Introduction
Osteoporosis is a metabolic bone disease resulting
from a disturbance of normal bone remodeling that shifts the
balance to bone resorption over bone formation, causing bone loss
and fractures (1). Osteoporosis
affects ~25 million individuals in the United States alone and it
is estimated that females >50 years-old possess an 11–18%
lifetime risk of suffering a hip fracture (2). Numerous attempts to develop novel
agents capable of preventing and/or treating bone diseases have
been investigated (3). Currently,
antiresorptive agents are extensively used, however, more highly
efficacious resorptive inhibitors with excellent safety and
efficacy are required. Anabolic agents that stimulate bone
formation are less well investigated in comparison to
antiresorptive agents (4). With
advances in the understanding of osteoblast differentiation and
bone formation, continuous trials to develop anabolic agents have
been performed (5,6).
The estrogen-deficient ovariectomy (OVX) model is
useful for the evaluation of osteoporotic drugs, as several
parameters are decreased by OVX within 4–6 weeks of surgery. The
OVX rat model was selected for the present study as it shares
numerous similarities with postmenopausal bone loss (7) and is recommended by the US Food and
Drug Administration as a test species for evaluating the long-term
skeletal safety and efficacy of osteoporosis therapies (8).
Calcium (Ca) salts have a reported therapeutic
benefit against osteoporosis (9).
Ca supplements are an important alternative source of Ca and
minimize bone loss during aging (10,11).
The bioavailability of Ca varies in Ca supplements and can be
affected by disintegration, solubility, chelate formation and
food-drug interactions (12,13).
Polycan, a commercial β-glucan obtained from Aureobasidium
pullulans SM-2001, is primarily comprised of β-1,3/1,6-glucan
and other organic materials, including amino acids, mono- or
di-unsaturated fatty acids (linoleic and linolenic acids) and
fibrous polysaccharides (14).
Polycan has been reported to induce antiosteoporotic effects via
inhibiting bone loss, accelerating bone formation (15), promoting fracture healing (16) and exhibiting anti-inflammatory
effects (17).
Therefore, a mixed formula consisting of water
soluble Ca salts and Polycan is expected to exhibit synergic
antiosteoporotic effects, providing a potential novel preventive or
therapeutic regime for osteoporosis. In the present study,
compositions of Polycan and calcium lactate-gluconate (CaLG) were
investigated with the aim of identifying a composition that
exhibited the most beneficial effects and synergism in OVX-induced
osteoporotic rats. Polycan and CaLG single formulas (100 mg/kg
each), and three doses (50, 100 and 200 mg/kg) of three mixed
formulas [polycan:CaLG (PCLG)=1:99, 5:95 and 10:90] were orally
administered daily to OVX osteoporotic rats for 84 days. Changes in
body weight (BW), femur indices, Ca and inorganic phosphorus (P)
content, bone mineral density (BMD), failure load (FL),
histological profiles and histomorphometrical analyses were
assessed. The results of the test materials were compared with
those of risedronate sodium (5 mg/kg), a pyridinyl bisphosphonate
that binds to bone hydroxyapatites and inhibits osteoclast-mediated
bone resorption (18).
Materials and methods
Animals
A total of 140 Sprague-Dawley specific pathogen-free
female rats (six-weeks old; Japan SLC, Inc., Komagane, Japan) were
allocated into polycarbonate cages (four per cage) in a temperature
(20–25°C) and humidity (30–35%) controlled room. The rats were
allowed to acclimatize for 12 days. The light:dark cycle was 12 h
and food (Samyang Foods Co., Ltd., Wonju, Korea) and water were
supplied ad libitum. OVX-induced osteoporotic rats (n=132)
were used for the study, with eight rats used as the sham controls.
Eight rats per group (total 14 groups; n=112) were selected based
on their BW during the first week following OVX surgery. Briefly,
excluding overweight and underweight rats, the rats were grouped
into a total of 14 groups. First, after arranging the rats in order
of weight, the heaviest 14 rats were randomly assigned to each of
14 groups. The 14 next heaviest rats were then randomly assigned to
each of the 14 groups. Therefore, 112 rats were randomly assigned
to each of the 14 groups. The animal experiments were performed in
accordance with the US National Institutes of Health Guidelines for
the Care and Use of Laboratory Animals (19) and the study was approved by the
Institute of Laboratory Animal Resources of Daegu Haany University
(Gyeongsan, Korea).
Drug preparation and administration
Polycan and CaLG were supplied by Glucan Corp.
(Busan, Korea). Polycan and CaLG single formulas (100 mg/kg each),
and three doses (50, 100 and 200 mg/kg) of three mixed formulas
[polycan:CaLG (PCLG)=1:99, 5:95 and 10:90] were dissolved in
distilled water and orally administered at a volume of 5 ml/kg,
daily for 84 days from the first week following OVX. Risedronate
sodium (Pharmaceutical Works Polpharma S.A., Starogard, Poland) was
dissolved in distilled water and orally administered by gastric
gavage at a concentration of 5 mg/kg.
OVX surgery
Bilateral OVX was performed under Zoletil (Virbac,
Carros, France) anesthesia in all the OVX groups, as previously
described (11). In the sham
controls, the bilateral ovaries were exposed, but not removed, and
the incision was closed with skin sutures.
BW changes
Changes in BW were calculated one day prior to OVX,
during OVX, six days following OVX, at the initiation of
administration and at each subsequent week using an automatic
electronic balance (Precisa Gravimetrics AG, Dietikon,
Switzerland). During OVX and at the initiation of administration
and termination of treatment, the experimental animals were fasted
overnight (water was not included; ~12 h) to minimize BW changes
due to feeding.
Assessment of bone weight
Animals were sacrificed by exsanguination under
anesthesia and the wet and dry weights of the right femur were
calculated, as previously described (15). Following weighing, the bones were
measured using an electronic digital caliper (Mitutoyo Corp.,
Kawasaki-shi, Japan).
Blood and urine collection
For blood analysis, 10-ml blood samples were
collected from the vena cava at sacrifice and the serum was
separated. All serum samples were frozen at −75°C prior to use. For
urinalysis, urine was collected over 24 h following the final
treatment dose and centrifuged (Thermo Scientific Sorvall Legend
Mach 1.6R; Thermo Fisher Scientific Inc., Waltham, MA, USA) at ~600
× g for 10 min to remove any sediments.
