Introduction
The development of gastric cancer involves several
factors, among which Helicobacter pylori infection is the
most important (1). H.
pylori is a type of micro-aerobic, Gram-negative, spiral
bacterium, which resides between the gastric mucous layer and the
gastric surface. H. pylori secretes urease, which damages
the gastric mucosal barrier. The H. pylori
lipopolysaccharide inhibits the binding of laminin to its receptor,
resulting in gastric mucosal injury (2). The vacuolating toxin gene of H.
pylori can change ion permeability, leading to cell
degeneration (3), and damage the
gastric mucosa, causing erosion or ulceration. H. pylori
expresses cytotoxin-associated gene (Cag) A, which generates a
cytotoxic effect and induces inflammatory and immune responses. The
deformation and necrosis of mucosal cells and inflammatory
infiltration can be observed in H. pylori-infected lesions,
and specific antibodies can be detected in serum (4,5).
Matrix metalloproteinases (MMPs) are highly
homologous, zinc-dependent endopeptidases that can degrade the
basement membrane. To date, 19 types of MMPs have been identified.
All MMPs are secreted by mesenchymal cells in the form of a
protease precursor, and can be inhibited by tissue inhibitors of
metalloproteinases (TIMPs). It has been found that MMPs are
important in tumor invasion, metastasis, cardiovascular disease and
diabetes, and are closely associated with the degradation of the
extracellular matrix (ECM) of tumor cells (6).
Invasion and metastasis are the prominent features
of malignant tumors, and the degradation of the ECM is one of the
key steps involved in these processes. Matrix degradation primarily
depends on proteolytic enzymes. An increasing number of studies
have shown that the invasion and metastasis of tumor cells are
closely associated with MMP production. In vitro and in
vivo studies have shown that MMPs are functional in various
stages of tumor progression, influencing tumor genesis, growth,
angiogenesis and metastasis (7–9). The
interaction of MMPs with the basement membrane is the initiating
signal of tumor invasion and metastasis.
As mentioned above, tumor invasion and metastasis
involve the secretion of MMPs. MMP-1 predominantly degrades the
stroma, which is closely associated with local invasion and
metastasis (10,11). MMP-10 is considered to be the most
important factor in human tumor cells that can activate other MMP
(such as MMP-1) precursors (12).
MMP-10 can also degrade certain interstitial proteins, contributing
to metastasis (13).
In this study, in order to determine the effect of
H. pylori infection on gastric cancer, the associations
among H. pylori infection, the expression of MMP-1 and
MMP-10 and the invasion and metastasis of gastric cancer were
investigated. H. pylori infection and MMP-1 and MMP-10
expression were detected in gastric cancer and chronic gastritis
specimens. The association between H. pylori infection and
the clinical pathological features of gastric cancer were then
analyzed. Additionally, the expression of MMP-1 and MMP-10
following H. pylori infection in MGC-803 cells was
studied.
Materials and methods
Cell line and reagents
The MGC-803 human gastric cancer cell line, a poorly
differentiated gastric adenocarcinoma, was provided by the
Department of School of Biological Science, Shandong Normal
University (Jinan, China) and preserved in the Central Laboratory
of the First Affiliated Hospital of Nanhua University (Hengyang,
China). MGC-803 cells were cultured in high-glucose Dulbecco’s
modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine
serum, 100 U/ml penicillin and 100 mg/ml streptomycin at 37°C in a
humidified incubator with 5% CO2.
A Streptavidin-peroxidase (SP) immunohistochemistry
kit, hematoxylin stain and 3,3′ diaminobenzidine (DAB) chromogenic
agent were all purchased from Fuzhou Maixin Biotechnology
Development Co., Ltd. (Fuzhou, China). Mouse anti-human MMP-1 and
MMP-10 monoclonal antibodies for immunohistochemistry and western
blotting were purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). Anti-mouse immunoglobulin G (IgG) was purchased
from Boster Biological Technology, Ltd. (Wuhan, China). The ELISA
kit was obtained from Bio-Check, Inc. (Foster City, CA, USA) and
the bicinchoninic acid assay reagent was purchased from Pierce
Chemical Co. (Rockford, IL, USA).
