Introduction
Anemia is a common comorbidity in patients with
chronic heart failure (CHF) (1–4) and
is associated with increased morbidity. The pathogenesis of anemia
in CHF is complex and is associated with a number of factors,
including renal dysfunction, plasma volume fluctuation,
inflammation, hematinic deficiencies and drug treatment (5). Initial data from a large
observational study of patients with CHF attending cardiology
clinics showed that 33% had a low hemoglobin (Hb), using the most
commonly employed definition of the World Health Organization
(5). Notably, anemia has been
described as an independent predictor of the risk of mortality and
hospitalization due to diastolic dysfunction and left ventricular
(LV) dysfunction in patients with CHF; diastolic and LV dysfunction
may both result in disability or mortality (6–8).
A number of preliminary studies have reported that
the correction of low Hb concentrations using
erythropoiesis-stimulating proteins (ESPs) may improve cardiac and
renal function and reduce the requirement for hospitalization and
diuretics in patients with CHF (9–10).
Erythropoietin (EPO) is an erythropoiesis-stimulating, 165-amino
acid glycoprotein hormone secreted by the kidney in response to
hypoxia and has a pivotal role in promoting an increase in red
blood cells. EPO acts by stimulating the generation and release of
cells from the bone marrow, thus improving the oxygen-carrying
capacity of the blood. EPO performs additional functions beyond
that of hematopoiesis, including cardioprotection (11).
Most interventional studies conducted in patients
with CHF and anemia have shown an improvement in exercise tolerance
and functional status in response to treatment; however, these were
uncontrolled studies or controlled studies without a placebo.
Furthermore, these studies were relatively short in duration and
not designed to provide conclusive data on the safety or clinical
efficacy of ESP treatment. In addition, the small sample sizes
produced underpowered results. In this study, a meta-analysis was
performed to explore the safety and therapeutic effects of ESPs in
patients with anemia and CHF.
Materials and methods
Selection of published studies
The databases EMBASE (www.embase.com) and
MEDLINE (www.ncbi.nlm.nih.gov/pubmed) were
searched from their inception to July 2013, and clinical studies
that evaluated the effects of ESPs among patients with CHF were
systematically identified. No language restriction was applied.
Search terms included ‘heart failure’ or ‘congestive heart failure’
or ‘chronic heart failure’ or ‘CHF’ combined with ‘recombinant