Serum osteocalcin and bone-specific
alkaline phosphatase (bALP) level measurement
Serum osteocalcin levels were detected by
radioimmunoassay using an Osteocalcina Myria kit (Technogenetics,
Milan, Italy) and a gamma counter (COBRA II, Packard, USA). Serum
bALP levels were detected via enzyme immunoassay (EIA) using a
Metra™ bALP kit (Quidel Corp., San Diego, CA, USA) and an
enzyme-linked immunosorbent assay (ELISA) reader (Sirio S; Radim
SpA, Pomezia RM, Italy).
Urine deoxypyridinoline (Dpd)/creatinine
ratio measurement
Urine Dpd was detected using an EIA with a Metra™
DPD kit (Quidel Corp.) and an ELISA Reader (Sirio S; Radim). The
urine creatinine levels were detected via a Jaffe method using
Sicdia creatinine reagents (Shin Yang Chemical Co., Ltd., Korea)
and an automated urine analyzer (Toshiba 2000FR; Toshiba, Tokyo,
Japan). The Dpd/creatinine ratio was calculated as follows:
Dpd/creatinine ratio (nM/g/day) = (Dpd levels/creatinine
levels).
Assessment of bone mineral content (BMC),
BMD and FL
BMC was measured in the tibias. Briefly, the tibias
were dried at 120°C for 8 h, carbonized at 800°C for 6 h in a
furnace (6000, Barnstead/Thermolyne, Dubuque, IA, USA) and
dissolved in nitric acid. In the diluted solution, the Ca and P
contents were calculated using orthocresolphthalein complexon
(Sigma-Aldrich, St. Louis, MO, USA) and enzyme methods (mg/g bone).
The BMD was measured in the femur using dual-energy X-ray
absorptiometry (g/cm2) bone strength was detected as
failure load (FL). FL of the midshaft region of the right femur was
measured in newtons by a three-point bending test to failure using
a computerized testing machine (Instron 6022; Instron, Canton, MA,
USA; speed 20 mm/min) (15).
Histological procedures
The left femur was separated, fixed in 10% neutral
buffered formalin and decalcified for five days. The samples were
then embedded, sectioned (3–4 μm) and stained with hematoxylin and
eosin. Histomorphometry analysis was performed for bone mass,
structure and resorption in a uniform area of the epiphyseal
regions (growth plate regions were excluded) using an automated
image analyzer (DMI-300; DMI, Korea). Cortical bone thickness was
measured in the epiphyseal neck and mid-shaft regions. For bone
mass and structure analysis, the trabecular bone volume (TBV),
thickness (Tbt), number (Tbn) and length (Tbl), as well as the
cortical bone thickness (Cbt), were measured as previously
described (2). Generally, the
degree of osteoporosis is measured by the osteoclast number (Ocn)
on the bone surface (cortical bone), or the number of eroded
surfaces. The problem caused by osteoporosis is fractures.
Therefore, in this study, determining Ocn in the epiphyseal region
(metaphyseal area) where fractures sometimes occur, as well as in
the midshaft of trabecula bone where numerous fractures occur, we
also evaluated the suppressive effects of polycalcium on bone
destruction. For the assessment of bone resorption, the Ocn in
uniform regions of the epiphyseal (number/epiphyseal) and the
osteoclast cell surface/bone surface (OS/BS) were detected as
previously described (20).
Statistical analysis
The results are expressed as the mean ± SD. Multiple
comparison tests for the different dose groups were conducted.
Variance homogeneity was examined using the Levene test. If the
Levene test indicated no significant deviations from the variance
homogeneity, the obtained data were analyzed by one-way analysis of
variance followed by the least-significant difference
multi-comparison test to determine which pairs of group comparison
were significantly different. In the cases where significant
deviations from the variance homogeneity were observed with the
Levene test, a non-parametric comparison test, Kruskal-Wallis H
test, was conducted. When a significant difference was observed
with the Kruskal-Wallis H test, the Mann-Whitney U-Wilcoxon Rank
Sum W test was conducted to determine the specific pairs of group
comparison which were significantly different. Statistical analyses
were conducted using SPSS for Windows (Release 14K; SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant result.
Results
BW assessment
Significant increases (P<0.01) in BW were
detected in all the OVX groups between day 7 and 14 following
initial administration, as compared with the sham control group. In
addition, the BW of all the ovariectomized rats significantly
increased during the administration of test material when compared
with the sham control rats. However, no significant differences in
BW were detected between the formula-administered groups and the
OVX control group (data not shown).
Bone weight assessment
Although a significant decrease (P<0.01) in the
relative wet femur weight was detected in the OVX group when
compared with the sham control group, significant increases
(P<0.01 or P<0.05) in the relative wet bone weights were
restricted in the risedronate sodium- and PCLG 1:99 200
mg/kg-treated rats as compared with the OVX control rats. A
significant decrease (P<0.01) in the absolute and relative ash
femur weights was detected in the OVX control group as compared
with the sham control group. The ash femur weight increased in the
formula-administered rats when compared with the OVX control rats.
Among the three types of mixed formulas, only PCLG 10:90 exhibited
favorable synergistic effects against OVX-induced bone weight loss
when compared with equal doses of the Polycan single formula. More
potent inhibitory effects on bone weight loss were detected in the
PCLG 10:90 mixed formula 50 mg/kg-treated rats as compared with the
Polycan single formula 100 mg/kg-treated rats (Table I).
 | Table IFemur weights after 84 days of
repeated oral administration of test materials in osteoporotic
rats. |
Table I
Femur weights after 84 days of
repeated oral administration of test materials in osteoporotic
rats.