Patient samples
Between 2005 and 2009, 80 cases of surgically
resected and pathologically diagnosed gastric cancer paraffin
specimens from the First Affiliated Hospital of Nanhua University
were collected. The patients with gastric cancer were aged between
23 and 78 years, with a mean age of 53.2±12.7 years, and included
58 males and 22 females. Prior written and informed consent was
obtained from every patient and the study was approved by the
Ethics Review Board of Nanhua University. Of the 80 cases of
gastric cancer, there were 20 cases of highly differentiated
carcinoma, 20 cases of moderately differentiated carcinoma and 40
cases of poorly differentiated carcinoma. A total of 20 cases of
gastric cancer exhibited lymph node metastasis. With regard to the
extent of infiltration, there were 15 cases of early gastric cancer
with gastric mucosal tissue infiltration limited to the mucosa or
submucosal layer (regardless of lymph node metastasis) and 65 cases
of advanced gastric cancer. Forty specimens of chronic superficial
gastritis were collected as the control group. All specimens were
fixed in formalin and embedded in paraffin. Specimens were sliced
into 5-μm sections.
Infection of MGC-803 cells with H.
pylori
A total of 1×105 MGC-803 cells were
seeded in T75 tissue culture flask and 6 ml serum-free DMEM was
added. After 24 h of synchronization, the culture medium was
discarded. H. pylori culture medium was then added.
According to the method described in a previous study (4), bacteria density was adjusted to
1×108 CFU/ml, and two-fold diluted with serum-free DMEM.
The MGC-803 cells were then co-cultured with H. pylori for
6, 12, 24 and 48 h.
ELISA
Fasting blood (2 ml) was collected from all
patients. For H. pylori IgG detection, ELISA was performed
according to the manufacturer’s instructions (Bio-Check, Inc.).
H. pylori IgG levels >20 U/ml were considered to be a
positive result.
Immunohistochemistry
Immunohistochemical staining (the SP method) was
conducted to detect the protein expression of MMP-1 and MMP-10 in
gastric cancer and chronic gastritis samples. Briefly, the samples
were fixed in formaldehyde and embedded in paraffin. The sections
were dewaxed, rehydrated in graded alcohols and processed prior to
incubation with antibodies. Subsequent to blocking, the sections
were incubated with primary antibodies at 37°C in the dark for 1 h.
Secondary antibodies were then added and incubated in dark for 30
min following washing with phosphate-buffered saline (PBS). The
sections were then developed with DAB chromogenic reagent and
counterstained with hematoxylin. The working concentration of MMP-1
and MMP-10 primary antibody was 1:200. PBS was used in the negative
control group instead of primary antibody. Positive staining for
MMP-1 and MMP-10 was located in the cytoplasm. A total of ≥10
high-power fields were randomly selected (magnification, ×200), and
≥1,000 cells were counted. The staining intensity and percentage of
positive cells was scored in each slice. Staining intensity was
defined as 0 points for no staining, 1 point for pale yellow, 2
points for brownish yellow and 3 points for tan. The positive
staining cell ratio was defined as 0 points for no staining, 1
point for <30%, 2 points for 30–60% and 3 points for >60%.
The combined points total for the staining intensity and positive
staining ratio was furthermore defined as follows: 0–2 points, weak
positive or negative; 3–4 points, positive; and 5–6 points, strong
positive. The rate of positive staining was calculated using the
following formula: Positive rate = (weak positive case number +
strong positive case number)/total cases × 100%.
Western blotting
At 6, 12, 24 and 48 h after H. pylori
infection, total proteins were extracted from MGC-803 cells and
separated using SDS-PAGE. The proteins were then transferred onto a
nitrocellulose membrane. Subsequent to blocking with non-fat milk,
the membrane was incubated with monoclonal mouse anti-MMP-1 and
-MMP-10 primary antibodies overnight at 4°C. The membrane was then
washed and incubated with the anti-mouse IgG secondary antibody at
37°C for 1 h, prior to being developed using enhanced
chemiluminescence plus reagent (Amersham, GE Healthcare Life
Sciences, Piscataway, NJ, USA). β-actin was used as an internal
control.
Statistical analysis
SPSS 13.0 (SPSS, Inc., Chicago, IL, USA) software
was used for statistical analysis. The immunohistochemical results
were compared using a χ2 test. The Spearman’s rho
correlation test was used to evaluate the correlations between
H. pylori infection and MMP-1 and MMP-10 expression in
gastric cancer. P<0.05 was considered statistically
significant.