erythropoietin’ or ‘darbepoetin’ or ‘erythropoietin’ or
‘erythropoiesis’. The titles and abstracts of studies identified in
the automated search were scanned to exclude any irrelevant
articles. The full texts of the remaining articles were read to
determine whether they contained relevant information on the topic
of interest. Cited references of retrieved and review articles were
assessed to confirm that the assembled list of relevant
publications was complete.
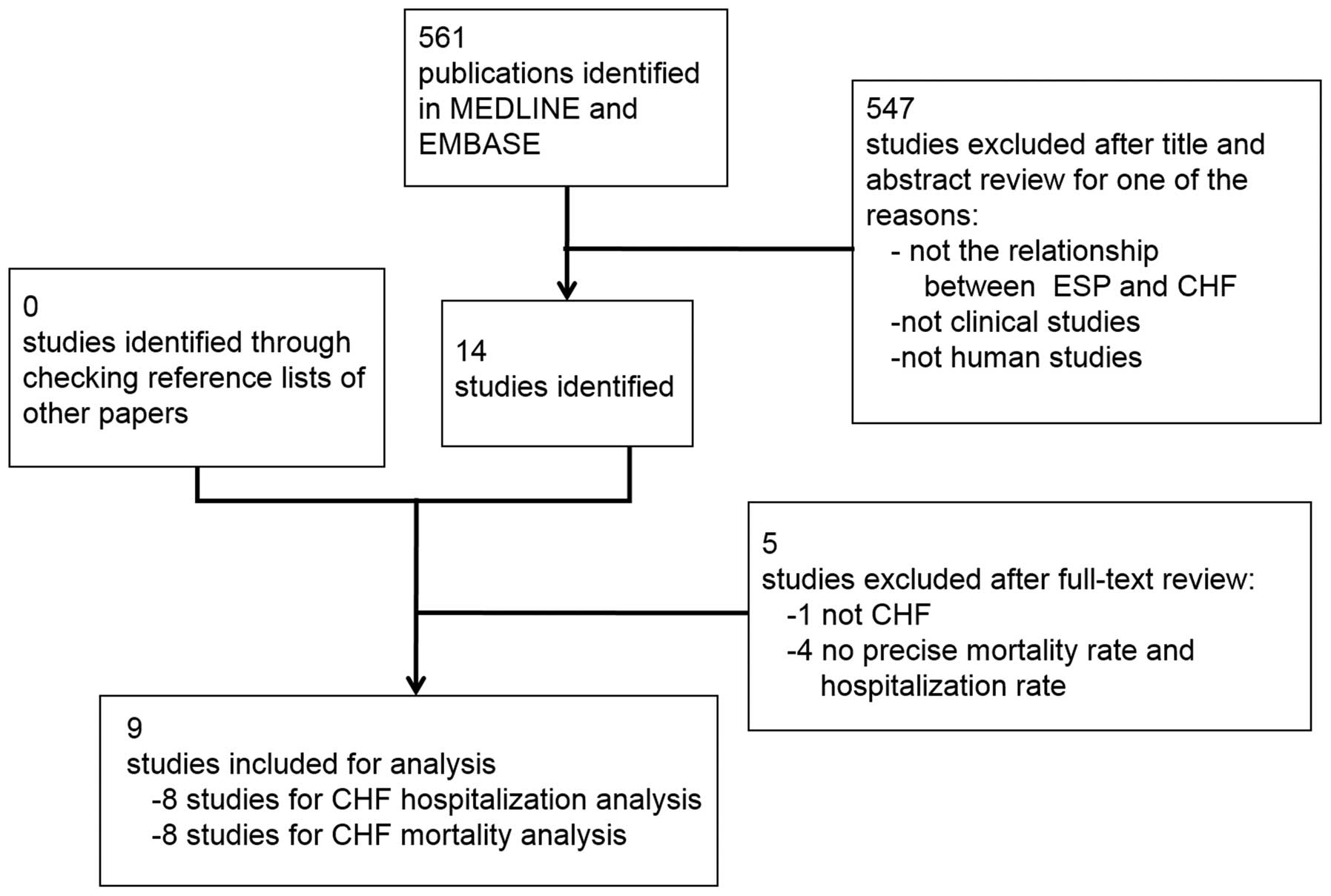
Fig. 1 illustrates
the search and selection process. The titles and abstracts of the
primary 561 publications identified were reviewed and 547 were
discarded for one of the following reasons: (i) The study did not
discuss the association between ESPs and HF; (ii) the study was not
a clinical study; (iii) the study was not a human study.
Bibliographies were also searched for publications not identified
in the database searches but no more additional publications were
found. In total, the full texts of 14 articles were read. If the
type of HF was not CHF (e.g. systolic HF) (12) or no precise mortality or
hospitalization rate could be calculated (13–16),
the publications were excluded. Nine publications were ultimately
selected for the meta-analysis.
Data extraction and quality
assessment
The eligibility of all studies retrieved from the
databases was independently evaluated and the relevant data from
each study were extracted using a unified data form. The items
included in the data form were as follows: (i) Study name (with
first author’s name and year of publication); (ii) journal name;
(iii) location; (iv) study design; (v) study population (case and
control); (vi) inclusion criteria; (vii) exclusion criteria; (viii)
range for follow-up; (ix) end-points. Two separate lists from two
independent authors were compared, and disagreements were resolved
by consensus. Relative risks (RRs) were recorded or calculated.
Each study was evaluated for quality according to
the guidelines provided by the United States Preventive Task Force.
The following characteristics were assessed: (i) Duration of
follow-up (>6 months); (ii) adequacy of follow-up (reporting
loss rate of follow-up); (iii) definition of anemia; (iv) full
specification of outcome; (v) study sample representative for the
unexposed population; (vi) full specification of clinical and
demographic variables; (vii) explanation of sample selection;
(viii) clear inclusion and exclusion criteria. Studies were graded
as ‘poor’ quality if they met <5 criteria, ‘fair’ if they met
5–6 criteria and ‘good’ if they met >7 criteria. Outcomes
assessed were mortality and CHF hospitalization rate.
