Introduction
A number of environmental and clinical risk factors
are associated with essential hypertension (EH), including dietary
intake of sodium, alcohol intake, lack of exercise, a poor diet,
obesity, insulin-resistant diabetes and hyperlipidemia (1). Although these factors explain a
substantial proportion of hypertension susceptibility, it is
estimated that up to 60% of the variation in hypertension risk is
due to the genetic makeup of an individual (2).
The association between hypertension and
hyperhomocysteinemia and the risk of cardiovascular disease (CVD)
has been previously studied (3).
The three major enzymes involved in homocysteine (Hcy) metabolism
are methylenetetrahydrofolate reductase (MTHFR), methionine
synthase (MS) and cystathionine β-synthase (CBS). A study has
revealed that the 677T MTHFR allele is associated with increased
levels of plasma Hcy in ethnic Europeans (4). A study of a population in the
Midwestern region of the USA demonstrated that a 68-bp insertion in
the CBS gene (844ins68) and the A2756G transition of the MS gene
were associated with low levels of plasma Hcy (5). To the best of our knowledge, a total
of 132 mutations in CBS have been identified to date with the
majority of these being missense mutations. The most frequent among
these is I278T (c.833T>C), commonly found in Caucasians, for
which the clinical manifestation is mild and vitamin B6-responsive
(6). While there has been major
focus on the association between CBS T833C polymorphism and
cerebral arterial thrombosis in the Chinese population (7,8), few
studies have investigated its association with hypertension.
The Kazakhs, a nomad population that inhabit, among
other areas, the north of Xinjiang province in northwest China, are
characterized by a higher prevalence of hypertension and higher
levels of blood pressure (BP) compared with other ethnic
populations residing in the same area (9). Since hypertension is a common disease
among the Kazakh people, the present study concentrated on the CBS
T833C polymorphism in order to investigate whether it was
correlated with hypertension in Kazakh patients.
Materials and methods
Subjects
The sample size for the Kazakh hypertensive group
[systolic blood pressure (SBP) ≥140 mmHg or diastolic blood
pressure (DBP) ≥90 mmHg)] was 545 (male:female ratio, 409:136; mean
age, 49.23±7.56 years; SBP, 161.18±23.62 mmHg; DBP, 96.46±10.99
mmHg). The sample size for the Kazakh normotensive control group
(SBP <140 mmHg and DBP <90 mmHg) was 540 (male:female ratio,
385:155; mean age, 49.90±10.01 years, SBP, 120.92±11.85 mmHg; DBP,
76.91±7.94 mmHg). The subjects were selected from a
population-based cross sectional study performed during January
March 2010 and August 2012 among the Kazakh population. The study
concentrated on isolated populations in a homogeneous environment.
The criteria for the selection of hypertensive subjects were a SBP
≥140 mmHg, DBP ≥90 mmHg or anti-hypertension treatment (10). The criteria for the selection of
normotensive control subjects was a SBP <140 mmHg, DBP <90
mmHg and no history of anti-hypertensive medication. Individuals
with secondary hypertension (estimated by their history,
examinations and laboratory evaluation), serious arrhythmia,
coronary heart disease, liver and renal insufficiency, heart
failure, cerebrovascular accidents, acute and chronic infectious
diseases, cancer, and those using contraceptives were excluded from
the current study. All Kazakh subjects who participated in the
present study were residents of the Xinjiang autonomous region of
China, which has an established Kazakh population who are
considered to be less affected by the migration of the Han Chinese.
The characteristics of the subjects analyzed in the present study
are summarized in Table I. Written
consent was obtained from all subjects prior to data collection and
measurements. The current study was approved by the Ethics
Committee of the First Affiliation of Xinjiang Medical University
(Xinjang, China).
 | Table IComparison of clinical characteristics
in the Kazakh subjects. |
Table I
Comparison of clinical characteristics
in the Kazakh subjects.
