Introduction
The pomegranate (Punica granatum) is a small,
fruit-bearing tree that has been cultivated and naturalized since
ancient times throughout the entire Mediterranean region. One of
its extracts, pomegranate juice (PJ), is consumed around the world.
A previous study revealed that PJ contains certain constituents
that appear to have beneficial therapeutic properties, including
antioxidative effects (1).
Consequently, the protective effect of PJ consumption on various
diseases is receiving considerable attention at present in all
medical fields. The anticancer, antimicrobial, antiproliferative
and cardioprotective effects of PJ and its metabolites have also
been demonstrated in numerous previous studies (2–5).
Pomegranate contains a number of polyphenols, including
anthocyanins, minor flavonoids and punicalagin, which is the most
important member of the ellagitannins family. The punicalagin is
the largest polyphenol among the pomegranate ellagitannins and it
is responsible from most of the antioxidant activity of the PJ
(6). The potent antioxidant
effects of polyphenols have been demonstrated in clinical and
experimental studies (7,8). Thus, the pomegranate and its extracts
have powerful antioxidant effects, which have also been revealed
for other fruit juices, including grape, blackberry and branberry
juice, and in green tea (9).
Testicular torsion is a rarely observed, serious
urologic emergency, usually encountered in prepubertal males, which
requires immediate surgical examination for proper treatment. It
has been hypothesized that treatment for testicular torsion may
lead to long-term infertility due to ischemia/reperfusion (I/R)
injury which occurs following surgical correction of the torsed
testis (10). Oxidative stress
(OS) is a complex phenomenon that causes tissue damage and occurs
due to an excessive production of reactive oxygen species (ROS)
that are not adequately eliminated by the natural antioxidant
defence mechanisms in the body. OS may cause significant damage to
tissues by inducing differentiation in the cell membranes leading
to irreversible cellular damage (11). In this context, OS increases the
levels of malondialdehyde (MDA) and protein carbonyls (PCs) in the
tissues, which are the end products of lipid peroxidation and
protein oxidation, respectively.
Under physiological conditions, the enzymes
belonging to the natural antioxidant defence system, including
superoxide dismutase (SOD), catalase and glutathione peroxidase
(GSH-Px), eliminate the ROS, which are released as a result of
normal cellular metabolism (12).
Excess levels of ROS particularly target unsaturated fatty acids,
which play an important role in the constitution of cell membranes.
Since the membranes of sperm contain a high abundance of
unsaturated fatty acids (13),
these cells are severely affected by peroxidation damage caused by
ROS. Lipid peroxidation destroys the structure of the lipid matrix
in the membranes of spermatozoa and is associated with the loss of
motility and defects in membrane integrity (14).
In the present experimental study, a rat model of
testicular torsion-induced OS was established. The biochemical and
histopathological effects of daily PJ consumption on the OS
parameters and sperm concentrations of the rats were subsequently
evaluated.
Materials and methods
Study animals
Upon obtaining consent from the Gaziosmanpasa
University Local Ethics Committee for Animal Experiments (Tokat,
Turkey), 21 male Wistar albino rats (aged 5–6 months) were
purchased Gaziosmanpasa University Experimental Medicine Research
Center (Tokat, Turkey)for use in the present study. The
experimental animals were housed at 18–22°C, under a 12 h
light/dark cycle throughout the study period. Surgical procedures
were performed under ketamine/xylazine anesthesia in sterile
conditions. All rats were sacrificed following the experimental
procedures.
Group allocation
The rats were randomly divided into three groups,
each group consisting of seven rats, as follows: i) control group,
which underwent a sham surgical procedure to determine the basal
values for biochemical and histopathological evaluations; ii) I/R
group, designed to study the effects of the testicular
torsion-detorsion process on the ipsilateral testicle and; iii)
PJ+I/R group, designed to determine the effect of PJ on OS
indicators and sperm parameters following unilateral testicular
torsion-detorsion. In the PJ+I/R group, the rats were given 0.4
ml/day commercially-available PJ (Ersu® Fruit and Food Industries
Inc., Konya, Turkey) orally over a period of eight weeks prior to
surgery.