Results
MMP-1 and MMP-10 protein expression in
gastric cancer is higher than that in chronic gastritis
To determine MMP-1 and MMP-10 protein expression in
gastric cancer and chronic gastritis, immunohistochemical staining
was performed. Representative immunohistochemical staining results
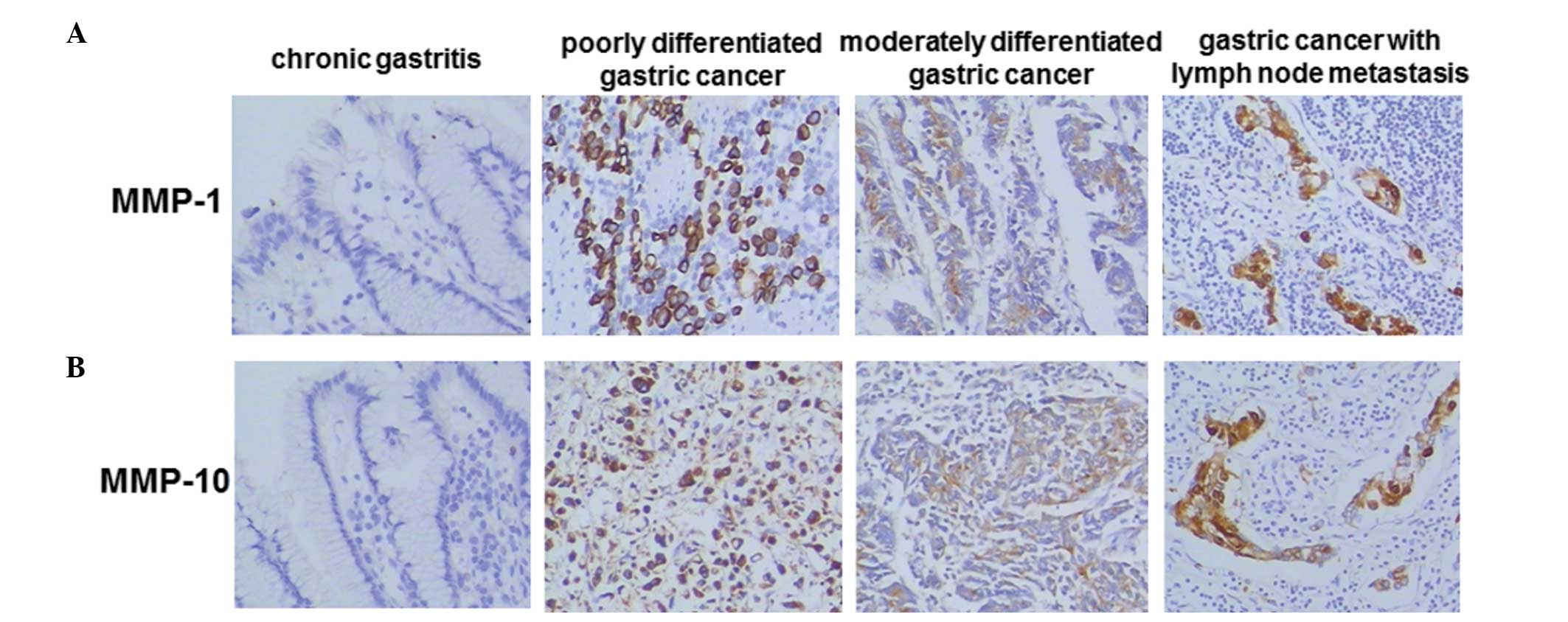
are shown in Fig. 1 and
quantitative results are shown in Table I. As shown in Fig. 1, MMP-1 and MMP-10 expression was
not detected in the chronic gastritis specimens. However, positive
staining for MMP-1 and MMP-10 was detected in the poorly and
moderately differentiated gastric cancer and gastric cancer with
lymph node metastasis specimens. The positive staining rate was
calculated as described in the Materials and methods section. As
shown in Table I, the
MMP-1-positive rate in patients with gastric cancer, metastatic
gastric cancer and chronic gastritis was 78.8% (63/80), 95.0%
(19/20) and 25.0% (10/40), respectively. The MMP-10-positive rate
in the patients with gastric cancer, metastatic gastric cancer and
chronic gastritis was 83.4% (67/80), 95.0% (19/20) and 30.0%
(12/40), respectively. Statistically, the expression levels of
MMP-1 and MMP-10 were significantly higher in the gastric cancer
specimens than those in the chronic gastritis specimens
(P<0.05). Furthermore, compared with levels in the gastric
cancer specimens, the expression levels of MMP-1 and MMP-10 in the
metastatic gastric cancer specimens were significantly higher
(P<0.05). Therefore, these results show that expression levels
of MMP-1 and MMP-10 were significantly increased in gastric cancer
and particularly in metastatic gastric cancer.