Statistical analysis
To compute a summary RR with a 95% confidence
interval (CI), a study-specific, most-adjusted RR and its 95% CI
was used in all analyses. Publication bias was assessed by visual
inspection of the funnel plots created by plotting the RR to
standard error (SE) for all nine studies.
Inter-study heterogeneity was examined using
Cochran’s Q and I2 statistics. The I2
statistic assesses the percentage of variability in the effect
estimates that is due to heterogeneity rather than chance. A
fixed-effects model was used if P<0.10 or I2 >50%
for heterogeneity, otherwise a random-effects model was utilized.
The RR was transformed to a natural log scale and the SEs were
calculated. The analysis was performed using RevMan 5.2 software
(The Nordic Cochrane Centre, The Cochrane Collaboration,
Copenhagen, 2012).
Results
Literature search, study characteristics
and quality assessment
Following the exclusion of duplicates, 561
potentially relevant studies were reviewed, with nine clinical
studies meeting the inclusion criteria and subsequently being
analyzed (Fig. 1). The baseline
characteristics of the studies included are summarized in Table I. The nine studies, published
between 2001 and 2011, followed 750 patients with CHF and anemia
receiving ESP treatment for between three months and one year. The
inclusion criteria for anemia differed from Hb (17–23)
to hematocrit (Hct) (24). Five
studies used EPO as the ESP, while the remaining four used
darbepoetin-α. All studies were conducted in Europe with a ‘fair’
quality score.
 | Table ISummarized information of included
studies. |
Table I
Summarized information of included
studies.
| First author
(year) | Study design | No. of patients
(ESP/Placebo) | Inclusion
criteria | Exclusion
criteria | ESP | Follow-up | Quality |
|---|
| Silverberg
(2001) | Single-center study,
randomized, open-label | 32 (16/16) | LVEF: 40%
Hb: 10–11.5 g/dl | Secondary causes of
anemia | EPO | 8.2 months | Fair |
| Mancini (2003) | Single-center study,
randomized, single-blind, placebo-controlled | 23 (15/8) | NYHA:
III/IV
Hct <35% | Non-ambulatory
patients, continuous inotropic agents, iron deficiency anemia, sCr
>2.5 mg/dl | α-EPO | 3 months | Fair |
| Palazzuoli
(2006) | Single-center study,