Surgical procedure
In the control group rats, the testicle was exposed
through a midline incision, a 4-0 silk suture was fixed through the
tunica albuginea and the testicle was replaced into the scrotum
with no additional intervention. In the rats in the I/R and PJ+I/R
groups, the tunica vaginalis was opened and the right testis
exposed to allow surgical intervention. It was rotated 720° in a
clockwise direction and maintained in this torsed position by
fixing the testicle to the scrotum with a 4-0 silk suture. After 1
h, the spermatic cord was detorsed and orchiectomy was performed on
the right side. The resected testicular tissue specimens were
separated into two parts for histopathological (sperm parameters)
and biochemical (tissue MDA and SOD level) analyses. Furthermore,
~5-cm3 blood samples were obtained from the inferior
vena cava of the rats to determine the blood levels of MDA and
SOD.
Biochemical analyses
Blood samples were drawn into heparin-free tubes for
biochemical analysis. Following centrifugation (2,000 × g for 15
min at 4°C), the serum samples were frozen and stored at −70°C
until required. Commercially-available chemical supplies (Sigma;
St. Louis, MO, USA) were used for the determination of the
following parameters in the serum samples.
Serum antioxidant enzyme analysis
Total (Cu-Zn and Mn) SOD [enzyme commission (EC)
number 1.15.1.1) activity was determined according to a previous
method described by Sun et al (15). The principle of the method is based
on the inhibition of nitroblue tetrazolium (NBT) reduction by the
xanthine-xanthine oxidase system as a superoxide generator.
Activity was assessed in the ethanol phase of the supernatant
following the addition of 1.0 ml ethanol/chloroform mixture (5:3,
v/v) to the same volume of sample and centrifugation (1,500 × g for
10 min). One unit of SOD was defined as the amount causing a 50%
inhibition of the NBT reduction rate. The SOD activity was
expressed in U/ml.
Determination of the levels of MDA
The level of tissue thiobarbituric acid reactive
substance (TBARS) was determined by a method based on a reaction
with thiobarbituric acid (TBA) at 90–100°C that was previously
described by Esterbauer and Cheeseman (16). In the TBA test reaction, MDA or
MDA-like substances and TBA react to produce a pink pigment with an
absorption maximum at 532 nm. The reaction was performed at pH 2–3
and 90°C for 15 min. The sample was mixed with a double volume of
cold 10% (w/v) trichloroacetic acid to precipitate the protein. The
precipitate was pelleted by centrifugation (1,500 × g for 10 min)
and an aliquot of the supernatant was reacted with an equal volume
of 0.67% (w/v) TBA in a boiling water bath for 10 min. Following
cooling, the absorbance at 532 nm was measured (GBC Cintra 10e
UV/VIS Spectrophotometer, Victoria, Australia). Results are
expressed in ng/ml, according to the graphic standard prepared from
measurements with a standard solution
(1,1,3,3-tetramethoxypropane).
Histological examination
The rat testes were fixed in Bouin’s solution
(Polysciences Europe GmbH, Eppelheim, BadenWürttemberg, Germany)
and passed through an increasing alcohol series. The testicular
tissue was processed for paraffin embedding following treatment
with xylene and paraffin. Subsequently, 5- and 20-μm-thick sections
were obtained with a rotary microtome (LeicaRM 2135, Leica
Instruments, Nussloch, Germany). The sections were randomly sampled
and stained with Periodic acid-Schiff (PAS). Finally, the
spermatogenic cells in the seminiferous tubules, including the
spermatids, spermatocytes and spermatogonia, were counted with an
optical fractionator (Stereology workstation: Trinokulermicroskop
Leica DM 2500, Leica Instruments; motorizedstage: Ludlelectronics,
New York, NY, USA; digitalmicrocator: Heidenhain, Traunreut,
Germany, StereoInvestigator: MBF Biosciences, Williston, VT, USA).
The spermatogenic cell concentrations are expressed in number of
cells/mm3).
Statistical analysis
Kruskal-Wallis one-way analysis of variance was used
to compare the biochemical and sperm parameters among groups. For
multiple comparisons, the Bonferroni adjusted Mann-Whitney U test
was performed. Data are expressed as the median and interquartile
range (IQR). P<0.05 was considered to indicate a statistically
significant difference. Analyses were carried out using a
commercially-available software (IBM SPSS Statistics 19; IBM,
Armonk, NY, USA).