 | Table IMMP-1 and MMP-10 expression in
specimens of gastric cancer and chronic gastritis. |
Table I
MMP-1 and MMP-10 expression in
specimens of gastric cancer and chronic gastritis.
| Type of MMP and
specimen | Cases (n) | Immunohistochemical
score | P-value |
|---|
|
|---|
| Weak positive, 0–2
(n) | Positive, 3–4
(n) | Strong positive, 5–6
(n) |
|---|
| MMP-1 |
| Gastric cancer | 80 | 17 | 19 | 44 | 0.004a |
| Metastatic gastric
cancer | 20 | 1 | 1 | 18 | <0.001b |
| Chronic
gastritis | 40 | 30 | 9 | 1 | <0.001c |
| MMP-10 |
| Gastric cancer | 80 | 13 | 19 | 48 | 0.036 a |
| Metastatic gastric
cancer | 20 | 1 | 2 | 17 | <0.001b |
| Chronic
gastritis | 40 | 28 | 10 | 2 | <0.001c |
H. pylori infection rate in gastric
cancer is higher than that in chronic gastritis
To detect the H. pylori infection level in
gastric cancer and chronic gastritis specimens, fasting blood was
collected and H. pylori IgG levels were measured by ELISA.
There were 62 cases of H. pylori infection out of 80 gastric
cancer cases with a positive rate of 77.5%, and 13 cases of H.
pylori infection out of 40 chronic gastritis cases with a
positive rate of 32.5%. Significantly higher levels of H.
pylori infection were observed in the gastric cancer specimens
than those in the chronic gastritis specimens (P<0.05, data not
shown). This result suggests that there was significant H.
pylori infection in gastric cancer.
Correlation between H. pylori infection,
MMP-1 and MMP-10 protein expression and gastric cancer pathological
characteristics
To determine the roles of H. pylori infection
and MMP-1 and MMP-10 protein expression in gastric cancer, the
association between these factors and the clinical pathological
characteristics of gastric cancer were analyzed. As shown in
Table II, positive H.
pylori infection and positive expression of MMP-1 and MMP-10
were associated with lymph node metastasis and clinical stage
(P<0.05), but not with age, gender or differentiation degree
(P>0.05). To further analyze the associations among H.
pylori infection and MMP-1 and MMP-10 expression, correlation
analyses were performed. It was revealed that MMP-1 and MMP-10
expression was significantly positively correlated in gastric
cancer (r=0.8321, P<0.05; data not shown). H. pylori
infection and MMP-1 and MMP-10 expression were also significantly
positively correlated (r=0.8718, P<0.05 and r=0.5477, P<0.05,
respectively; data not shown). Thus, these data suggest that
associations exist among H. pylori infection, MMP-1
expression and MMP-10 expression in the development and metastasis
of gastric cancer.
 | Table IICorrelation analysis of
Helicobacter pylori infection, MMP-1 and MMP-10 protein
expression and gastric cancer pathological characteristics. |
Table II
Correlation analysis of
Helicobacter pylori infection, MMP-1 and MMP-10 protein
expression and gastric cancer pathological characteristics.
| | H. pylori
infection | | MMP-1 expression | | MMP-10
expression | |
|---|
| |
| |
| |
| |
|---|
| Parameter | Cases (n) | Positive (n) | Negative (n) | P-value | Positive (n) | Negative (n) | P-value | Positive (n) | Negative (n) | P-value |
|---|
| Age in years | | | | 0.217 | | | 0.432 | 67 | 13 | 0.157 |
| ≥50 | 61 | 49 | 12 | | 48 | 13 | | 53 | 8 | |
| <50 | 19 | 13 | 6 | | 15 | 4 | | 14 | 5 | |
| Gender | | | | 0.597 | | | 0.532 | | | 0.098 |
| Male | 58 | 45 | 13 | | 46 | 12 | | 51 | 7 | |
| Female | 22 | 17 | 5 | | 17 | 5 | | 16 | 6 | |
| Lymph node
metastasis | | | | <0.001a | | | <0.001a | | | <0.001a |
| With | 52 | 48 | 4 | | 48 | 4 | | 50 | 2 | |
| Without | 28 | 14 | 14 | | 15 | 13 | | 17 | 11 | |
| Clinical stage | | | | <0.001a | | | <0.001a | | | <0.001a |
| Early | 15 | 4 | 11 | | 5 | 10 | | 7 | 8 | |
| Middle | 65 | 58 | 7 | | 58 | 7 | | 60 | 5 | |
| Differentiation
degree | | | | 0.395 | | | 0.500 | | | 0.500 |
| Poorly | 40 | 32 | 8 | | 31 | 9 | | 33 | 7 | |
| Highly and
moderately | 40 | 30 | 10 | | 32 | 8 | | 34 | 6 | |
MMP-1 and MMP-10 expression in H.