| Wet weights | Ash weights |
|---|
|
|
|
|---|
| Groups | Absolute (g) | Relative (% of
BW) | Absolute (g) | Relative (% of
BW) |
|---|
| Controls |
| Sham | 0.839±0.048 | 0.324±0.016 | 0.364±0.018 | 0.141±0.010 |
| OVX | 0.861±0.042 | 0.264±0.019a | 0.324±0.015a | 0.099±0.006a |
| Risedronate
sodium | 0.893±0.086 | 0.297±0.032bc | 0.390±0.039bc | 0.129±0.011bc |
| Single formula (100
mg/kg) |
| Polycan | 0.889±0.067 | 0.277±0.023a | 0.355±0.020d | 0.111±0.007ad |
| CaLG | 0.910±0.064b | 0.282±0.024a | 0.356±0.023c | 0.110±0.008ad |
| Polycan:CaLG mixed
formula (1:99; mg/kg) |
| 200 | 0.916±0.056b | 0.292±0.027bd | 0.362±0.023c | 0.115±0.004ac |
| 100 | 0.886±0.047 | 0.282±0.033a | 0.349±0.024d | 0.110±0.004ac |
| 50 | 0.888±0.037 | 0.280±0.015a | 0.354±0.021d | 0.112±0.009ac |
| Polycan:CaLG mixed
formula (5:95; mg/kg) |
| 200 | 0.893±0.041 | 0.275±0.026a | 0.372±0.026c | 0.115±0.012ac |
| 100 | 0.894±0.081 | 0.269±0.020a | 0.359±0.030c | 0.108±0.007ac |
| 50 | 0.898±0.051b | 0.272±0.042a | 0.351±0.032d | 0.106±0.017a |
| Polycan:CaLG mixed
formula (10:90; mg/kg) |
| 200 | 0.907±0.059b | 0.288±0.018a | 0.382±0.019c | 0.122±0.012ac |
| 100 | 0.907±0.053b | 0.278±0.019a | 0.370±0.018c | 0.114±0.005ac |
| 50 | 0.883±0.054 | 0.280±0.019a | 0.357±0.021c | 0.113±0.009ac |
Serum biochemistry
Serum osteocalcin levels in the OVX control group
significantly increased (P<0.01), while the serum bALP levels
significantly decreased (P<0.01) when compared with the sham
control group. However, a significant decrease (P<0.01 or
P<0.05) in serum osteocalcin levels with an elevation in bALP
levels was observed in all the treatment groups with the exception
of the risedronate sodium-treated group, in which a significant
decrease (P<0.01) in serum osteocalcin levels was detected with
no changes to serum bALP levels. Among the three types of mixed
formulas, only the PCLG 10:90 formula exhibited favorable
synergistic effects against OVX-induced serum osteocalcin and bALP
level changes when compared with equal doses of the CaLG single
formula. Similar inhibitory effects on the serum osteocalcin and
bALP level changes were detected in the PCLG 10:90 mixed formula 50
mg/kg-treated rats as compared with the CaLG single formula 100
mg/kg-treated rats (Table
II).
 | Table IISerum osteocalcin and bALP levels
after 84 days of repeated oral administration of test materials in
osteoporotic rats. |
Table II
Serum osteocalcin and bALP levels
after 84 days of repeated oral administration of test materials in
osteoporotic rats.
| Groups | Serum osteocalcin
(ng/ml) | Serum bALP
(U/l) |
|---|
| Controls |
| Sham | 1.24±0.17 | 1.66±0.21 |
| OVX | 2.20±0.27a | 0.95±0.15a |
| Risedronate
sodium | 1.58±0.09ab | 1.03±0.15ab |
| Single formula (100
mg/kg) |
| Polycan | 1.88±0.10ab | 1.18±0.18ab |
| CaLG | 1.77±0.09ab | 1.25±0.09ab |
| Polycan:CaLG mixed
formula (1:99; mg/kg) |
| 200 | 1.72±0.16ab | 1.29±0.14ab |
| 100 | 1.79±0.13ab | 1.24±0.12ab |
| 50 | 1.88±0.09ab | 1.15±0.13ab |
| Polycan:CaLG mixed
formula (5:95; mg/kg) |
| 200 | 1.66±0.18ab | 1.31±0.15ab |
| 100 | 1.74±0.14ab | 1.26±0.11ab |
| 50 | 1.84±0.12ab | 1.19±0.11ab |
| Polycan:CaLG mixed
formula (10:90; mg/kg) |
| 200 | 1.55±0.11ab | 1.41±0.12ab |
| 100 | 1.63±0.21ab | 1.33±0.12ab |
| 50 | 1.75±0.13ab | 1.28±0.15ab |
Urinalysis assessment
A significant increase (P<0.01) in urine Dpd
levels and the urine Dpd/creatinine ratio were observed in the OVX
control group when compared with the sham control group. However,
these values significantly decreased (P<0.01) in the test
material-treated rats when compared with the OVX control. No marked
changes in urine creatinine levels were detected in any
formula-tested groups compared with the Sham and OVX control
groups. Among the three types of mixed formulas, only the PCLG
10:90 mixed formula exhibited favorable synergistic effects when
compared with equal doses of the single Polycan formula. Similar
inhibitory effects on the urine Dpd levels and a reduction in the
Dpd/creatinine ratio were detected in the PCLG 10:90 mixed formula
50 mg/kg-treated rats as compared with the Polycan single formula
100 mg/kg-treated rats (Table
III).
 | Table IIIUrinalysis after 84 days of repeated
oral administration of test materials in osteoporotic rats. |
Table III
Urinalysis after 84 days of repeated
oral administration of test materials in osteoporotic rats.
| Groups | Dpd (nM) | Dpd/creatinine
ratio (nM/g/day) |
|---|
| Controls |
| Sham | 37.58±3.98 | 5972.95±746.51 |
| OVX | 67.08±3.90a |
10884.88±1642.09a |
| Risedronate
sodium | 49.67±5.69ac |
7887.86±966.21ac |
| Single formula (100
mg/kg) |
| Polycan | 54.33±6.00ac |
8839.59±1031.19ac |
| CaLG | 60.15±6.67ac |
9428.92±1668.14ad |
| Polycan:CaLG mixed
formula (1:99; mg/kg) |
| 200 | 52.68±5.89ac |
8333.03±1034.61ac |
| 100 | 56.40±5.66ac |
8833.37±1126.67ac |
| 50 | 58.41±5.31ac |
9167.76±1126.57ac |
| Polycan:CaLG mixed
formula (5:95; mg/kg) |
| 200 | 48.92±5.78ac |
7846.84±1210.32ac |
| 100 | 54.59±5.50ac |
8824.94±872.25ac |
| 50 | 57.48±3.90ac |
9095.29±1056.95ac |
| Polycan:CaLG mixed
formula (10:90; mg/kg) |
| 200 | 47.15±3.12ac |
7492.46±671.09bc |
| 100 | 51.04±5.00ac |
8190.17±1198.21ac |
| 50 | 53.07±4.72ac |
8567.11±1339.35ac |
Effects on BMC, BMD and FL
Femur Ca and P contents significantly decreased
(P<0.01) in the OVX control group when compared with the sham
control group. However, a significant increase (P<0.01) in the
femur Ca and P contents was observed in the formula-treated groups
when compared with the OVX control group, but with no change to the
bone Ca/P ratio. The PCLG 10:90 mixed formula exhibited the most
favorable synergistic effects against the OVX-induced decrease in
femur Ca and P content when compared with equal doses of the CaLG
single formula. Similar inhibitory effects were detected in the
PCLG 10:90 mixed formula 50 mg/kg-treated rats as compared with the
CaLG single formula 100 mg/kg-treated rats (Table IV).