Results
SOD activity and MDA levels in the serum
and testicular tissue
The levels of MDA and SOD in the serum and tissue
samples of the three groups of rats are presented in Table I. No statistically significant
differences were identified between the groups in terms of
testicular tissue weights. In the I/R group, the SOD activity and
MDA levels in the serum and testicular tissue were significantly
higher compared with those in the control group (P<0.001). The
consumption of PJ significantly reduced the SOD activity and MDA
levels in the serum and tissue compared with those in the I/R group
(P<0.001).
 | Table ILevels of superoxide dismutase (SOD)
and malondialdehyde (MDA) in the serum and testicular tissue of
rats from the different groups. |
Table I
Levels of superoxide dismutase (SOD)
and malondialdehyde (MDA) in the serum and testicular tissue of
rats from the different groups.
| Variable | Control | I/R | PJ+I/R | P-value |
|---|
| tSOD (U/ml) | 13.18
(7.86–17.54) | 29.37
(26.45–38.26)a | 12.04
(8.61–13.66) | <0.001 |
| tMDA (ng/ml) | 86.09
(66–139.71) | 270.37
(237.86–316.39)a | 81.84
(70.08–103.53) | <0.001 |
| sSOD (U/ml) | 26.76
(23.67–29.52) | 73.45
(61.66–81.47)a | 22.1 (16.9–25.2) | <0.001 |
| sMDA (ng/ml) | 1660.6
(1589.3–1747.0) | 2605.0
(2518.0–2894.8)a | 1592.3
(1467.8–1706.6) | <0.001 |
| Tissue weights
(g) | 0.23±0.03 | 0.23±0.02 | 0.23±0.03 | 0.860 |
Spermatogenic cell concentrations
The comparison of spermatogenic cell concentrations
in the three groups of rats is presented in Table II. The concentrations of
spermatids, spermatocytes and spermatogonia were significantly
reduced in the I/R group compared with those in the control group
(P<0.001). However, the consumption of PJ over a period of eight
weeks significantly improved the spermatid, spermatocyte and