pylori-infected gastric cancer cells is upregulated
To investigate the effect of H. pylori
infection on MMP-1 and MMP-10 expression, the expression levels of
MMP-1 and MMP-10 were detected in MGC-803 cells following H.
pylori infection. Western blotting was conducted at 6, 12, 24
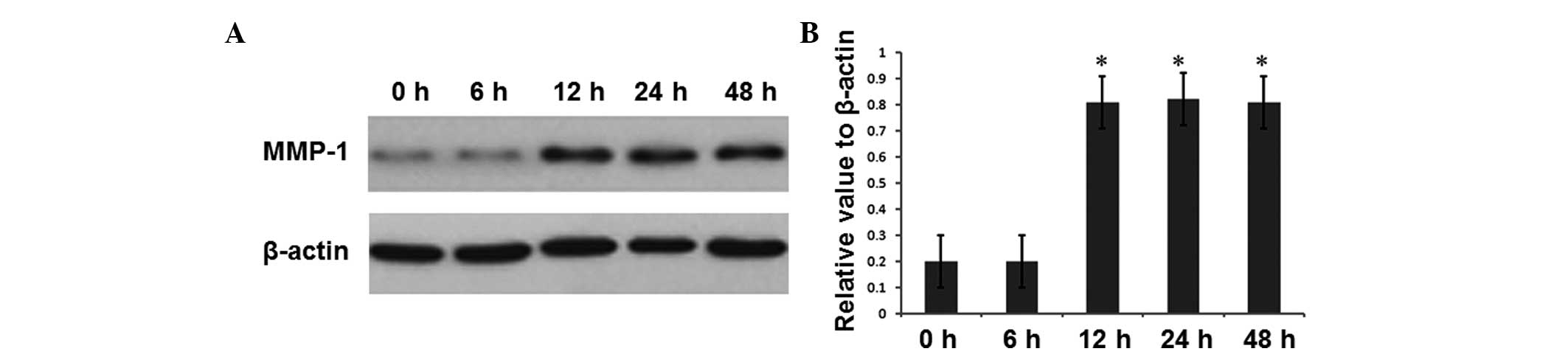
and 48 h of infection. The results for MMP-1 and MMP-10 are shown
in Figs. 2 and 3, respectively. As shown in Fig. 2, at 6 h of H. pylori
infection, the intracellular MMP-1 expression level was not
significantly changed, while at 12 h of H. pylori infection,
the intracellular MMP-1 expression was significantly increased
compared with that in the uninfected group at 0 h (P<0.05).
MMP-1 expression at 24 and 48 h was maintained at a similar level
to that at 12 h, which was also significantly increased compared
with expression in the uninfected group at 0 h (P<0.05).