 | Table IVBone Ca and P contents after 84 days
of repeated oral administration of test materials in osteoporotic
rats. |
Table IV
Bone Ca and P contents after 84 days
of repeated oral administration of test materials in osteoporotic
rats.
| Groups | Ca (mg/g bone) | P (mg/g bone) | Ca/P ratio |
|---|
| Controls |
| Sham | 178.29±8.15 | 103.08±4.83 | 1.73±0.02 |
| OVX |
120.92±12.96a | 69.51±7.27a | 1.74±0.07 |
| Risedronate
sodium |
148.22±10.61ab | 85.90±7.64ab | 1.73±0.08 |
| Single formula (100
mg/kg) |
| Polycan | 137.56±6.41ab | 80.12±3.28ab | 1.72±0.02 |
| CaLG | 140.73±5.45ab | 79.38±5.68ab | 1.78±0.10 |
| Polycan:CaLG mixed
formula (1:99; mg/kg) |
| 200 | 148.93±7.73ab | 84.44±4.16ab | 1.76±0.05 |
| 100 | 140.59±8.47ab | 79.82±5.21ab | 1.76±0.08 |
| 50 | 135.22±9.16ab | 78.23±3.97ab | 1.73±0.11 |
| Polycan:CaLG mixed
formula (5:95; mg/kg) |
| 200 |
153.21±10.77ab | 88.71±7.71ab | 1.73±0.05 |
| 100 | 141.15±7.75ab | 79.75±7.39ab | 1.78±0.11 |
| 50 | 137.39±5.34ab | 78.58±4.49ab | 1.75±0.07 |
| Polycan:CaLG mixed
formula (10:90; mg/kg) |
| 200 | 161.26±8.53ab | 93.47±3.77ab | 1.73±0.07 |
| 100 | 149.27±8.35ab | 86.21±4.93ab | 1.73±0.03 |
| 50 | 142.49±7.06ab | 81.22±3.21ab | 1.75±0.07 |
The BMD of the OVX control group decreased at all
the detection points when compared with the sham control group.
However, marked increases in the BMD of the measured regions were
evident in the formula administration groups when compared with the
OVX control group. PCLG 10:90 exhibited a favorable effect against
the OVX-induced femur BMD decrease when compared with equal doses
of the CaLG single formula. Similar inhibitory effects on the femur
BMD decrease were detected in the PCLG 10:90 mixed formula 50
mg/kg-treated rats as compared with the CaLG single formula 100
mg/kg-treated rats (Table V).
 | Table VBMD and FL after 84 days of repeated
oral administration of test materials in osteoporotic rats. |
Table V
BMD and FL after 84 days of repeated
oral administration of test materials in osteoporotic rats.
| Groups | Total | Epiphyseal
neck | Mid-shaft | FL (n) |
|---|
| Controls |
| Sham | 0.1207±0.0026 | 0.1327±0.0085 | 0.1017±0.0069 | 131.11±14.77 |
| OVX |
0.1097±0.0050a |
0.1159±0.0077a |
0.0914±0.0019a | 76.27±10.93a |
| Risedronate
sodium |
0.1326±0.0055ac |
0.1615±0.0188ac |
0.1066±0.0069c |
104.29±11.51ac |
| Single formula
(100mg/kg) |
| Polycan |
0.1169±0.0063c |
0.1287±0.0029c | 0.0985±0.0083 | 97.45±11.55ac |
| CaLG |
0.1175±0.0034c |
0.1360±0.0045c |
0.0999±0.0030c | 96.89±11.30ac |
| Polycan:CaLG mixed
formula (1:99; mg/kg) |
| 200 |
0.1175±0.0053c |
0.1433±0.0142c |
0.1019±0.0046c |
105.21±12.82ac |
| 100 |
0.1172±0.0040c |
0.1362±0.0059c |
0.0989±0.0056c | 96.89±16.03ac |
| 50 |
0.1157±0.0057bd |
0.1333±0.0081c |
0.0983±0.0065d | 94.48±10.91ac |
| Polycan:CaLG mixed
formula (5:95; mg/kg) |
| 200 |
0.1192±0.0042c |
0.1437±0.0091bc |
0.1056±0.0095c |
108.78±10.71ac |
| 100 |
0.1181±0.0035c |
0.1365±0.0080c |
0.1005±0.0068c | 94.47±16.98ac |
| 50 |
0.1169±0.0054c |
0.1334±0.0138d |
0.0982±0.0043c | 96.12±14.38ac |
| Polycan:CaLG mixed
formula (10:90; mg/kg) |
| 200 |
0.1216±0.0033c |
0.1418±0.0095c |
0.1090±0.0109c |
118.04±11.83ac |
| 100 |
0.1201±0.0031c |
0.1407±0.0079c |
0.1059±0.0065c |
109.68±14.94ac |
| 50 |
0.1193±0.0063c |
0.1381±0.0107c |
0.1020±0.0042c |
104.87±12.47ac |
The strength (FL) of the femur in the OVX control
group decreased compared with the sham control group. However,
increases in FL were detected in all the administration groups when
compared with the OVX control group. However, only the PCLG 10:90
mixed formula exhibited favorable synergism against OVX-induced
femur FL decrease when compared with equal doses of the Polycan
single formula. Similar inhibitory effects were detected in the
PCLG 10:90 mixed formula 50 mg/kg-treated rats as compared with the
Polycan single formula 100 mg/kg-treated rats (Table V).
Histopathological profile changes
Relatively well-developed trabecular and cortical
bone was observed in the femurs of the sham control group. However,
a classical osteoporotic histological profile was detected in the
OVX control group, including a marked loss of trabecular and
cortical bone and increased levels of connective tissue in the
periosteum of the cortical bone, resulting from the resorption of
osteoid tissues. These osteoporotic changes were markedly inhibited
by treatment with the test materials. Among the three types of
mixed formulas, only PCLG 10:90 exhibited synergistic effects
against the OVX-induced changes when compared with equal doses of
the Polycan single formula. Similar inhibitory effects were
detected in the PCLG 10:90 mixed formula 50 mg/kg-treated rats as
compared with the Polycan single formula 100 mg/kg-treated rats
(Fig. 1).
