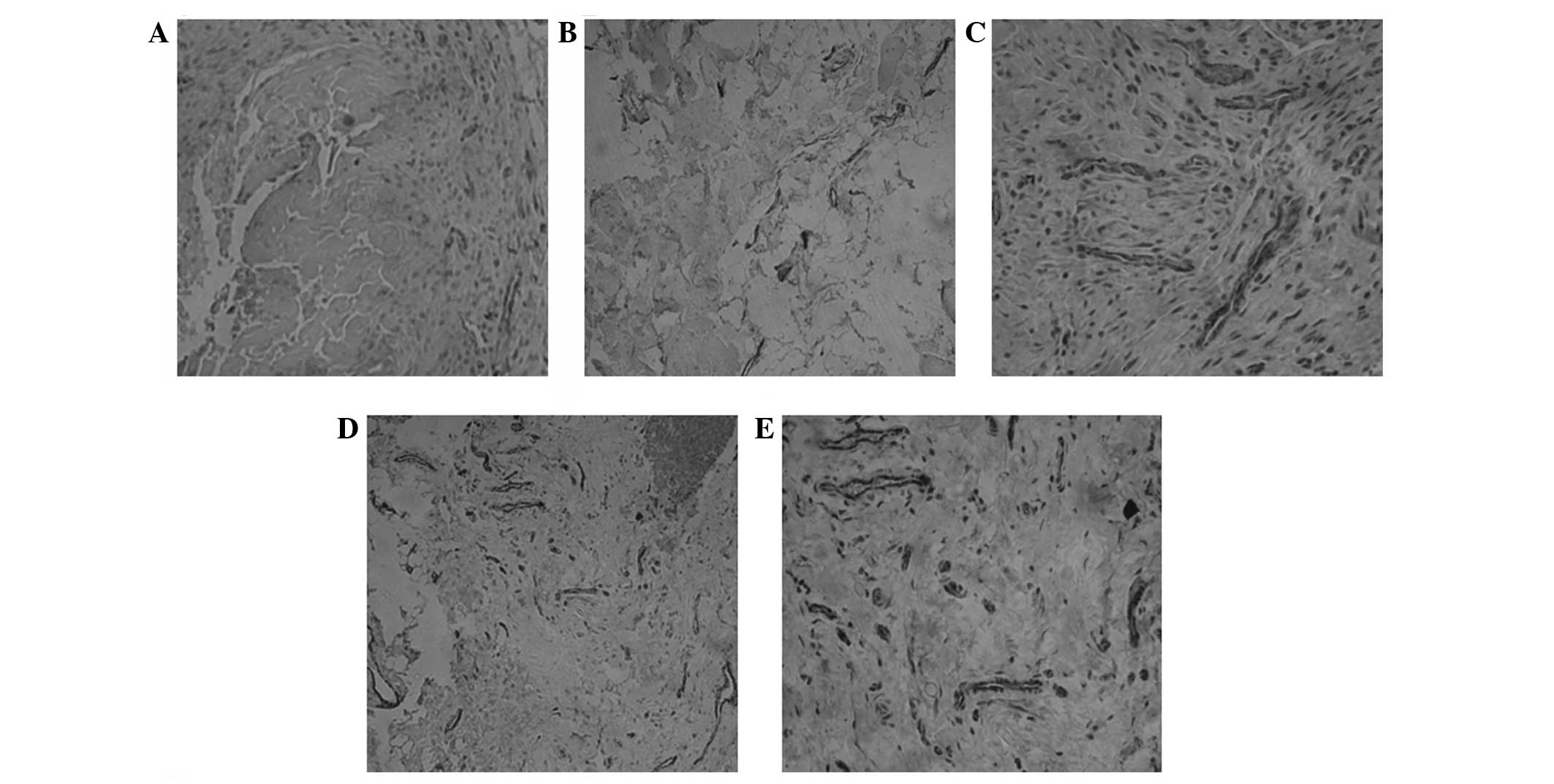 | Figure 2Immunohistochemical analysis of CD31
expression. Each rabbit was implanted at five different sites with
β-TCP only (group A), BMSCs and β-TCP (group B),
bioreactor-cultured BMSCs and β-TCP (group C), PRP-cultured BMSCs
and β-TCP (group D), and bioreactor- and PRP-cultured BMSCs and
β-TCP (group E), respectively. Three months following implantation,
samples were collected from implanted rabbits (n=10). Expression of
CD31 was analyzed using immunohistochemistry. Cells with brown
granules were considered positive cells. Representative
immunohistochemical results from (A) group A, (B) group B, (C)
group C, (D) group D and (E) group E (magnification, ×200). CD,
cluster of differentiation; TCP, tricalcium phosphate; BMSCs, bone
marrow mesenchymal stem cells; PRP, platelet-rich plasma. |
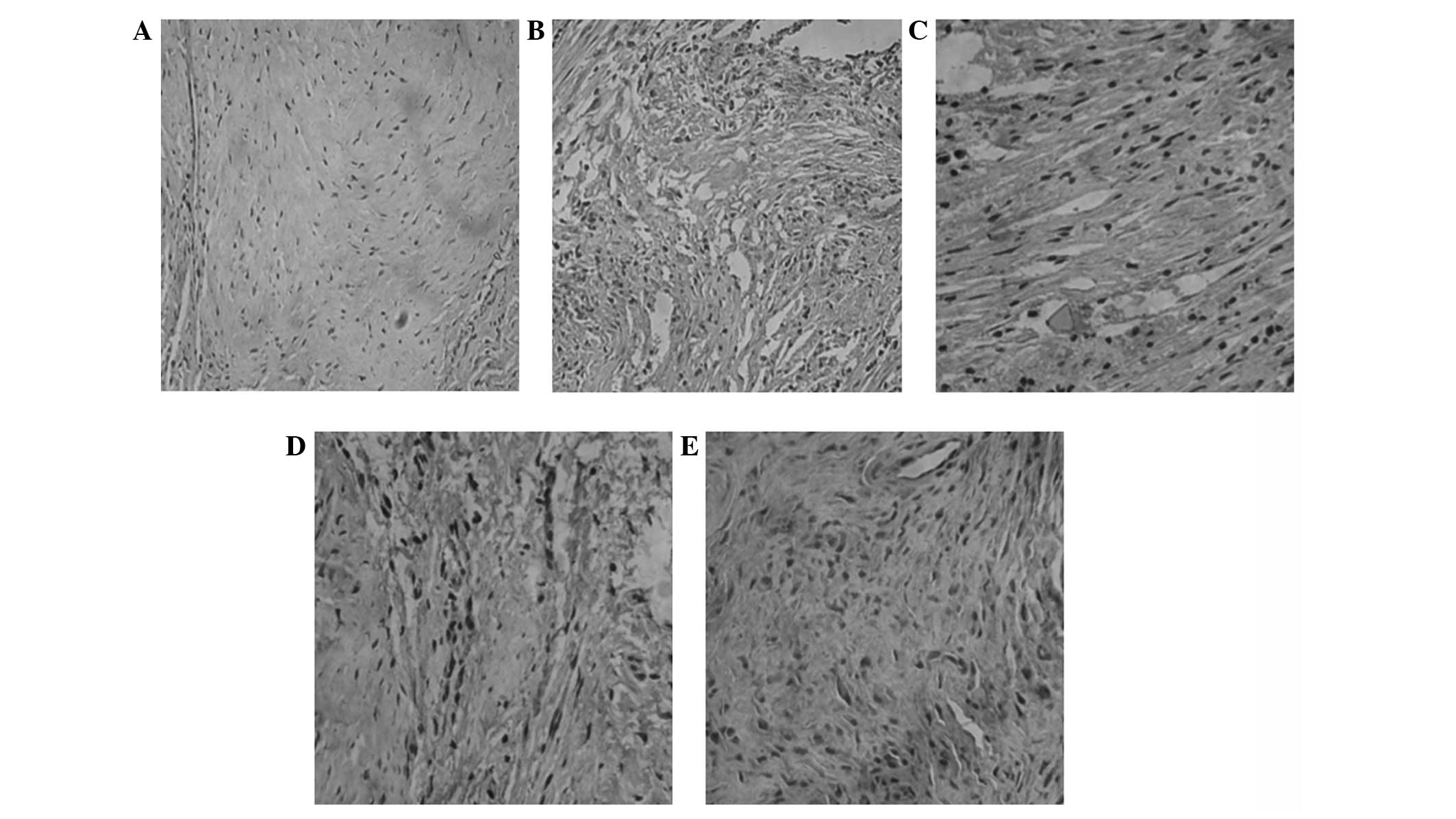 | Figure 3Immunohistochemical analysis of vWF
expression. Each rabbit was implanted at five different sites with
β-TCP only (group A), BMSCs and β-TCP (group B),
bioreactor-cultured BMSCs and β-TCP (group C), PRP-cultured BMSCs
and β-TCP (group D), and bioreactor- and PRP-cultured BMSCs and
β-TCP (group E), respectively. Three months following implantation,
samples were collected from implanted rabbits (n=10). vWF
expression was detected using immunohistochemistry. Cells with
brown granules were considered positive cells. Expression of vWF
was analyzed by immunohistochemistry. Representative
immunohistochemical results from (A) group A, (B) group B, (C)