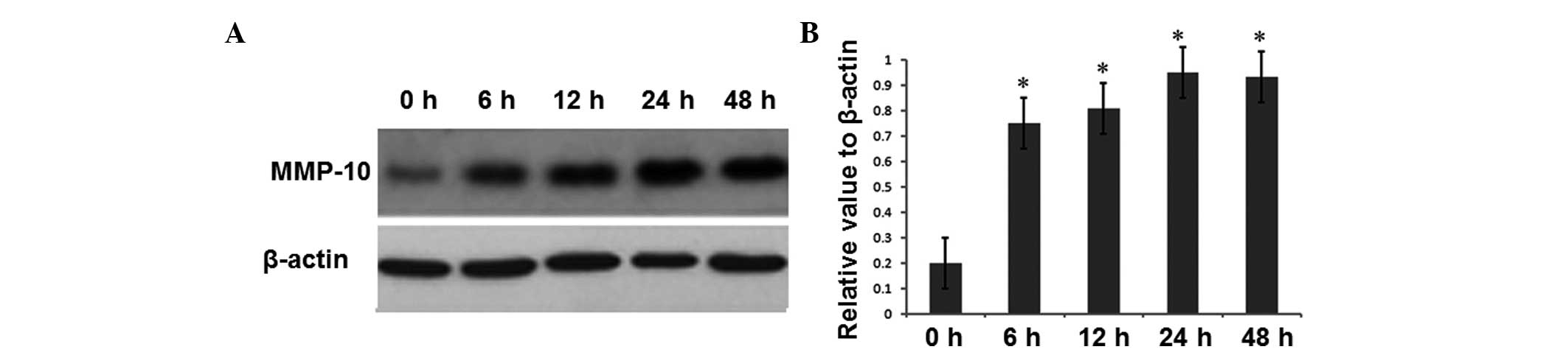
However, the intracellular MMP-10 expression was significantly
increased at 6 h of H. pylori infection compared with that
in the uninfected group at 0 h (P<0.05) (Fig. 3). Similarly, MMP-10 expression at
12, 24 and 48 h of H. pylori infection remained
significantly increased (P<0.05). These results indicate that
H. pylori infection promoted the expression of MMP-1 and
MMP-10 in gastric cancer cells.
Discussion
MMP-1 is predominantly involved in the degradation
of stromal components (10), and
is important in tumor invasion and metastasis (11). MMP-10 is considered an important
factor in the activation of other MMP (such as MMP-1) precursors in
human tumor cells (12). In
addition, MMP-10 can degrade proteins, including collagen III/IV/V,
gelatin, nidogen, laminin-l and proteoglycans, thereby contributing
to metastasis (13). This study
has shown that MMP-1 and MMP-10 expression was higher in patients
with gastric cancer with metastasis than in those with gastric
cancer without metastasis. MMP-1 and MMP-10 expression in patients
with gastric cancer and in those with metastatic gastric cancer was
significantly higher than that in patients with gastritis. The
positive expression of MMP-1 and MMP-10 was associated with lymph
node metastasis and clinical stage, but not with age, gender or
differentiation degree, indicating that MMP-1 and MMP-10 expression
was involved in gastric cancer metastasis. It was also found that
MMP-1 expression was correlated with MMP-10 expression, indicating
that these two different types of MMPs may play a synergic role in
gastric cancer genesis, invasion and metastasis. Thus, the
simultaneous detection of MMP-1 and MMP-10 may be important in the
evaluation of the prognosis and metastasis of gastric cancer.
Crawford et al (14) revealed that MMP-7 levels were
significantly increased in H. pylori-Cag-positive gastric
cancer cells, while not significantly increased in uninfected
cells. Gööz et al (15)
found that H. pylori can stimulate the secretion of MMP-1,
MMP-3 and TIMP-3 in gastric cancer cells in vitro. However,
TIMP-2 expression levels were not significantly changed. In the
present study, it was revealed that H. pylori infection was
significantly associated with MMP-1 and MMP-10 expression. Positive
H. pylori infection was also associated with lymph node
metastasis and clinical stage, and the number of specimens positive
for H. pylori infection in advanced gastric cancer was
significantly higher than that in early stage disease. This may be
a result of the destruction of the normal mucosal barrier following
H. pylori infection, stimulating MMP-1 and MMP-10
expression. MMPs can destroy the matrix-degrading balance, promote
cancer invasion through the histological barrier (constituted by
the basement membrane and ECM) into the surrounding tissues and
metastasis to distant tissues, causing gastric cancer
metastasis.
The present data showed that co-culturing H.
pylori with MGC-803 cells led to the secretion of MMP-1 and
MMP-10 by the MGC-803 cells, indicating that H. pylori
infection may be involved in metastasis through the upregulation of
MMP-1 and MMP-10 expression. At 6 h after H. pylori
infection, MMP-10 expression was upregulated, while at 12 h MMP-1
began to be expressed. MMP-1 and MMP-10 expression was maintained
at a high level at 24 and 48 h after H. pylori infection. As
shown in previous studies (12,16),
MMP-10 can activate precursors of other MMPs (including MMP-1). In
this study, the results indicate that in the H.
pylori-induced MMP secretion in gastric cancer cells, MMP-10
may be involved in the activation of MMP-1.