spermatogonia concentrations compared with those in the I/R group
(P=0.008).
 | Table IIComparison of the concentrations of
spermatogenic cells between groups. |
Table II
Comparison of the concentrations of
spermatogenic cells between groups.
| Cell type | Control | I/R | PJ+I/R | P-value |
|---|
| Spermatids | 32.22
(28.35–36.35) | 21.04
(17.16–28.01)a | 28.68
(24.86–37.24) | P<0.001 |
| Spermatocytes | 18.56
(15.53–21.27) | 11.53
(8.89–14.57)a | 16.22
(13.84–18.39) | P<0.001 |
| Spermatogonia | 8.48
(6.89–10.06) | 6.09
(4.10–7.35)a | 7.81 (6.41–9.28) | P=0.008 |
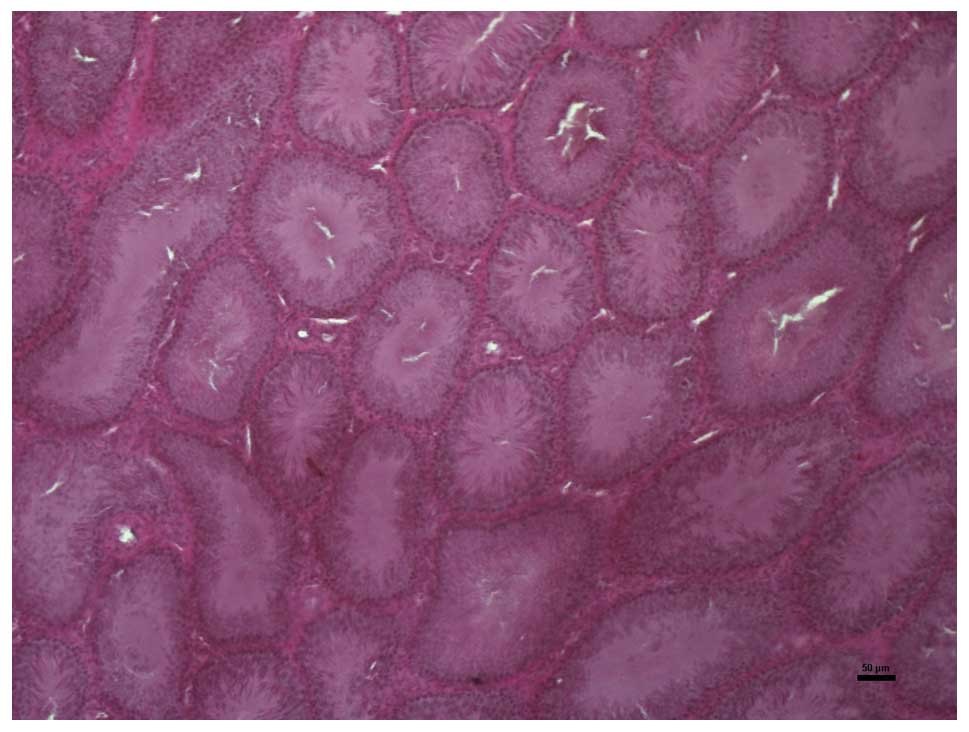
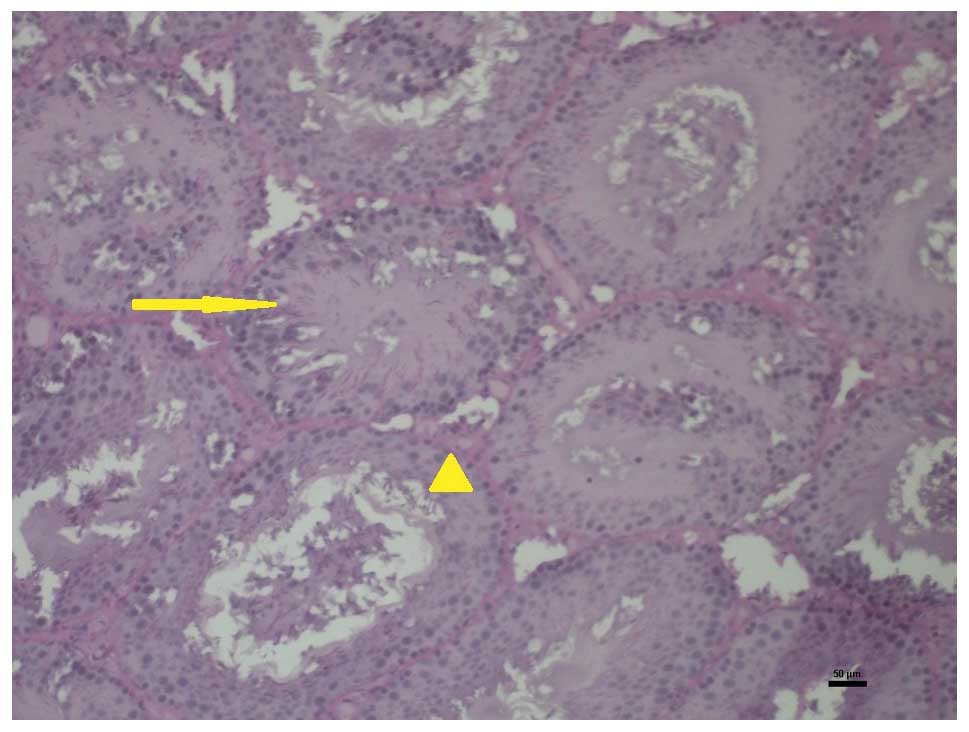
Histopathological analysis
In the histopathological analysis of the testicular
tissues, intensive vacuolization with irregularities in the germ
cell sequences were observed in the I/R group. A reduced incidence
of vacuolizations and regular germ cell formation, comparable to
that observed in the control group, were present in the PJ+I/R
group. Representative microscopic images of the testicular tissues
in the three groups are presented in Figs. 1–3.
Discussion
Since it contains a high amount of polyphenols, PJ
possesses strong antioxidant properties. The antioxidant effects of
polyphenols, particularly ellagitannins, have been previously
demonstrated in numerous studies (17–19).
Ellagitannins and other polyphenols are hydrolyzed into ellagic
acid (EA), which is responsible for their antioxidant activities
in vivo (20). An in
vitro study has revealed the ability of EA to easily pass
through the mitochondrial membrane (21). In the present study, testicular
torsion-detorsion caused elevations in the levels of SOD and MDA,
which are the indirect indicators of oxidative injury. However,
pretreatment with PJ over eight weeks decreased the levels of SOD
and MDA in the serum and testicular tissue.
