Introduction
Agranulocytosis, a rare antithyroid drug-induced
complication that may be life threatening, is characterized by a
marked reduction in the number of circulating granulocytes and a
neutrophil count of <0.5×109/l (1). Agranulocytosis usually occurs within
the first 2–3 months of treatment (2); however, certain cases have
demonstrated that agranulocytosis may occur following long-term
treatment (3). Previously reported
cases of agranulocytosis have been due to continuous antithyroid
drug (ATD) treatment; however, in the present study a case of
ATD-induced agranulocytosis occurring following the discontinuation
of methimazole (MMI) treatment for 4 months is presented. This
report, to the best of our knowledge, is the first time that a case
of agranulocytosis following discontinued MMI has been
reported.
Case report
This study was approved by the Ethics Committee of
People’s Hospital Of New District Longhua Shenzhen (Shenzhen,
China). Written informed consent was obtained from the patient. A
27-year-old female was admitted to People’s Hospital Of New
District Longhua Shenzhen in May 2013 with complaints of fever,
sore throat and hypodynamia for three days. Three days prior to
admission, the patient started to feel cold and had a fever,
although the temperature was not taken, and the patient experienced
a sore throat, dizziness and hypodynamia with unknown causes. Two
days prior to admission, the patient started vomiting twice a day;
the amount of vomit was unknown. The patient did not have a
headache, chest distress, cardiopalmus, abdominal pain, diarrhea,
cough or expectoration. One day prior to admission, the patient
visited the local Community Health Service, and the results from
the laboratory tests were as follows: white blood cell (WBC) count,
1.28×109/l; neutrophils, 0.04×109/l;
hemoglobin, 143 g/l; and platelets, 231×109/l. The
patient was prescribed cefathiamidine, vitamin C intravenous drip,
once daily (qd) and 4 compound Coptidis Rhizome capsules, twice
daily (bid). However, the patient’s condition did not improve and
the patient was transferred to People’s Hospital Of New District
Longhua Shenzhen the following day.
The patient had a history of hyperthyroidism. Four
years previously, the patient had been diagnosed with
hyperthyroidism, and prescribed methimazole [MMI; 10 mg; three
times a day (tid)] and propanolol (10 mg; tid). After one month,
the dose of MMI was gradual reduced to 10 mg, bid; after 4 months,
MMI was reduced to 10 mg, qd; and after 6 months, MMI was reduced
to 5 mg, qd, and this dosage was maintained for 18 months. The
propanolol treatment was initiated to maintain the heart rate; when
the heart rate returned to normal, the propanolol treatment was
discontinued. During the initial treatment, blood samples were
analyzed once a week; following this, they were analyzed once a
month, and the results of these blood tests were normal. The course
of treatment was 2 years. The patient made a full recovery from
hyperthyroidism and MMI was discontinued. Six months previously,
the patient’s hyperthyroid symptoms returned, and the patient was
prescribed 10 mg MMI, tid and 10 mg propanolol, tid, at a local
hospital. After two months of treatment, the patient refused to
continue taking the medicine as she considered it to be
ineffective. The patient had no history of hypertension, diabetes,
kidney disease or other chronic diseases, or tuberculosis,
hepatitis, typhoid fever or other infectious diseases. In addition,
the patient had no history of trauma surgery and no a history of
medicine or food allergies. The previous vaccination history of the
patient was unknown. On admission to hospital, the results from the
physical examination were as follows: temperature, 38.6°C; pulse,
138 beats/min; respiratory rate, 30 breaths/min and blood pressure,
137/94 mmHg. No proptosis was observed and general superficial
lymph node enlargement was not palpable. The patient had pharyngeal
congestion, a 2-fold enlarged thyroid, bilateral tonsil enlargement
of II degree, and visible purulent secretions on the left side of
the tonsil. The bilateral thyroid enlargement was of II degree, but
no vascular murmurs were audible. The patient had a mild hand
tremor; however, no rash or jaundice was observed. The results from
the blood tests showed an absolute neutrophil count of zero and a
total WBC count of 0.50×109/l. The differential count
showed 2.2% neutrophils (reference range, 45.0–73.0%), 95%
lymphocytes (reference range, 20.0–40.0%), 0.7% monocytes
(reference range, 5.0–11.0%), 0% eosinophils (reference range,
0.5–5.0%) and 0.8% basophils (reference range, 0.0–1.0%). The
hemoglobin concentration and platelet count were normal at 134 g/l
(reference range, 110–150 g/l) and 190×109/l (reference
range, 100–360×109/l), respectively. The erythrocyte
sedimentation rate was 33 mm/h (reference range, 0–10 mm/h).