| Characteristic | Hypertension | Normotension | t/χ2 | P-value |
|---|
| N (male/female) | 545 (409/136) | 540 (385/155) | 1.93 | 0.16 |
| Age (years) | 49.23±7.56 | 49.90±10.01 | 0.56 | 0.57 |
| Smoking (n/%) | 390/71.6 | 325/60.2 | 15.61 | 0.02 |
| Drinking (n/%) | 387/71.0 | 330/61.1 | 11.85 | 0.01 |
| BMI
(kg/m2) | 27.31±3.94 | 24.98±3.28 | 4.84 | 0.01 |
| SBP (mmHg) | 161.18±23.62 | 120.92±11.85 | 15.72 | 0.01 |
| DBP (mmHg) | 96.46±10.99 | 76.91±7.94 | 15.25 | 0.01 |
| FPG (mmol/l) | 5.83±1.17 | 5.56±1.67 | 2.06 | 0.04 |
| UA (mmol/l) | 294.69±59.20 | 282.27±34.87 | 1.88 | 0.06 |
| TC (mmol/l) | 4.48±0.98 | 3.72±1.25 | 5.35 | 0.01 |
| TG (mmol/l) | 3.38±1.54 | 2.69±1.53 | 3.50 | 0.01 |
| HDL (mmol/l) | 1.21±0.33 | 1.19±0.24 | 0.04 | 0.97 |
| LDL, (mmol/l) | 2.73±0.65 | 2.10±0.58 | 7.85 | 0.01 |
| Hcy (μmol/l) | 17.19±6.11 | 13.77±5.66 | 4.24 | 0.01 |
Study design and procedures
The blood pressure measurements were performed by
trained and certified observers three times for each subject
following at least 10 min of rest in a sitting position. Weight and
height were measured using standard techniques with the
participants in light clothing and barefoot. Body mass index (BMI)
was calculated as weight (kg)/height (m2). Blood samples
from the patients were collected in the morning following overnight
fasting from the antecubital vein. The samples were divided into
aliquots, separated within 30 min and stored at −80°C until
transportation for analysis. An enzyme-linked immunosorbent assay
(ELISA) was used to measure the levels of Hcy (11). In addition to performing a routine
blood examination that included lipid profiling, and glucose level,
blood/urine electrolyte and anthropometric measurements, all
subjects completed a set of questionnaires that included questions
on demographic information, personal history, family history of
disease and lifestyle.
DNA isolation
Genomic DNA was extracted from the blood sample of
each subject using the PAXgene™ gene blood DNA kit
(Qiagen, Hilden, Germany/BD Biosciences, Franklin Lakes, NJ,
USA).
Detection of the CBS T833C
polymorphism
The CBS T833C polymorphism was analyzed through
amplification refractory mutation system polymerase chain reaction
(PCR) analysis (12). The forward
primer (P1) was (5′-GGA GAA GTG TCC TGG ATG CA-3′), the wild type
reverse primer (P2) was (5′-CCC TTC GGG ATC CAC CCC AA-3′) and the
mutant type reverse primer (P3) was (5′-CCC TT CGG GAT CC AC CCC
AG-3′). The PCR reaction was conducted with a final volume of 20 μl
in two tubes. The 10 μl reaction mix (Tarcine BioMed, Inc.,
Beijing, China), 0.5 μl each primer, 8 μl ddH2O and 1 μl
genomic DNA were used for the amplification. Cycling conditions