group C, (D) group D and (E) group E (magnification, ×200). vWF,
von Willebrand Factor; TCP, tricalcium phosphate; BMSCs, bone
marrow mesenchymal stem cells; PRP, platelet-rich plasma. |
 | Table IResults of histological scoring in
each group for CD31. |
Table I
Results of histological scoring in
each group for CD31.
| Case number | Group A | Group B | Group C | Group D | Group E |
|---|
| 1 | 0 | 2 | 3 | 4 | 6 |
| 2 | 1 | 2 | 3 | 3 | 6 |
| 3 | 0 | 3 | 2 | 5 | 7 |
| 4 | 2 | 4 | 4 | 6 | 7 |
| 5 | 1 | 1 | 3 | 3 | 6 |
| 6 | 3 | 2 | 5 | 5 | 5 |
| 7 | 2 | 3 | 2 | 5 | 4 |
| 8 | 1 | 4 | 1 | 6 | 6 |
| 9 | 3 | 2 | 3 | 7 | 4 |
| 10 | 3 | 3 | 2 | 5 | 5 |
Discussion
In the present study, BMSCs were three-dimensionally
cultured in a perfusion bioreactor. This culture model has certain
advantages compared with traditional planar two-dimensional culture
models. Firstly, the culture medium in traditional two-dimensional
cell culture models requires intermittent replacement, whilst in a
three-dimensional bioreactor the culture medium flows, and
therefore does not need to be replaced frequently. However, the
flow medium may stimulate cells mechanically and simulate an in
vivo cell stress environment. Therefore, the mechanical
characteristics of the cells are similar to the biological changes
that occur in vivo (14–16).
In addition, cells may be fully combined with or adhered to the
three-dimensional scaffold materials in the bioreactor, which means
that the cell culture is similar to a three-dimensional growth
(17,18). Furthermore, vascularized bone
constructed by a three-dimensional culture model has better