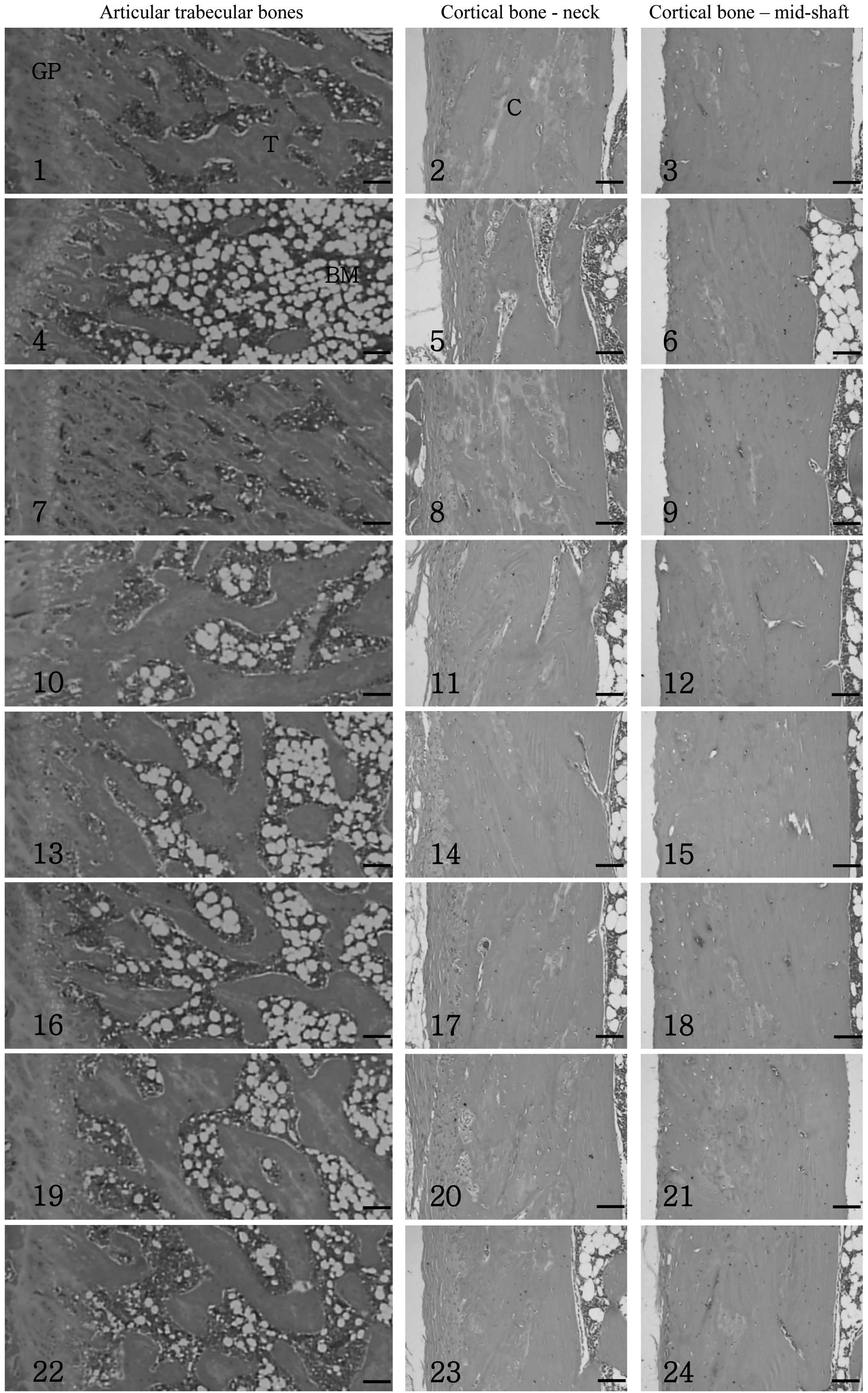 | Figure 1Histological profiles of the femur in
osteoporotic rats in the sham control (1–3), OVX
control (4–6), risedronate sodium (7–9),
Polycan (10–12) and CaLG (13–15)
single formula and PCLG 1:99 200 (16–18),
100 (19–21) and 50 mg/kg (22–24)
mixed formula groups (hematoxylin and eosin; scale bars, 160 μm).
OVX, ovariectomy; CaLG, calcium lactate-gluconate; PCLG,
Polycan:CaLG. Histological profiles of the femur in osteoporotic
rats in the PCLG 5:90 200 (25–27),
100 (28–30) and 50 mg/kg (31–33)
and the PCLG 10:90 200 (34–36),
100 (37–39) and 50 mg/kg (40–42) mixed formula groups (hematoxylin
and eosin; scale bars, 160 μm). Relatively well-developed
trabecular and cortical bone was observed in the femur of the sham
control group. However, a classical osteoporotic histological
profile was detected in the OVX control group with a marked
histological decrease of trabecular and cortical bone and an
increase of connective tissues in the periosteum of the cortical
bone, resulting from the resorption of osteoid tissues. These
osteoporotic changes were markedly inhibited by treatment with all
the test materials used. OVX, ovariectomy; CaLG, calcium
lactate-gluconate; PCLG, Polycan:CaLG. |
Significant reductions (P<0.01) in the TBV, Tbn,
Tbt, Tbl and Cbt (at the epiphyseal and mid-shaft) were detected in
the OVX control group when compared with the sham control group.