In conclusion, H. pylori infection is not
only the primary factor of gastric cancer that causes mucosal
injury, promotes tumor suppressor gene mutation and activates
oncogenes, but may also be involved in invasion and metastasis by
promoting MMP secretion, leading to gastric cancer deterioration
and a decrease in survival time. H. pylori infection may be
involved in gastric cancer metastasis through the mechanism of
upregulating the expression of MMP-1 and MMP-10.
Acknowledgements
This study was supported by the Project of Hunan
Provincial Education Department (grant no. 09A077).
References
|
1
|
Piazuelo MB, Epplein M and Correa P:
Gastric cancer: an infectious disease. Infect Dis Clin North Am.
24:853–869. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pizzi M, Saraggi D, Fassan M, Megraud F,
Di Mario F and Rugge M: Secondary prevention of epidemic gastric
cancer in the model of Helicobacter pylori-associated gastritis.
Dig Dis. 32:265–274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pandey R, Misra V, Misra SP, Dwivedi M,
Kumar A and Tiwari BK: Helicobacter pylori and gastric cancer.
Asian Pac J Cancer Prev. 11:583–588. 2010.PubMed/NCBI
|
|
4
|
Borlace GN, Butler RN and Brooks DA:
Monocyte and macrophage killing of Helicobacter pylori:
relationship to bacterial virulence factors. Helicobacter.
13:380–387. 2008.PubMed/NCBI
|
|
5
|
Zhang Q, Li Y, Li X, Zhou W, Shi B, Chen H
and Yuan W: PARP-1 Val762Ala polymorphism, CagA+ H.
pylori infection and risk for gastric cancer in Han Chinese
population. Mol Biol Rep. 36:1461–1467. 2009.PubMed/NCBI
|
|
6
|
Rydlova M, Holubec L Jr, Ludvikova M Jr,
et al: Biological activity and clinical implications of the matrix
metalloproteinases. Anticancer Res. 28:1389–1397. 2008.PubMed/NCBI
|
|
7
|
Scherer RL, McIntyre JO and Matrisian LM:
Imaging matrix metalloproteinases in cancer. Cancer Metastasis Rev.
27:679–690. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Orlichenko LS and Radisky DC: Matrix
metalloproteinases stimulate epithelial-mesenchymal transition
during tumor development. Clin Exp Metastasis. 25:593–600. 2008.
View Article : Google Scholar
|
|
9
|
Gentner B, Wein A, Croner RS, et al:
Differences in the gene expression profile of matrix
metalloproteinases (MMPs) and their inhibitors (TIMPs) in primary
colorectal tumors and their synchronous liver metastases.
Anticancer Res. 29:67–74. 2009.
|
|
10
|
Shim KN, Jung SA, Joo YH and Yoo K:
Clinical significance of tissue levels of matrix metalloproteinases
and tissue inhibitors of metalloproteinases in gastric cancer. J
Gastroenterol. 42:120–128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujimoto D, Hirono Y, Goi T, Katayama K
and Yamaguchi A: Prognostic value of protease-activated receptor-1
(PAR-1) and matrix metalloproteinase-1 (MMP-1) in gastric cancer.
Anticancer Res. 28:847–854. 2008.PubMed/NCBI
|
|
12
|
Frederick LA, Matthews JA, Jamieson L, et
al: Matrix metalloproteinase-10 is a critical effector of protein
kinase Ciota-Par6alpha-mediated lung cancer. Oncogene.
27:4841–4853. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dannewitz B, Edrich C, Tomakidi P, et al:
Elevated gene expression of MMP-1, MMP-10, and TIMP-1 reveal
changes of molecules involved in turn-over of extracellular matrix
in cyclosporine-induced gingival overgrowth. Cell Tissue Res.
325:513–522. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Crawford HC, Krishna US, Israel DA, et al:
Helicobacter pylori strain-selective induction of matrix
metalloproteinase-7 in vitro and within gastric mucosa.
Gastroenterology. 125:1125–1136. 2003. View Article : Google Scholar
|
|
15
|
Gööz M, Shaker M, Gööz P and Smolka AJ:
Interleukin 1beta induces gastric epithelial cell matrix
metalloproteinase secretion and activation during Helicobacter
pylori infection. Gut. 52:1250–1256. 2003.PubMed/NCBI
|
|
16
|
Nakamura H, Fujii Y, Ohuchi E, Yamamoto E
and Okada Y: Activation of the precursor of human stromelysin 2 and
its interactions with other matrix metalloproteinases. Eur J
Biochem. 253:67–75. 1998. View Article : Google Scholar : PubMed/NCBI
|