physiological functions and mechanical properties compared with
bone constructed using a two-dimensional culture model.
In our previous study (19), BMSCs were used as seed cells and
β-TCP as a scaffold material, and a tissue-engineered osteochondral
composite was successfully constructed in a perfusion bioreactor.
This composite was used in the repair of osteochondral defects in
beagles. It was found that certain grafts did not completely
integrate into the areas of bone cartilage defect, indicating that
the constructed osteochondral composite was not completely
vascularized. In the present study, in the process of bone
construction in the three-dimensional perfusion bioreactor,
autologous PRP was used as a cytokine source to promote bone
formation and vascularization. The results demonstrated that the
number of blood vessels in the composite cultured using the
three-dimensional bioreactor and PRP was significantly higher
compared with that in the other control groups. Furthermore,
autologous PRP is a convenient, economical and sustainable source
of high-quality cytokines.
Acknowledgements
This study was supported by the Chinese national
science foundation (no. 81271966).
References
|
1
|
Song HJ, Lan BS, Cheng B, et al: Treatment
of early avascular necrosis of femoral head by small intestinal
submucosal matrix with peripheral blood stem cells. Transplant
Proc. 43:2027–2032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feng L, Wu H, EL, et al: Effects of
vascular endothelial growth factor 165 on bone tissue engineering.