These histomorphometrical indices for bone mass and structure
significantly increased in the test material-administered OVX rats
when compared with the OVX control rats. However, only the PCLG
10:90 mixed formula exhibited favorable synergistic effects against
OVX-induced femur histopathology when compared with equal doses of
the Polycan or CaLG single formulas. Similar inhibitory effects
were detected in the PCLG 10:90 mixed formula 50 mg/kg-treated rats
as compared with the Polycan or CaLG single formula 100
mg/kg-treated rats (Table
VI).
 | Table VIHistomorphometry of the femur after
84 days of repeated oral administration of test materials in
osteoporotic rats. |
Table VI
Histomorphometry of the femur after
84 days of repeated oral administration of test materials in
osteoporotic rats.
| Groups | TBV (%) | Tbn (n) | Tbl (mm) | Tbt (μm) | Ocn (n) | OS/BS (%) | Cbt-neck (μm) | Cbt-shaft (μm) |
|---|
| Controls |
| Sham | 48.36±5.98 | 28.63±3.78 | 7.35±0.69 | 465.03±46.70 | 8.50±2.45 | 2.48±0.51 | 1042.37±137.98 | 1264.96±215.64 |
| OVX | 20.10±2.07a | 8.25±2.43a | 3.21±0.40a |
185.42±28.75a | 30.63±4.75a | 22.17±3.81a |
549.82±82.24a |
743.76±63.80a |
| Risedronate
sodium | 53.28±8.94c | 31.50±3.02bc | 4.83±0.53bc |
174.59±16.86a | 36.25±7.01a | 7.11±1.23ac |
782.29±85.97ac |
852.60±80.20ad |
| Single formula (100
mg/kg) |
| Polycan | 36.49±3.77ac | 18.25±1.67ac | 5.19±0.60ac |
271.72±38.72ac | 18.23±2.70ac | 11.55±3.72ac |
788.03±24.83ac |
990.67±107.17bc |
| CaLG | 32.27±2.46ac | 15.88±1.96ad | 5.37±0.56ad |
305.26±37.91ac | 26.13±3.36a | 18.05±2.95a |
860.28±89.76ac |
1037.67±75.58bc |
| Polycan:CaLG mixed
formula (1:99; mg/kg) |
| 200 | 40.80±5.50bc | 20.38±1.69ac | 6.36±0.56ac |
315.14±23.92ac | 14.25±2.92ac | 9.26±1.72ac |
871.51±113.27bc |
1109.18±102.73c |
| 100 | 36.20±4.83ac | 18.38±1.92ac | 5.32±0.41ac |
307.22±16.19ac | 18.50±2.78ac | 13.38±3.90ac |
813.29±65.29ac |
1026.50±76.65bc |
| 50 | 29.72±2.74ac | 14.13±1.89ac | 4.91±0.28ac |
289.03±17.61ac | 20.13±3.04ac | 18.46±1.24ad |
753.68±85.50ac |
948.33±67.40ac |
| Polycan:CaLG mixed
formula (5:95; mg/kg) |
| 200 | 46.58±4.50c | 21.75±1.67ac | 6.47±0.54ac |
322.65±23.11ac | 14.00±4.34ac | 8.48±1.75ac |
947.07±67.10c |
1129.15±134.17c |
| 100 | 36.82±3.03a,c | 18.63±1.19ac | 5.45±0.68ac |
315.50±23.05ac | 18.25±2.49ac | 12.52±3.98ac |
869.35±82.56ac |
1027.62±78.15bc |
| 50 | 33.53±4.80ac | 16.75±1.28ac | 5.23±0.51ac |
293.91±16.64ac | 19.75±2.49ac | 15.70±3.54ac |
772.26±103.37ac |
989.81±77.08ac |
| Polycan:CaLG mixed
formula (10:90; mg/kg) |
| 200 | 50.28±3.06c | 27.88±2.03c | 7.70±0.66c |
395.20±28.97ac | 9.38±1.77c | 6.78±1.81ac |
995.82±136.21c |
1186.15±188.42c |
| 100 | 43.46±3.23c | 23.00±3.38ac | 6.79±0.50bc |
367.72±47.79ac | 13.38±3.74ac | 9.25±1.85ac |
968.71±86785c |
1126.91±145.68c |
| 50 | 38.10±3.59ac | 18.38±1.77ac | 6.19±0.75ac |
349.57±45.52ac | 17.25±3.85ac | 12.01±2.27ac |
882.55±45.04ac |
1040.69±101.95bc |
Significant (P<0.01) increases in Ocn and OS/BS
were detected in the OVX group compared with the Sham control
group. This means that the OVX model is good for the evaluation of
osteoporosis and that osteoporosis was well induced by OVX in this
study. However, decreased Ocn and OS/BS values were observed in the
material-administered group compared with the OVX control group.
Although a similar Ocn was detected in the risedronate-treated and
OVX control groups, the OS/BS significantly (P<0.01) decreased.
Only the PCLG 10:90 mixed formula exhibited favorable synergistic
effects against the OVX-induced femur histopathological bone
resorption changes when compared with equal doses of the Polycan
single formula. Similar inhibitory effects were detected in the
PCLG 10:90 mixed formula 50 mg/kg-treated rats as compared with the
Polycan single formula 100 mg/kg-treated rats (Table VI).
Discussion
Bone remodeling by osteoblasts and osteoclasts is a
crucial determinant of increasing bone mass under pathological
conditions, including bone disorders (21). As favorable antiosteoporotic
effects of Polycan (15) and CaLG
(9) have been reported, the
present study aimed to select the optimal PCLG composition that
exhibited the most favorable efficacy on OVX-induced osteoporotic
rats. Polycan and CaLG single formulas (100 mg/kg each), and three
doses (50, 100 and 200 mg/kg) of three mixed formulas [polycan:CaLG
(PCLG)=1:99, 5:95 and 10:90] were orally administered daily for 84
days to OVX osteoporotic rats, and changes in the BW, serum
osteocalcin levels, urine Dpd/creatinine ratio, femur indices, BMC,
BMD, FL and histological and histomorphometrical analyses were
assessed.
Alterations in BW were detected in all the OVX
groups, which is considered as a general sign of estrogen
deficiency (22). In the present
study, no marked changes in BW were detected in any of the treated
groups when compared with the OVX control group. Therefore, Polycan
and CaLG single and mixed formulas were hypothesized to not affect
OVX-induced BW increase.
Although it is generally accepted that changes in BW
are not a critical index for detecting the efficacy of
antiosteoporotic agents (23), an
increased ash bone weight is considered a valuable marker of
antiosteoporotic activity (24).
The PCLG 10:90 mixed formula exhibited favorable inhibitory effects
on OVX-induced ash bone weight decrease, providing direct evidence
that an appropriate mixture of Polycan and CaLG induces synergistic
antiosteoporotic effects.