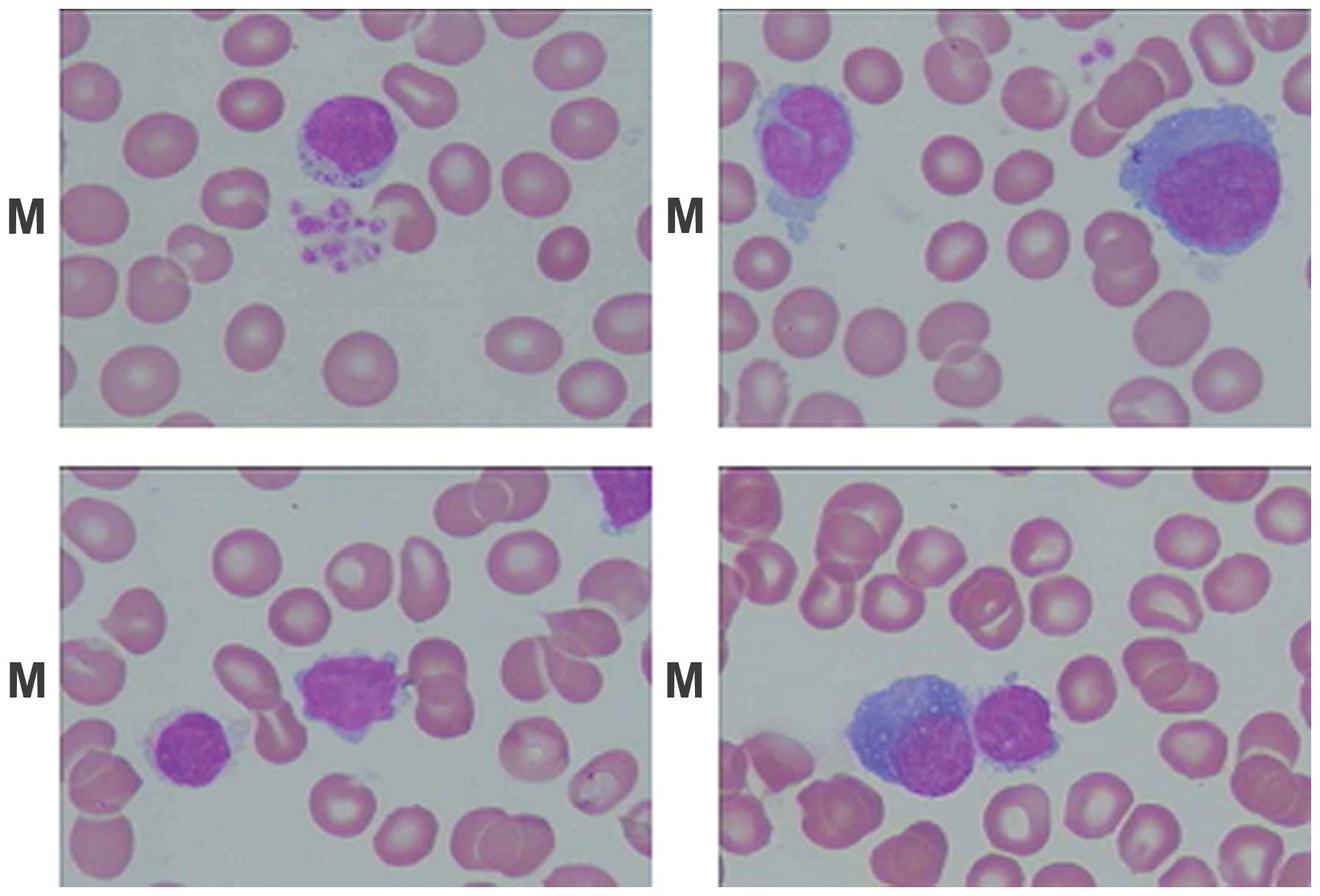
Results from the marrow biopsy showed hyperplasia with karyocyte
proliferation. The granulocyte precursors were normal; however, the
ratio of granulocyte to erythrocyte (G:E) counts was low. The ratio
of leukomonocytes was 79% (reference range, 15.74–29.82%) and the
heterology ratio of leukomonocytes was 3% (Fig. 1). Thyroidal function tests were as
follows: free T3, 12.58 pmol/l (reference range, 3.80–6.00 pmol/l);
free T4>80.45 pmol/l (reference range, 7.86–14.41 pmol/l); and
thyroid-stimulating hormone (TSH), 0.01 mIU/l (reference range,
0.34–5.60 mIU/l). The results from the ultrasonograph showed
diffuse goiter and normal liver. The results from the
electrocardiogram (ECG) revealed sinus tachycardia, and chest X-ray
showed no abnormalities. Based on these results, the patient was
diagnosed with acute agranulocytosis, diffuse toxic goiter, thyroid
crisis and acute tonsillitis.