included an initial denaturation at 94°C for 5 min followed by 35
cycles with a fast denaturation at 94°C for 60 sec, an annealing
step at 60°C for 45 sec and an extension step at 72°C for 60 sec,
with a final incubation for 7 min at 72°C. The amplification
reaction was followed by digestion with the reaction enzyme, MspA1I
(Fermentas Inc., Beijing, China) at 37°C for 24 h and
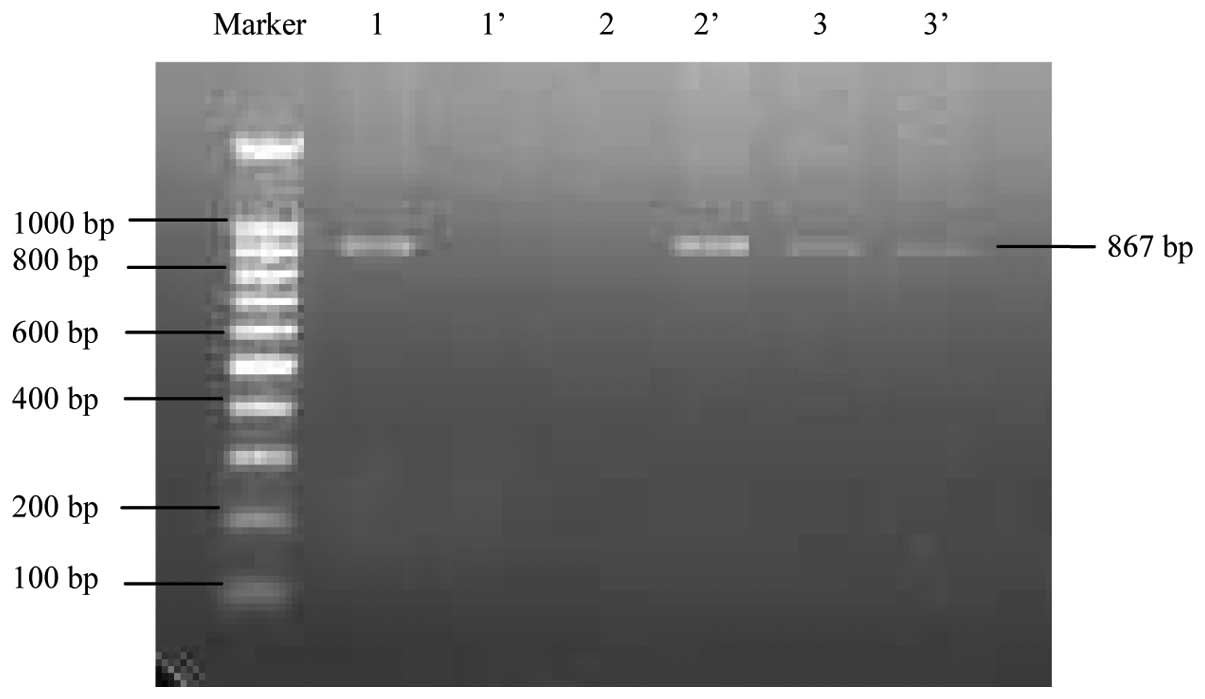
electrophoresis on a 2% agarose gel. The PCR product was 867 base
pairs in size (Fig. 1). CBS TT and
CC genotypes were identified as only one band (P1 vs. P2 display
respectively), while the CT genotype was identified as two bands
(P1 and P2 displays).
Statistical analysis
Statistical analysis was performed using SPSS
software, version 16.0 (SPSS, Inc., Chicago, IL, USA). The
Student’s t-test or one-way analysis of variance (ANOVA) was used
to compare numeric variables. The χ2 test was used to
determine any deviation in genotype distribution from
Hardy-Weinberg equilibrium and any differences in the frequency of
the CBS T833C polymorphism. The correlation between risk factors
and hypertension was assessed using logistic regression analysis.
The odds ratio (OR) with a 95% confidence interval (CI) was
determined. P<0.05 was considered to indicate a statistically
significant difference.
Results
Profile of the clinical characteristics
of the study groups
The demographic and clinical characteristics of the
study population are shown in Table
I. Age and genders were comparable in the two groups. The
levels of smoking and drinking, BMI, SBP, DBP, fasting plasma
glucose (FPG), total cholesterol (TC), triglycerides (TG), low
density lipoprotein (LDL) and Hcy were significantly higher in the
hypertensive group compared with those in the normotensive group
(P<0.05). The levels of uric acid (UA) and high density
lipoprotein (HDL) were not observed to be statistically different
between the two groups (P>0.05).
CBS T883C genotype and allele frequency
distribution in the Kazakh population
The distribution of the CBS T883C genotype
identified in the two groups did not significantly deviate from the
Hardy-Weinberg equilibrium. CBS T883C genotypes in the hypertensive
and control subjects are shown in Table II. No statistical difference in
genotype distribution was identified between the hypertensive and
control groups (P=0.36) and no significant difference in allele
frequency of the CBS T883C polymorphism was observed between the
two groups (68.9 vs. 65.7%, respectively, P=0.12).
 | Table IIGenotype distribution and allele
frequency of the CBS T833C polymorphism in the Kazakh
population. |
Table II
Genotype distribution and allele
frequency of the CBS T833C polymorphism in the Kazakh
population.