Although variability in the methods for measuring
bone turnover and formation exist among previous studies, serum
osteocalcin levels are the generally accepted marker of bone
turnover, while bALP levels are considered to demonstrate bone
formation (25). However, since
osteocalcin is a vitamin K-dependent α-carboxyglutamic acid
released by osteoblasts, serum osteocalcin levels are also regarded
as an indicator of bone formation (26). In the present study, serum
osteocalcin levels in the OVX control group markedly increased
compared with those in the sham control group, indicating an
increased bone turnover. A marked reduction in the serum
osteocalcin levels was detected in all the treated rats when
compared with the untreated OVX control, indicating an inhibition
of bone turnover. In addition, the decrease in serum bALP levels
was significantly inhibited by the Polycan and CaLG single or mixed
formulas, providing indirect evidence that the formulas facilitate
bone formation. The PCLG 10:90 mixed formula exhibited the most
favorable inhibitory effects on the OVX-induced serum osteocalcin
and bALP level changes when compared with the single Polycan or
CaLG formulas, and thus achieved the most promising synergistic
antiosteoporotic effects.
Urine Dpd is a marker of bone resorption that is
sensitive to creatinine, an indicator of kidney status (27). The Dpd/creatinine ratio was
employed in the present study as an index of bone resorption during
osteoporosis (25). The urine
Dpd/creatinine ratio significantly increased in the OVX rats, but
was inhibited by the formula treatments, indicating that the test
materials inhibited the bone resorption induced by OVX. Similarly,
the PCLG 10:90 mixed formula exhibited the most favorable
inhibitory effects as compared with the single formulas of Polycan
or CaLG.
Generally, the BMC significantly decreases during
osteoporosis. With regard to BMC, the Ca and P contents represent
the most markedly decreased mineral contents during osteoporosis,
while the Ca/P ratio does not generally change (28). The Polycan and CaLG single or mixed
formulas significantly increased the content of Ca and P in the
femur, indicating that the formulas preserve bone mass and are
likely to increase bone strength. In addition, favorable inhibitory
effects on OVX-induced BMC decreases were detected with the PCLG
10:90 mixed formula as compared with equal doses of the Polycan or
CaLG single formulas.
BMD is a marker of bone quantity and generally
decreases in osteoporotic animals. The BMD of bone provides
reliable information regarding the efficacy of antiosteoporotic
agents (29), thus, provides a
diagnostic profile of bone quantity (30). All the test materials administered
in the present study demonstrated favorable inhibitory effects on
the decrease in BMD induced by OVX, regardless of the detection
regions.
The FL directly indicates cortical bone strength
(31) and is an important
indicator of the efficacy of antiosteoporotic agents (32). All the test materials administered
in the present study exhibited favorable effects on bone strength.
In particular, the PCLG 10:90 mixed formula demonstrated the most
favorable inhibitory effects on OVX-induced BMD and bone strength
decreases.
The efficacy of various antiosteoporotic agents has
been evaluated through bone histology analysis (33,34).
Microscopic bone analysis was used to assess bone morphology
(31,35). In the osteoporotic animals, the
histological profiles were clearly altered regardless of cause,
particularly in the trabecular and cortical bone. All the test
materials exhibited clear inhibitory effects on these histological
changes in the OVX-induced osteoporotic rats.
During osteoporosis, a number of histomorphometrical
indices for bone mass decrease, while bone resorption generally
increases, providing reliable information to predict the efficacy
of antiosteoporotic agents (36).
Following treatment with the Polycan and CaLG single or mixed
formulas, changes in the histomorphometrical indices for bone mass,
structure and resorption were markedly inhibited. These
observations provide evidence of the favorable antiosteoporotic
effects that these formulas exhibit. The PCLG 10:90 mixed formula
demonstrated the most favorable inhibitory effects on the
OVX-induced histopathological changes as compared with the single
formulas of Polycan or CaLG. Calcium is critical for improving bone
health, and its intake is generally recommended. However, the
consumption of sufficient calcium is not the only factor required
for preventing osteoporosis or bone fracture. If patients receive
polycalcium, intake of less than the daily recommended amount of
calcium results in suppression of bone destruction and prevention
of fractures (FL growth).
Acknowledgements
This study was conducted at the Global Healthcare
Industry RIS Center with a grant from the Ministry of Knowledge
Economy, Republic of Korea (no. 70007205).
References
|
1
|
Sakai A, Nishida S, Okimoto N, Okazaki Y,
Hirano T, Norimura T, Suda T and Nakamura T: Bone marrow cell
development and trabecular bone dynamics after ovariectomy in ddy
mice. Bone. 23:443–451. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Melton LJ 3rd, Chrischilles EA, Cooper C,
Lane AW and Riggs BL: How many women have osteoporosis? JBMR
Anniversary Classic JBMR, Volume 7, Number 9, 1992. J Bone Miner
Res. 20:886–892. 2005.PubMed/NCBI
|
|
3
|
Rodan GA and Martin TJ: Therapeutic
approaches to bone diseases. Science. 289:1508–1514. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gowen M, Emery JG and Kumar S: Emerging
therapies for osteoporosis. Expert Opin Emerg Drugs. 5:1–43. 2000.
View Article : Google Scholar
|
|
5
|
Endo I and Matsumoto T: New approved drugs
for osteoporosis in Japan. Clin Calcium. 22:870–876.
2012.PubMed/NCBI
|
|
6
|
Lakatos P: Pharmacologic treatment of
osteoporosis - 2011. Orv Hetil. 152:1320–1326. 2011.(In
Hungarian).
|
|
7
|
Kalu DN: The ovariectomized rat model of
postmenopausal bone loss. Bone Miner. 15:175–191. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
US Food and Drug Administration.
Guidelines for preclinical and clinical evaluation of agents used
in the prevention or treatment of postmenopausal osteoporosis.
Rockville, MD, USA: pp. 2–22. 1994
|
|
9
|
Heaney RP, Recker RR, Watson P and Lappe
JM: Phosphate and carbonate salts of calcium support robust bone
building in osteoporosis. Am J Clin Nutr. 92:101–105. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aloia JF, Vaswani A, Yeh JK, Ross PL,
Flaster E and Dilmanian FA: Calcium supplementation with and
without hormone replacement therapy to prevent postmenopausal bone
loss. Ann Intern Med. 120:97–103. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han SY, Lee JR, Kwon YK, Jo MJ, Park SJ,
Kim SC, Lee HS and Ku SK: Ostreae Testa prevent ovariectomy-induced
bone loss in mice by osteoblast activations. J Ethnopharmacol.