Under normal conditions, sperm cells produce high
amounts of ROS as a result of their physiological metabolism
(22). As testicular tissue
contains a high amount of unsaturated fatty acids, it is very
sensitive to the detrimental effects of ROS. Lipid peroxidation is
able to damage the structure of the lipid matrix in sperm
membranes, decrease intracellular levels of ATP leading to reduced
sperm viability, cause axonemal damage and increase mid-piece
morphological defects (23. However, testicular tissue contains
numerous antioxidant enzymes and ROS scavengers that protect its
spermatogenic function from OS and in particular from peroxidative
damage, which is the most important cause of spermatogenic
dysfunction (24,25). This antioxidant defence system
scavenges considerable numbers of ROS in order to protect sperm
cells from their harmful effects and preserves only a small number
of ROS to maintain normal cell function. Without this defence
system, the ROS cause thinning of the germ layer, decreased
spermatogenic cell density and sperm motility, and an increased
production of abnormal sperm cells (26).
The current study demonstrated that testicular
torsion-detorsion induced OS in testicular tissue and caused a
reduction in the concentrations of all spermatogenic cell types,
including spermatogonia, spermatocytes and spermatids. The
reduction in spermatogenic cell concentration, which occurred
without any reduction in testicular tissue weight, may indicate
that OS was the most important reason for this pathology. However,
the daily consumption of PJ over a period of eight weeks prior to
the torsion-detorsion surgery resulted in a reduction in the OS
parameters and a significant increase in the spermatogenic cell
concentration.
Similarly, in a previous experimental study, Türk
et al revealed that the daily consumption of PJ over a
period of seven weeks caused a reduction in OS parameters and a
marked increase in the levels of spermatogenic cells and the
thickness of the germ layer (27).
Furthermore, Mansour et al demonstrated that the daily
consumption of PJ at various doses for a period of six weeks
resulted in a significiant increase in serum glutathione peroxidase
(GSH-Px) and catalase activities in rats. The study also noted that
PJ consumption significantly increased epididymal sperm
concentration and sperm motility parameters and decreased abnormal
sperm production (28).
In the current experimental study, it was indicated
that testicular torsion-detorsion caused OS, which may have led to
reduced sperm concentrations in the rat testes. However, the daily
consumption of PJ prior to the torsion-detorsion surgery resulted
in a reduction in the parameters of OS and produced an increment in
spermatogenic cell concentrations.
References
|
1
|
Aviram M, Dornfeld L, Kaplan M, Coleman R,
Gaitini D, Nitecki, et al: Pomegranate juice flavonoids inhibit
low-density lipoprotein oxidation and cardiovasculardiseases:
studies in atherosclerotic mice and in humans. Drugs Exp Clin Res.
28:49–62. 2002.PubMed/NCBI
|
|
2
|
Seeram NP, Adams LS, Henning SM, Niu Y,
Zhang Y, Nair MG and Heber D: In vitro antiproliferative,
apoptotic and antioxidant activities of punicalagin, ellagic acid
and a total pomegranate tannin extract are enhanced in combination
with other polyphenols as found in pomegranate juice. J Nutr
Biochem. 16:360–367. 2005. View Article : Google Scholar
|
|
3
|
Aslam MN, Lansky EP and Varani J:
Pomegranate as a cosmeceutical source: pomegranate fractions
promote proliferation and procollagen synthesis and inhibit matrix
metalloproteinase-1 production in human skin cells. J
Ethnopharmacol. 103:311–318. 2006.
|
|
4
|
Aviram M and Dornfeld L: Pomegranate juice
consumption inhibits serum angiotensin converting enzyme activity
and reduces systolic blood pressure. Atherosclerosis. 158:195–198.
2001. View Article : Google Scholar
|
|
5
|
Aviram M, Rosenblat M, Gaitini D, Nitecki
S, Hoffman A, Dornfeld L, et al: Pomegranate juice consumption for
3 years by patients with carotid artery stenosis reduces common
carotid intima-media thickness, blood pressure and LDL oxidation.
Clin Nutr. 23:423–433. 2004.
|
|
6
|
Seeram N, Lee R, Hardy M and Heber D:
Rapid large scale purification of ellagitannins from pomegranate
husk, a byproduct of the commercial juice industry. Sep Purif
Technol. 41:49–55. 2005. View Article : Google Scholar
|
|
7
|
Hermann DD: Naturoceutical agents in the
management of cardiovascular disease. Am J Cardiovasc Drugs.