| Genotype/allele | Hypertension
(n=545) | Control (n=540) |
χ2-value | P-value |
|---|
| TT | 283 (51.9%) | 260 (48.1%) | 2.03 | 0.36 |
| CT | 185 (33.9%) | 190 (35.2%) | | |
| CC | 77 (14.1%) | 90 (15.4%) | | |
| T | 751 (68.9%) | 710 (65.7%) | 2.46 | 0.12 |
| C | 339 (31.1%) | 370 (34.3%) | | |
Comparing the clinical characteristics in
the CBS T883C genotype
The clinical characteristics in the CBS T883C
genotypes of the Kazakh subjects are shown in Table III. BMI, TG and Hcy were
significantly different for the CBS CC genotype than for the CBS TT
and CT genotypes (P<0.05). SBP, DBP, FPG, UA, TC, HDL and LDL
were not significantly different between the three genotypes.
 | Table IIIComparison of clinical characteristics
in the CBS T833C genotypes in the Kazakh subjects. |
Table III
Comparison of clinical characteristics
in the CBS T833C genotypes in the Kazakh subjects.
| Characteristic | TT | TC | CC | F-value | P-value |
|---|
| BMI
(kg/m2) | 25.58±3.39 | 26.73±4.12 | 28.19±4.07a | 6.85 | 0.01 |
| SBP (mmHg) | 145.57±27.78 | 144.62±28.60 | 142.06±27.25 | 0.21 | 0.82 |
| DBP (mmHg) | 88.43±13.35 | 89.14±14.40 | 86.88±13.83 | 0.33 | 0.72 |
| FPG (mmol/l) | 5.65±0.88 | 5.80±1.21 | 5.79±0.76 | 0.66 | 0.51 |
| UA (mmol/l) | 288.87±39.05 | 295.29±67.09 | 276.97±35.65 | 1.59 | 0.21 |
| TC (mmol/l) | 4.02±1.20 | 4.32±1.16 | 4.29±0.95 | 1.97 | 0.14 |
| TG (mmol/l) | 2.43±1.43 | 3.08±1.28 | 3.34±1.28a | 9.19 | 0.01 |
| HDL (mmol/l) | 1.18±0.31 | 1.19±0.32 | 1.04±0.27 | 1.69 | 0.19 |
| LDL (mmol/l) | 2.43±0.65 | 2.56±0.73 | 2.44±0.75 | 0.93 | 0.39 |
| Hcy (μmol/l) | 15.36±5.73 | 15.62±6.32 | 16.31±6.70a | 5.95 | 0.01 |
Multivariate analysis of the CBS T833C
polymorphism in the Kazakh population
Table IV shows the
multivariate analysis of risk factors relating to hypertension and
CBS T833C under a conditional logistic regression model. The
present study revealed that BMI, TC, smoking, level of plasma Hcy
and a family history of hypertension were independently correlated
with a significant predisposition to hypertension following
adjustments for related risk factors including age, gender and
drinking. The CBS T833C polymorphism was not found to be correlated
with a significant predisposition to hypertension.
 | Table IVMultivariate analysis for the CBS
T833C polymorphism in the Kazakh population according to a
conditional logistic regression model. |
Table IV
Multivariate analysis for the CBS
T833C polymorphism in the Kazakh population according to a
conditional logistic regression model.
| Variable | β-coefficient | SE | Wald | 95% CI | P-value |
|---|
| BMI | 0.26 | 0.07 | 12.75 | 1.29 (1.12,
1.49) | 0.01 |
| Age | 0.05 | 0.03 | 2.90 | 1.05 (0.99,
1.10) | 0.09 |
| Gender | 0.24 | 0.50 | 0.23 | 1.26 (0.48,
3.36) | 0.63 |
| Family history of
hypertension | 2.10 | 0.58 | 13.07 | 8.19 (2.62,
25.65) | 0.01 |
| Smoking | 2.98 | 0.81 | 13.70 | 9.68 (4.06,
25.39) | 0.01 |
| Drinking | 0.63 | 0.70 | 0.81 | 1.88 (0.48,
7.45) | 0.37 |
| FPG | −0.13 | 0.28 | 0.21 | 0.88 (0.51,
1.51) | 0.65 |
| TC | 0.55 | 0.22 | 6.39 | 1.72 (1.13,
2.63) | 0.01 |
| TG | −0.22 | 0.65 | 0.73 | 1.06 (0.48,
2.36) | 0.54 |
| Hcy | 0.81 | 0.37 | 4.74 | 2.25 (1.09,
4.69) | 0.03 |
| CBS T833C | 0.04 | 0.03 | 1.58 | 1.04 (0.98,
1.11) | 0.21 |
Discussion
Hcy is an amino acid derived from the demethylation
of methionine. Hyperhomocysteinemia is associated with an increased
risk of several complex diseases, including CVD (13). Hao et al (14) revealed a high prevalence of
hyperhomocysteinemia in Chinese adults, with 18% having Hcy
concentrations >16.0 mmol/l. This was especially prevalent among
males in the north of China where 28% of adults (40% of males) had
Hcy levels above this, a four-fold increase compared with that in
the south. Published data suggest that concentrations of plasma Hcy
vary depending on age and have significant ethnic and gender
differences (15–17). Since the fruit and vegetable intake
of people in China relies on local and seasonal produce, the intake
of green-leaf vegetables with a high folate content is much higher
in the south than in the north of the country. The Kazakhs, as a