114:400–405. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pak CY, Poindexter J and Finlayson B: A
model system for assessing physicochemical factors affecting
calcium absorbability from the intestinal tract. J Bone Miner Res.
4:119–127. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Whiting SJ and Pluhator MM: Comparison of
in vitro and in vivo tests for determination of availability of
calcium from calcium carbonate tablets. J Am Coll Nutr. 11:553–560.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seo HP, Kim JM, Shin HD, Kim TK, Chang HJ,
Park BR and Lee JW: Production of −1,3/1,6-glucan by
Aureobasidium pullulans SM-2001. Kor J Biotechnol Bioeng.
17:376–380. 2002.
|
|
15
|
Shin HD, Yang KJ, Park BR, Son CW, Jang HJ
and Ku SK: Antiosteoporotic effect of Polycan, beta-glucan from
Aureobasidium, in ovariectomized osteoporotic mice.
Nutrition. 23:853–860. 2007. View Article : Google Scholar
|
|
16
|
Lee HS, Cho HR, Moon SB, Shin HD, Yang KJ,
Park BR, Jang HJ, Kim LS and Ku SK: Effect of β-glucan from
Aureobasidium pullulans on rat rib fracture healing. Lab
Anim Res. 24:39–44. 2008.
|
|
17
|
Kim HD, Cho HR, Moon SB, Shin HD, Yang KJ,
Park BR, Jang HJ, Kim LS, Lee HS and Ku SK: Effect of exopolymers
from Aureobasidium pullulans on formalin-induced chronic paw
inflammation in mice. J Microbiol Biotechnol. 16:1954–1960.
2006.
|
|
18
|
Harris ST, Watts NB, Genant HK, McKeever
CD, Hangartner T, Keller M, Chesnut CH 3rd, Brown J, Eriksen EF,
Hoseyni MS, Axelrod DW and Miller PD: Effects of risedronate
treatment on vertebral and nonvertebral fractures in women with
postmenopausal osteoporosis: a randomized controlled trial.
Vertebral Efficacy With Risedronate Therapy (VERT) Study Group.
JAMA. 282:1344–1352. 1999. View Article : Google Scholar
|
|
19
|
World Health Organization Regional Office
for the Western Pacific, . Research Guidelines for Evaluating the
Safety and Efficacy of Herbal Medicines. WHO Regional Publications;
pp. 5–34. 1993
|
|
20
|
Parfitt AM, Drezner MK, Glorieux FH, Kanis
JA, Malluche H, Meunier PJ, Ott SM and Recker RR: Bone
histomorphometry: standardization of nomenclature, symbols, and
units. Report of the ASBMR Histomorphometry Nomenclature Committee.
J Bone Miner Res. 2:595–610. 1987. View Article : Google Scholar
|
|
21
|
Manolagas SC, Kousteni S and Jilka RL: Sex
steroids and bone. Recent Prog Horm Res. 57:385–409. 2002.
View Article : Google Scholar
|
|
22
|
Lorden JF and Caudle A: Behavioral and
endocrinological effects of single injections of monosodium
glutamate in the mouse. Neurobehav Toxicol Teratol. 8:509–519.
1986.PubMed/NCBI
|
|
23
|
Yamamoto M, Fisher JE, Gentile M, Seedor
JG, Leu CT, Rodan SB and Rodan GA: The integrin ligand echistatin
prevents bone loss in ovariectomized mice and rats. Endocrinology.
139:1411–1419. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie F, Wu CF, Zhang Y, Yao XS, Cheung PY,
Chan AS and Wong MS: Increase in bone mass and bone strength by
Sambucus williamsii HANCE in ovariectomized rats. Biol Pharm
Bull. 28:1879–1885. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ke HZ, Foley GL, Simmons HA, Shen V and
Thompson DD: Long-term treatment of lasofoxifene preserves bone
mass and bone strength and does not adversely affect the uterus in
ovariectomized rats. Endocrinology. 145:1996–2005. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ederveen AG and Kloosterboer HJ: Tibolone,
a steroid with a tissue-specific hormonal profile, completely
prevents ovariectomy-induced bone loss in sexually mature rats. J
Bone Miner Res. 14:1963–1970. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blanqué R, Cottereaux C and Gardner CR:
Phasic production of urinary pyridinium crosslinks in mice: the
effect of ovariectomy. Calcif Tissue Int. 68:102–108.
2001.PubMed/NCBI
|
|
28
|
Tanaka S, Shimizu M, Debari K, Furuya R,
Kawawa T and Sasaki T: Acute effects of ovariectomy on wound
healing of alveolar bone after maxillary molar extraction in aged
rats. Anat Rec. 262:203–212. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Syed Z and Khan A: Bone densitometry:
applications and limitations. J Obstet Gynaecol Can. 24:476–484.
2002.PubMed/NCBI
|
|
30
|
Diez F: Guidelines for the diagnosis of
osteoporosis by densitometric methods. J Manipulative Physiol Ther.
25:403–415. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamaguchi K, Yada M, Tsuji T, Kuramoto M
and Uemura D: Suppressive effect of norzoanthamine hydrochloride on
experimental osteoporosis in ovariectomized mice. Biol Pharm Bull.
22:920–924. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Horcajada-Molteni MN, Crespy V, Coxam V,
Davicco MJ, Rémésy C and Barlet JP: Rutin inhibits
ovariectomy-induced osteopenia in rats. J Bone Miner Res.
15:2251–2258. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Miyakoshi N, Sato K, Tamura Y, Tsuchida T,
Kudo T and Kasukawa Y: Evaluation of long-term sequential changes
in bone mass and strength following withdrawal of incadronate
disodium (YM175) in ovariectomized rats. J Orthop Sci. 6:167–176.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Glatt M, Pataki A, Evans GP, Hornby SB and
Green JR: Loss of vertebral bone and mechanical strength in
estrogen-deficient rats is prevented by long-term administration of
zoledronic acid. Osteoporos Int. 15:707–715. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Heikkinen T, Puoliväli J and Tanila H:
Effects of long-term ovariectomy and estrogen treatment on maze
learning in aged mice. Exp Gerontol. 39:1277–1283. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Weinreb M, Patael H, Preisler O and
Ben-Shemen S: Short-term healing kinetics of cortical and
cancellous bone osteopenia induced by unloading during the
reloading period in young rats. Virchows Arch. 431:449–452. 1997.
View Article : Google Scholar : PubMed/NCBI
|