randomized, double-blind, placebo-controlled | 38 (20/18) | NYHA:
III/IV
Hb <11 g/dl | Secondary causes of
anemia, isolated diastolic HF, <12 weeks MI, sCr <5.0
mg/dl | β-EPO | 3 months | Fair |
| Ponikowski
(2007) | Multi-center study,
randomized, double-blind, placebo-controlled | 41 (19/22) | Symptomatic
CHF
LVEF <40%
Hb: 9–12 g/dl | Blood transfusion or
ESP within 12 weeks, sCr >3.0 mg/dl | α-DPO | 26 weeks | Fair |
| Van Veldhuisen
(2007) | Multi-center study,
randomized, double-blind, placebo-controlled | 165 (56 WD+54
FD/55) | Symptomatic
CHF
LVEF <40%
Hb: 9–12.5 g/dl | sCr >3.0
mg/dl | α-DPO | 26 weeks | Fair |
| Ghali (2008) | Multi-center study,
randomized, double-blind, placebo-controlled | 319 (162/157) | NYHA: II–IV
LVEF <40%
Hb <12.5 g/dl | sCr >3.0
mg/dl | α-DPO | 1 year | Fair |
| Parissis (2009) | Single-center study,
randomized, single-blind, placebo-controlled | 30 (15/15) | | sCr >2.5
mg/dl | α-DPO | 3 months | Fair |
| Palazzuoli
(2009) | Single-center study,
randomized, placebo-controlled, double-blind | 58 (29/29) | NYHA: III–IV, Hb
<11.5 g/dl, LVEF <40% | Intestinal bleeding,
gastric lesions, vitamin B12 deficiency, severe renal failure | β-EPO | 1 year | Fair |
| Palazzuoli
(2011) | Single-center study,
randomized, double-blind, placebo-controlled | 42 (13 α-EPO+14
β-EPO/25) | NYHA:
III–IV
Hb <11.5 g/dl
Cr clearance: 30–60 ml/min | Isolated diastolic
dysfunction, more than moderate valvular disease, recent MI,
modifiable causes of anemia severe renal failure, gastrointestinal
bleeding | α-EPO/β-EPO | 1 year | Fair |
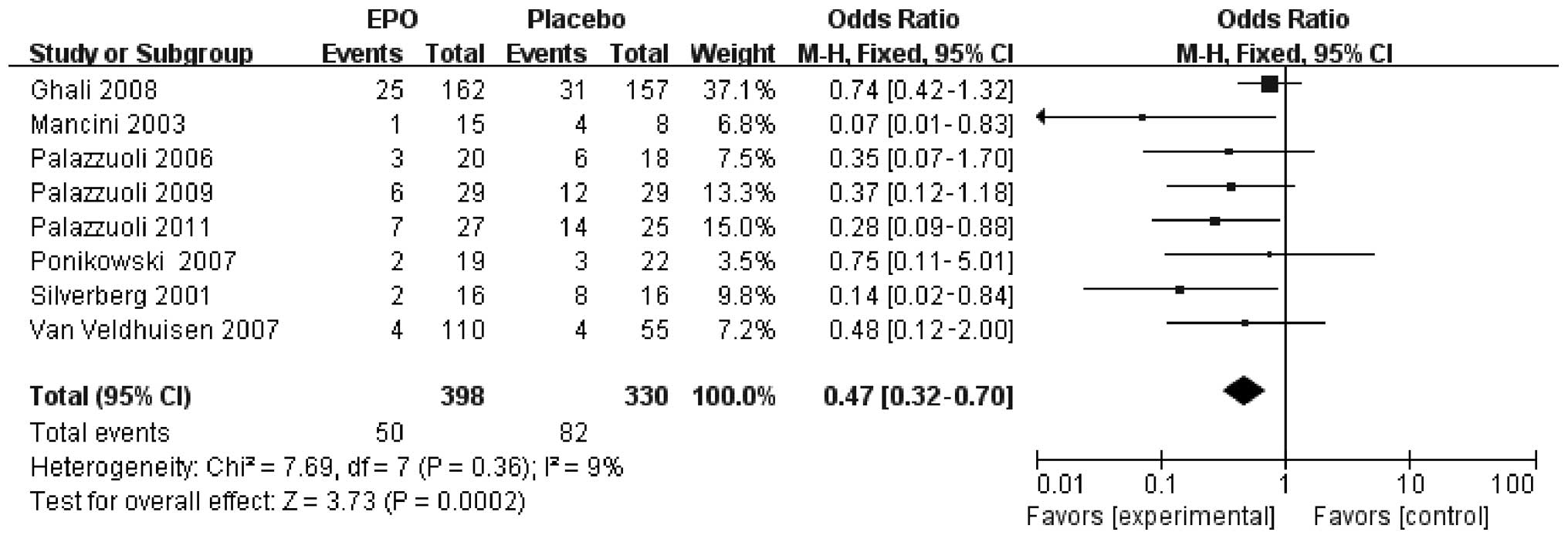
Eight of the nine studies reported detailed CHF
hospitalization. Of the 398 patients with CHF in the ESP treatment
group, 50 were admitted to hospital (12.6%), whilst 82 of the 330
placebo-treated patients were hospitalized (24.8%). A significantly
reduced hospitalization risk was identified for patients with CHF
treated with ESPs compared with those treated with placebo (RR,
0.47; 95% CI, 0.32–0.70; P=0.0002) (Fig. 2). With regard to the outcomes, no
statistically significant differences in heterogeneity were found
between the included studies for hospitalization (I2=9%,
P=0.36), and there was no obvious indication of publication
bias.