nomad population, inhabit the north of Xinjiang province in
northwest China and 99% of them are herdsmen. Their diet consists
of a higher intake of salt than of green-leaf vegetables. The
present study identified that not only traditional risk factors,
including the levels of smoking and drinking, BMI, SBP, DBP, FPG,
UA, TC, TG, HDL and LDL, but also Hcy were significantly higher in
subjects with hypertension than in those with normotension
(17.19±6.11 vs. 13.77±5.66 μmol/l, respectively). Thus, close
monitoring to control cardiovascular risk factors is recommended in
the Kazakh population.
CBS, one of the three major enzymes involved in Hcy
metabolism, catalyzes the initial step of the transsulfuration
pathway, which condenses Hcy and serine to produce cystathionine
and ultimately cysteine (18).
Kozich and Kraus (19) used a
bacterial expression system and western blot analysis to
demonstrate that unstable CBS subunits were formed by clones
containing the I278T (c.833T>C) mutation. They suggested that
the substitution of hydrophobic isoleucine by a more hydrophilic
threonine residue may affect the CBS conformation or the
interaction of the subunits, which may result in an unstable
tetrameric CBS. The human CBS gene is located at chromosome 21q22.3
(20). A total of 132 mutations in
CBS have been identified to date, with the majority of these being
missense mutations. Furthermore, 92 disease-associated mutations
have been identified in the CBS gene (21). The most frequently occurring of
these is I278T (c.833T>C), which substitutes threonine for
isoleucine at codon 278. A number of studies investigating the CBS
T833C polymorphism have focused on CVD (22,23).
A meta-analysis by Ding et al indicated that the CBS T833C
polymorphism is associated with an increased risk of stroke, and
that the C allele is likely to be an important risk factor for
stroke (24). Fewer studies,
however, have investigated its association with hypertension,
especially in the Kazakh population.
To the best of our knowledge, the current study is
the first study to demonstrate a correlation between the CBS T833C
allele and hypertension in the Kazakh population (21). The present study confirmed the
presence of the CBS T833C polymorphism in the Kazakh population and
detected the frequency of the TT, CT and CC genotypes, which were
51.9, 33.9 and 14.1%, respectively, in the hypertensive group, and
48.1, 35.2 and 15.4%, respectively, in the normotensive group. No
statistically significant difference was identified in the genotype
distribution between the two groups. Furthermore, the frequency of
the T allele genotype in subjects with hypertension (68.9%) was not
statistically different from that of subjects with normotension
(65.7%), which demonstrated that a single gene mutation does not
have a significant influence on hypertension in the Kazakh
population. When comparing the clinical characteristics among
different CBS T833C genotypes, the present study demonstrated
significant correlations not only between CBS T833C and BMI and TG,
but also between CBS T833C and Hcy. The C allele was correlated
with increased BMI, TG and Hcy. Multivariate logistic analyses
revealed that BMI, TC, smoking, level of plasma Hcy and a family
history of hypertension, but not CBS T833C, were independent risk
factors for hypertension in the Kazakh population in Xinjiang. This
indicated that not only the T833C mutation of the CBS gene but
other factors, including age, smoking and the levels of TG and LDL,
were associated with Hcy metabolism.
Numerous studies have focused on investigating the
association between the methylene tetrahydrofolate reductase
(MTHFR) C677T genetic polymorphism and primary hypertension in the
Chinese population. Zong et al (25) demonstrated that the homozygous
C677T mutation of the MTHFR gene was a major heredity factor for
increased levels of Hcy and H-type hypertension in the Chinese
population, and that this was also related to gender. However,
other studies did not observe the same association between the Hcy
gene polymorphisms and primary hypertension (26–28).