On admission to People’s Hospital Of New District
Longhua Shenzhen, the patient was prescribed oxygen, ECG,
hydrocortisone to improve the patient’s stress response,
propranolol to inhibit T4 transformation into T3 and to inhibit
excitatory effects on the heart, and intravenous antibiotics
(piperacillin and tazobactam) to control infection.
Granulocyte-colony stimulating factor (GCSF) was administered to
raise neutrophil numbers, with an initial dose of 100 μg/day. Due
to poor response and sustained agranulocytosis, the dosage was
increased to 300 μg/day after six days. After two days, the
neutrophil count was increased to 0.02×109/l; after four
days, the neutrophil count was increased to 0.07×109/l;
and after six days, the neutrophil count was increased to
2.46×109/l (Table I).
After 10 days of treatment, the neutrophil count was increased to
4.53×109/l, and the patient’s symptoms were generally
improved; the patient no longer had a fever, sore throat or
hypodynamia. When discharged from the hospital, the patient was
prescribed oral I-131.
 | Table IChanges of selective indices observed
in the patient during treatment. |
Table I
Changes of selective indices observed
in the patient during treatment.
| Parameters
studied | Day-1 | Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 10 |
|---|
| WBC count
(x109/l) | 1.28 | 0.50 | 0.50 | 0.57 | 0.92 | 1.42 | 2.22 | 5.86 | 6.53 |
| Neutrophil ratio
(%) | 3.5 | 2.2 | 1.3 | 3.9 | 4.7 | 5.2 | 30.1 | 42 | 65 |
| Neutrophil count
(x109/l) | 0.05 | 0.0 | 0.01 | 0.02 | 0.04 | 0.07 | 0.67 | 2.46 | 4.53 |
| HGB (g/l) | 134 | 134 | 111 | 111 | 111 | 107 | 121 | 117 | 119 |
| PLT
(x109/l) | 231 | 190 | 153 | 146 | 147 | 125 | 133 | 110 | 130 |
| Free T3
(pmol/l) | | | 12.58 | | | | | | |
| Free T4
(pmol/l) | | | 80.45 | | | | | | |
| TSH (mIU/l) | | | 0.01 | | | | | | |
Discussion
ATDs, in particular thioamides, including MMI,
propylthiouracil and carbimazole, have adverse hematological
effects, ranging from mild leucopenia to agranulocytosis and
aplastic anemia. The incidence of ATD-induced agranulocytosis in
patients with hyperthyroidism is rare; however, serious and
potentially life-threatening adverse effects may occur, mainly due
to severe systemic infection, if appropriate medical intervention
is not administered immediately. ATD-induced agranulocytosis
usually occurs within 2 or 3 months of ATD treatment; however, in
certain cases this may be delayed. By reviewing previous studies,
it was identified that the previously reported cases all occurred
following continuous ATD treatment. In the present study, a case of
ATD-induced agranulocytosis following treatment with MMI that was
discontinued for four months is presented.
ATD-induced agranulocytosis is mediated by a variety
of mechanisms, including direct toxic effects and immunological
reactions. ATDs readily penetrate the marrow, affecting oxygen and
glucose utilization of leukocytes through their oxidized
metabolites (4). Toxic effects
require between 20 and 40 days of exposure, and the onset is
insidious. It is usually dose- and concentration-dependent
(5), and is associated with
continuous administration. However, the present report is not in
accordance with this. In addition, damage to stem cells or
granulocytic precursors in the bone marrow prevents the
differentiation of granulocytes, without affecting the peripheral
pool of neutrophils.