2:173–196. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Seeram NP, Aviram M, Volkova N, Zhang Y,
Henning SM, Nair M and Heber D: Dietary polyphenols derived from
pomegranates are potent antioxidants: evaluation in various in
vitro models of antioxidation. Presented at 228th National
Meeting of the American Chemical Society; pp. Abstract AGFD442004,
http://oasys2.confex.com/acs/228nm/techprogram/P787337.HTMuri.
|
|
9
|
Gil MI, Tomás-Barberán FA, Hess-Pierce B,
Holcroft DM and Kader AA: Antioxidant activity of pomegranate juice
and its relationship with phenolic composition and processing. J
Agric Food Chem. 48:4581–4589. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Turner TT, Bang HJ and Lysiak JL: The
molecular pathology of experimental testicular torsion suggests
adjunct therapy to surgical repair. J Urol. 172:2574–2578. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aitken RJ, Baker MA and Sawyer D:
Oxidative stress in the male germ line and its role in the
aetiology of male infertility and genetic disease. Reprod Biomed
Online. 7:65–70. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tremellen K: Oxidative stress and male
infertility - a clinical perspective. Hum Reprod Update.
14:243–258. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sanocka D and Kurpisz M: Reactive oxygen
species and sperm cells. Reprod Biol Endocrinol. 2:122004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Lamirande E, Jiang H, Zini A, Kodama H
and Gagnon C: Reactive oxygen species and sperm physiology. Rev
Reprod. 2:48–54. 1997.PubMed/NCBI
|
|
15
|
Sun Y, Oberley LW and Li Y: A simple
method for clinical assay of superoxide dismutase. Clin Chem.
34:497–500. 1988.PubMed/NCBI
|
|
16
|
Esterbauer H and Cheeseman KH:
Determination of aldehydic lipid peroxidation products:
malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 186:407–421.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Manach C, Mazur A and Scalbert A:
Polyphenols and prevention of cardiovascular diseases. Curr Opin
Lipidol. 16:77–84. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cerdá B, Espín JC, Parra S, Martínez P and
Tomás-Barberán FA: The potent in vitro antioxidant ellagitannins
from pomegranate juice are metabolised into bioavailable but poor
antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the
colonic microflora of healthy humans. Eur J Nutr. 43:205–220.
2004.
|
|
19
|
Cerdá B, Llorach R, Cerón JJ, Espín JC and
Tomás-Barberán FA: Evaluation of the bioavailability and metabolism
in the rat of punicalagin, an antioxidant polyphenol from
pomegranate juice. Eur J Nutr. 42:18–28. 2003.PubMed/NCBI
|
|
20
|
Larrosa M, Tomás-Barberán FA and Espín JC:
The dietary hydrolysable tannin punicalagin releases ellagic acid
that induces apoptosis in human colon adenocarcinoma Caco-2 cells
by using the mitochondrial pathway. J Nutr Biochem. 17:611–625.
2006. View Article : Google Scholar
|
|
21
|
Seeram NP, Lee R and Heber D:
Bioavailability of ellagic acid in human plasma after consumption
of ellagitannins from pomegranate (Punica granatum L.)
juice. Clin Chim Acta. 348:63–68. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sikka SC: Oxidative stresss and role of
antioxidants in normal and abnormal sperm function. Front Biosci.
1:e78–e86. 1996.PubMed/NCBI
|
|
23
|
Henkel R: The impact of oxidants on sperm
functions. Andrologia. 37:205–206. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koksal IT, Usta M, Orhan I, Abbasoglu S
and Kadioglu A: Potential role of reactive oxygen species on
testicular pathology associated with infertility. Asian J Androl.
5:95–99. 2003.PubMed/NCBI
|
|
25
|
Turner TT and Lysiak JJ: Oxidative stress:
a common factor in testicular dysfunction. J Androl. 29:488–498.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alkan I, Simşek F, Haklar G, Kervancioğlu
E, Ozveri H, Yalçin S and Akdaş A: Reactive oxygen species
production by the spermatozoa of patients with idiopathic
infertility: relationship to seminal plasma antioxidants. J Urol.
157:140–143. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Türk G, Sönmez M, Aydin M, Yüce A, Gür S,
Yüksel M, Aksu EH and Aksoy H: Effects of pomegranate juice
consumption on sperm quality, spermatogenic cell density,
antioxidant activity and testosterone level in male rats. Clin
Nutr. 27:289–96. 2008.PubMed/NCBI
|
|
28
|
Mansour SW, Sangi S, Harsha S, Khaleel MA,
Ibrahim AR and Eldin TA: Sensibility of male rats fertility against
olive oil, Nigella sativa oil and pomegranate extract. Asian
Pac J Trop Biomed. 3:563–568. 2013. View Article : Google Scholar : PubMed/NCBI
|