Though the study by Zong et al revealed that Hcy was an
independent risk factor of primary hypertension in the Kazakh
population, it did not reveal that the CBS T833C mutations were
associated with primary hypertension in the Kazakh population,
which further demonstrates that a single factor and/or gene locus
is responsible for hypertension, in particular, in the Kazakh
population in Xinjiang. In addition, due to the complexity of the
Hcy metabolic pathway, the Hcy metabolism may have been affected by
certain interactions between the three enzymes involved. Therefore,
further large-scale studies are required to be carried out among
the Kazakh population in the future, in particular examining
polygenes and multiple mutations.
Acknowledgements
This study was funded by grant no. 2012211A082 from
the Natural Science Foundation of Xinjiang (Xinjiang, China).
References
|
1
|
Fowdar JY, Lason MV, Szvetko AL, Lea RA
and Griffiths LR: Investigation of homocysteine-pathway-related
variants in essential hypertension. Int J Hypertens.
2012:1909232012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singh M, Mensah GA and Bakris G:
Pathogenesis and clinical physiology of hypertension. Cardiol Clin.
28:545–559. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Towfighi A, Markovic D and Ovbiagele B:
Pronounced association of elevated serum homocysteine with stroke
in subgroups of individuals: a nationwide study. J Neurol Sci.
298:153–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chambers JC, Obeid OA, Refsum H, Ueland P,
Hackett D, Hooper J, Turner RM, Thompson SG and Kooner JS: Plasma
homocysteine concentrations and risk of coronary heart disease in
UK Indian Asian and European men. Lancet. 355:523–527. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsai MY, Bignell M, Yang F, Welge BG,
Graham KJ and Hanson NQ: Polygenic influence on plasma
homocysteine: association of two prevalent mutations, the 844ins68
of cystathionine β-synthase and A2756G of methionine synthase, with
lowered plasma homocysteine levels. Atherosclerosis. 149:131–137.
2000.PubMed/NCBI
|
|
6
|
Kruger WD, Wang L, JHee KH, Singh RH and
Elsas LJ II: Cystathionine β-synthase deficiency in Georgia (USA):
correlation of clinical and biochemical phenotype with genotype.
Hum Mutat. 22:434–441. 2003.
|
|
7
|
Wu JM, Wang TG, Li YQ, Song XW, Liu YY,
Yun HR, Zhong ZY and Zhou TH: Genetic mutations of homocysteine
metabolism related enzymes in patients with ischemic stroke. Yi
Chuan. 26:298–302. 2004.(In Chinese).
|
|
8
|
Lv YL, Li JZ, Mi MR, Wei HX and Liu XD:
Correlation of cystathionine β synthase gene polymorphisms and risk
of cerebral arterial thrombosis in Chinese population: a
meta-analysis. Chin J Evid-based Med. 6:674–677. 2010.(In
Chinese).
|
|
9
|
Li NF, Zhang JH, Chang JH, Yang J, Wang
HM, Zhou L and Luo WL: Association of genetic variations of the
prostasin gene with essential hypertension in the Xinjiang Kazakh
population. Chin Med J (Engl). 124:2107–2112. 2011.PubMed/NCBI
|
|
10
|
Liu LS; Writing Group of 2010 Chinese
Guidelines for the Management of Hypertension. 2010 Chinese
guidelines for the management of hypertension. Zhonghua Xin Xue
Guan Bing Za Zhi. 39:579–615. 2011.(In Chinese).
|
|
11
|
Frantzen F, Faaren AL, Alfheim I and
Nordhei AK: Enzyme conversion immunoassay for determining total
homocysteine in plasma or serum. Clin Chem. 44:311–316.
1998.PubMed/NCBI
|
|
12
|
Pepe G, Vanegas OC, Rickards O, Giusti B,
Comeglio P, Brunelli T, Marcucci R, Prisco D, Gensini GF and Abbate
R: World distribution of the T833C/844INS68 CBS in cis double
mutation: a reliable anthropological marker. Hum Genet.