Wall et al (6) observed that in vitro
peripheral lymphocyte transformation in response to ATD and
circulating antibodies against neutrophils were significant in
patients with ATD-induced agranulocytosis compared with control
patients (6). Using direct
immunofluorescence tests, a transient autoantibody response in
patients with agranulocytosis has been shown to be induced by
propylthiouracil (7). This
ATD-induced specific immune-mediated response reacted not only with
mature granulocytes, but also affected mature blood cells and
myeloid progenitor cell growth. These results suggest an
immune-mediated mechanism rather than direct toxic effects of the
drug. The immune-mediated destruction of mature neutrophils was the
first mechanism to be identified as a cause of ATD-induced
agranulocytosis. Sprikkelman et al (8) described four different immunological
mechanisms that may be responsible (8). Firstly, antibodies may develop
against the antithyroid drug when it is bound to the cell membrane
of the granulocyte, resulting in an accelerated destruction of the
granulocyte. Secondly, antibodies may target the drug/metabolite
complex that has been adsorbed to the neutrophil granulocyte in the
presence of plasma component. Thirdly, the drug may trigger the
production of auto-antibodies. Finally, the interaction of a
granulocyte antigen and drug may induce the production of
antibodies. In addition, other immunological reactions include the
immunoglobulin E (IgE)-mediated hypersensitivity reaction,
drug-induced IgG and IgM responses and antineutrophil cytoplasm
antibody (ANCA)-associated immune injury, which may contribute to
agranulocytosis (9–11). In the present case, the patient was
diagnosed with agranulocytosis after MMI was discontinued for four
months. A review of previous studies found no similar report.
Although ATD-induced agranulocytosis is considered to be mediated
primarily by immunological mechanisms, this does not explain the
pathogenesis of the present patient, and further investigation is
required.
In conclusion, ATD-induced agranulocytosis is a rare
but potentially fatal idiosyncratic reaction. It is not usually
possible to predict which patients are likely to be susceptible, so
conducting a routine complete blood cell count is suggested, and
informing the patient of the common symptoms of agranulocytosis may
contribute to an early diagnosis. Admission to hospital and
treatment with GCSF may accelerate neutrophil recovery. The present
case report aims to increase the awareness of the onset
agranulocytosis from discontinued MMI treatment, and warn that MMI
should be used with caution.
References
|
1
|
Andres E, Dali-Youcef N, Serraj K and
Zimmer J: Recognition and management of drug-induced
blood-cytopenias: the example of idiosyncratic drug-induced
thrombocytopenia. Expert Opin Drug Saf. 8:183–190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tajiri J and Noguchi S: Antithyroid
drug-induced agranulocytosis: special reference to normal white
blood cell count agranulocytosis. Thyroid. 14:459–462. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tamai H, Takaichi Y, Morita T, Komaki G,
et al: Methimazole-induced agranulocytosis in Japanese patients
with Graves’ disease. Clin Endocrinol (Oxf). 30:525–530. 1989.
|
|
4
|
Waldhauser L and Uetrecht J: Oxidation of
propylthiouracil to reactive metabolites by activated neutrophils.
Implications for agranulocytosis. Drug Metab Dispos. 19:354–359.
1991.PubMed/NCBI
|
|
5
|
Pisciotta AV: Immune and toxic mechanisms
in drug-induced agranulocytosis. Semin Hematol. 10:279–310.
1973.PubMed/NCBI
|
|
6
|
Wall JR, Fang SL, Kuroki T, Ingbar SH and
Braverman LE: In vitro immunoreactivity to propylthiouracil,
methimazole, and carbimazole in patients with Graves’ disease: a
possible cause of antithyroid drug-induced agranulocytosis. J Clin
Endocrinol Metab. 58:868–872. 1984.PubMed/NCBI
|
|
7
|
Toth EL, Mant MJ, Shivji S and Ginsberg J:
Propylthiouracil induced agranulocytosis: an unusual presentation
and a possible mechanism. Am J Med. 85:725–727. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sprikkelman A, de Wolf JT and Vellenga E:
The application of hematopoietic growth factors in drug-induced
agranulocytosis: a review of 70 Cases. Leukemia. 8:2031–2036.
1994.PubMed/NCBI
|
|
9
|
Sun MT, Tsai CH and Shih KC: Antithyroid
drug-induced agranulocytosis. J Chin Med Assoc. 72:438–441. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akamizu T, Ozaki S, Hiratani H, et al:
Drug-induced neutropenia associated with anti-neutrophil
cytoplasmic antibodies (ANCA): possible involvement of complement
in granulocyte cytotoxicity. Clin Exp Immunol. 127:92–98. 2002.
View Article : Google Scholar
|
|
11
|
Harper L, Chin L, Daykin J, et al:
Propylthiouracil and carbimazole associated- antineutrophil
cytoplasmic antibodies (ANCA) in patients with Graves’ disease.
Clin Endocrinol (Oxf). 60:671–675. 2004.
|