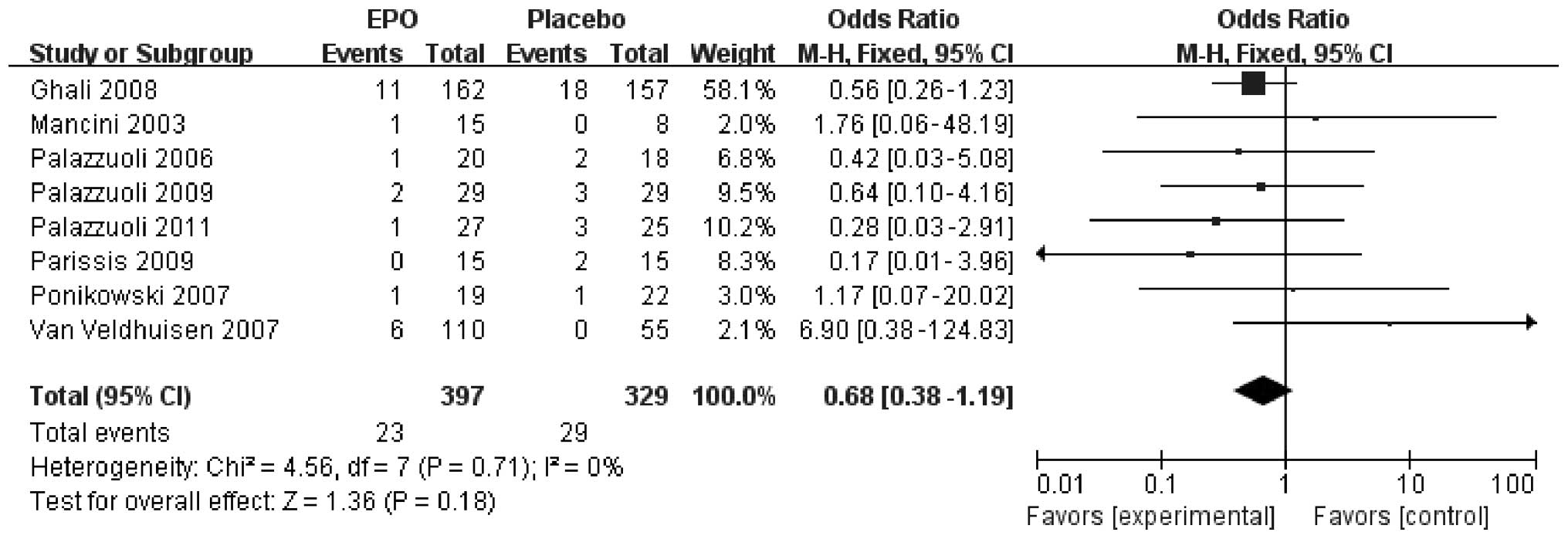
Eight of the nine studies reported details of CHF
mortality rate. Of the 397 patients with CHF in the ESP treatment
group, 23 patients succumbed during the study (5.8%), compared with
29 of the 329 placebo-treated patients (8.8%). This resulted in a
moderate reduction, without reaching statistical significance, in
mortality risk for patients with CHF treated with ESPs (RR, 0.68;
95% CI, 0.38–1.19; P=0.18) compared with those treated with placebo
(Fig. 3). No statistically
significant differences in heterogeneity were found between the
included studies for hospitalization (I2=0%, P=0.71),
and there was no obvious indication of publication bias.
The baseline and achieved Hb and Hct levels and red
blood cell count of the different studies are shown in Table II. Patients treated with ESPs
showed enhanced recovery from anemia (P<0.05). Furthermore,
patients with CHF treated with ESPs exhibited improved exercise
capacity with regard to exercise duration, volume of oxygen
consumption and distance walked during exercise, as compared with
controls (Table III). Following
treatment with ESPs, a reduction in brain natriuretic peptide (BNP)
and creatinine was observed in patients with CHF compared with
controls (Table IV). It was
additionally detected that ESP treatment significantly improved the
New York Heart Association (NYHA) class and slowed the
deterioration of LV ejection fraction (LVEF) in patients with CHF
(Table V).
 | Table IIComparison of anemia following ESP
treatment. |
Table II
Comparison of anemia following ESP
treatment.