104:126–129. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Malinowska A and Chmurzynska A:
Polymorphism of genes encoding homocysteine metabolism-related
enzymes and risk for cardiovascular disease. Nutr Res. 29:685–695.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hao L, Ma J, Zhu J, Stampfer MJ, Tian Y,
Willett WC and Li Z: High prevalence of hyperhomocysteinemia in
Chinese adults is associated with low folate, vitamin B-12, and
vitamin B-6 status. J Nutr. 137:407–413. 2007.PubMed/NCBI
|
|
15
|
Must A, Jacques PF, Rogers G, Rosenberg IH
and Selhub J: Serum total homocysteine concentrations in children
and adolescents: results from the third National Health and
Nutrition Examination Survey (NHANES III). J Nutr. 133:2643–2649.
2003.
|
|
16
|
Jacques PF, Rosenberg IH, Rogers G, Selhub
J, Bowman BA, Gunter EW, Wright JD and Johnson CL: Serum total
homocysteine concentrations in adolescent and adult Americans:
results from the third National Health and Nutrition Examination
Survey. Am J Clin Nutr. 69:482–489. 1999.
|
|
17
|
Malinow MR, Ducimetiere P, Luc G, Evans
AE, Arveiler D, Cambien F and Upson BM: Plasma homocyst(e)ine
levels and graded risk for myocardial infarction: findings in two
populations at contrasting risk for coronary heart disease.
Atherosclerosis. 126:27–34. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miles EW and Kraus JP: Cystathionine
β-synthase: structure, function, regulation, and location of
homocystinuria-causing mutations. J Biol Chem. 279:29871–29874.
2004.
|
|
19
|
Kozich V and Kraus JP: Screening for
mutations by expressing patient cDNA segments in E. coli:
homocystinuria due to cystathionine β-synthase deficiency. Hum
Mutat. 1:113–123. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Münke M, Kraus JP, Ohura T and Francke U:
The gene of cystathionine β-synthase (CBS) maps to the subtelomeric
region on human chromosome 21q and to proximal mouse chromosome 17.
Am J Hum Genet. 42:550–559. 1988.
|
|
21
|
Kraus JP, Janosík M, Kozich V, Mandell R,
et al: Cystathionine β-synthase mutations in homocystinuria. Hum
Mutat. 13:362–375. 1999.
|
|
22
|
Carnicer R, Navarro MA, Arbonés-Mainar JM,
Arnal C, et al: Genetically based hypertension generated through
interaction of mild hypoalphalipoproteinemia and mild
hyperhomocysteinemia. J Hypertens. 25:1597–1607. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grobelny BT, Ducruet AF, DeRosa PA,
Kotchetkov IS, Zacharia BE, et al: Gain-of-function polymorphisms
of cystathionine β-synthase and delayed cerebral ischemia following
aneurysmal subarachnoid hemorrhage. J Neurosurg. 115:101–107.
2011.PubMed/NCBI
|
|
24
|
Ding R, Lin S and Chen D: The association
of cystathionine β synthase (CBS) T833C polymorphism and the risk
of stroke: a meta-analysis. J Neurol Sci. 312:26–30. 2012.
|
|
25
|
Zong YH, Li XY, Chen GL, Xu XP, Li JP, Huo
Y, Wei JH and Zhao LS: Homocysteine level and N5,
10-methylenetetrahydrofolate reductase gene polymorphism in
hypertensive population. J Clin Cardiol. 27:203–207. 2011.(In
Chinese).
|
|
26
|
Wang H, Wu GZ, Zhang Y, Zhang XY, Chen YZ
and Sikaer A: Association of homocysteine and its metabolic enzyme
genes polymorphisms with essential hypertension in Xinjiang
Kazakhs. J Clin Rehabil Tissue Eng Res. 33:6247–6252. 2010.(In
Chinese).
|
|
27
|
Wang H, Zhang Y, Zhang XY, Wu GZ, Chen YL
and Sikaer A: Relationship of homocysteine and methionine synthase
A2756G polymorphisms with essential hypertension in Kazak
nationality in Xinjiang. J Chin Pract Diagn Ther. 24:879–882.
2010.(In Chinese).
|
|
28
|
Zhang Y, Wang H, Zhang XY, Wang L and Wu
GZ: Relationship between homocysteine, methylene tetrahydrofolate
reductase C677T polymorphisms and essential hypertension in Kazak
nationality in Xinjiang. J Clin Cardiol. 28:570–573. 2012.(In
Chinese).
|