PLoS One. 8:e829452013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zou D, Zhang Z, Ye D, et al: Repair of
critical-sized rat calvarial defects using genetically engineered
bone marrow-derived mesenchymal stem cells overexpressing
hypoxia-inducible factor-1α. Stem Cells. 29:1380–1390.
2011.PubMed/NCBI
|
|
4
|
Marques LF, Stessuk T, Camargo IC, Sabeh N
Junior, Santos LD and Ribeiro-Paes JT: Platelet-rich plasma (PRP):
Methodological aspects and clinical applications. Platelets. Feb
10–2014.(Epub ahead of print).
|
|
5
|
Kim TH, Kim SH, Sándor GK and Kim YD:
Comparison of platelet-rich plasma (PRP), platelet-rich fibrin
(PRF), and concentrated growth factor (CGF) in rabbit-skull defect
healing. Arch Oral Biol. 59:550–558. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin BN, Whu SW, Chen CH, et al: Bone
marrow mesenchymal stem cells, platelet-rich plasma and
nanohydroxyapatite-type I collagen beads were integral parts of
biomimetic bone substitutes for bone regeneration. J Tissue Eng
Regen Med. 7:841–854. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marx RE, Carlson ER, Eichstaedt RM,
Schimmele SR, Strauss JE and Georgeff KR: Platelet-rich plasma:
Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 85:638–646. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim ES, Kim JJ and Park EJ: Angiogenic
factor-enriched platelet-rich plasma enhances in vivo bone
formation around alloplastic graft material. J Adv Prosthodont.
2:7–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yokota K, Ishida O, Sunagawa T, et al:
Platelet-rich plasma accelerated surgical angio-genesis in
vascular-implanted necrotic bone: an experimental study in rabbits.
Acta Orthop. 79:106–110. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tao H, Zhang C, Zeng B, Yuan T, Xu J and
Song W: Experimental study on the treatment of femur head necrosis
with tricalcium phosphate and platelet-rich plasma. Zhongguo Xiu Fu
Chong Jian Wai Ke Za Zhi. 19:170–173. 2005.(In Chinese).
|
|
11
|
Latalski M, Elbatrawy YA, Thabet AM, et
al: Enhancing bone healing during distraction osteogenesis with
platelet-rich plasma. Injury. 42:821–824. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Curi MM, Cossolin GS, Koga DH, et al:
Bisphosphonate-related osteonecrosis of the jaws - an initial case
series report of treatment combining partial bone resection and
autologous platelet-rich plasma. J Oral Maxillofac Surg.
69:2465–2472. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scala M, Gipponi M, Mereu P, et al:
Regeneration of mandibular osteoradionecrosis defect with platelet
rich plasma gel. In Vivo. 24:889–893. 2010.PubMed/NCBI
|
|
14
|
Klein-Nulend J, van der Plas A, Semeins
CM, et al: Sensitivity of osteocytes to biomechanical stress in
vitro. FASEB J. 9:441–445. 1995.PubMed/NCBI
|
|
15
|
Owan I, Burr DB, Turner CH, et al:
Mechanotransduction in bone: osteoblasts are more responsive to
fluid forces than mechanical strain. Am J Physiol. 273:C810–C815.
1997.PubMed/NCBI
|
|
16
|
Bakker AD, Soejima K, Klein-Nulend J and
Burger EH: The production of nitric oxide and prostaglandin E(2) by
primary bone cells is shear stress dependent. J Biomech.
34:671–677. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Kim UJ, Blasioli DJ, et al: In
vitro cartilage tissue engineering with 3D porous aqueous-derived
silk scaffolds and mesenchymal stem cells. Biomaterials.
26:7082–7094. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang W, Itaka K, Ohba S, et al: 3D
spheroid culture system on micropatterned substrates for improved
differentiation efficiency of multipotent mesenchymal stem cells.
Biomaterials. 30:2705–2715. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun S, Ren Q, Wang D, Zhang L, Wu S and
Sun XT: Repairing cartilage defects using chondrocyte and
osteoblast composites developed using a bioreactor. Chin Med J
(Engl). 124:758–763. 2011.PubMed/NCBI
|

