| First author
(year) | No. of patients
(ESP/Placebo) | Baseline Hb
(g/dl) | Hb at end (g/dl) | Baseline Hct
(g/dl) | Hct at end (%) | Baseline RBCs
(1/ml) | RBCs at end
(x10x/ml) |
|---|
|
|
|
|
|
|
|---|
| ESP | Placebo | ESP | Placebo | ESP | Placebo | ESP | Placebo | ESP | Placebo | ESP | Placebo |
|---|
| Silverberg
(2001) | 32 (16/16) | 10.3 | 10.9 | 12.9 | 10.8 | | | | | | | | |
| Mancini (2003) | 23 (15/8) | 11.0 | 10.9 | 14.3 | 11.5 | | | | | | | | |
| Palazzuoli
(2006) | 38 (20/18) | 10.4 | 10.6 | 12.4 | 10.5 | 30.0 | 32.0 | 36.4 | 31.0 | 3.3 | 3.4 | 4.2 | 3.2 |
| Ponikowski
(2007) | 41 (19/22) | 11.8 | 11.6 | 13.9 | 12.3 | | | | | | | | |
| Van Veldhuisen
(2007) | 165 (56 WD+54
FD/55) | 11.5 | 11.4 | 13.3 | 11.4 | | | | | | | | |
| Ghali (2008) | 319 (162/157) | 11.5 | 11.3 | 13.6 | 11.8 | | | | | | | | |
| Parissis
(2008) | 32 (21/11) | 11.0 | 11.4 | 12.8 | 11.8 | | | | | | | | |
| Kourea (2008) | 41 (21/20) | 10.9 | 11.4 | 12.8 | 11.7 | | | | | | | | |
| Palazzuoli
(2009) | 58 (29/29) | 9.6 | 9.3 | 11.9 | 10.5 | 30.8 | 31.6 | 34.1 | 32.3 | 3.2 | 3.2 | 3.8 | 3.3 |
| Parissis
(2009) | 30 (15/15) | 11.2 | 11.5 | 12.8 | 11.9 | | | | | | | | |
| Palazzuoli
(2011) | 42 (13 α-EPO/14
β-EPO/25) | 10.4/9.0 | 9.3 | 12.3/11.7 | 10.6 | 30.6/30.8 | 31.6 | 34.2/34.0 | 32.3 | 3.6/3.2 | 3.2 | 3.9/3.8 | 3.3 |
 | Table IIIComparison of exercise capacity
following ESP treatment. |
Table III
Comparison of exercise capacity
following ESP treatment.
| First author
(year) | No. of patients
(ESP/Placebo) | Baseline exercise
duration (sec) | Exercise duration
at end (sec) | Baseline
VO2 (ml/kg/min) | VO2 at
end (ml/kg/min) | Baseline distance
walked (m) | Distance walked at
end (m) |
|---|
|
|
|
|
|
|
|---|
| ESP | Placebo | ESP | Placebo | ESP | Placebo | ESP | Placebo | ESP | Placebo | ESP | Placebo |
|---|
| Mancini (2003) | 23 (15/8) | 590 | 542 | 657 | 459 | 11.0 | 10.0 | 12.7 | 9.5 | 362 | 283 | 405 | 321 |
| Palazzuoli
(2006) | 38 (20/18) | 348 | 348 | 468 | 360 | 12.8 | 12.5 | 15.1 | 12.0 | 278 | 285 | 356 | 266 |
| Ponikowski
(2007) | 41 (19/22) | 559 | 597 | 526 | 468 | 12.4 | 12.2 | 12.4 | 11.7 | | | | |
| Ghali (2008) | 319 (162/157) | 408 | 409 | 465.3 | 455.5 | | | | | | | | |
| Van Veldhuisen
(2007) | 165 (56 WD+54
FD/55) | | | | | | | | | 287 | 304 | 321 | 315 |
| Parissis
(2008) | 32 (21/11) | | | | | | | | | 227 | 214 | 296 | 167 |
| Kourea (2008) | 41 (21/20) | | | | | | | | | 201 | 237 | 274 | 204 |
| Parissis
(2009) | 30 (15/15) | | | | | | | | | 227 | 214 | 296 | 167 |
 | Table IVComparison of BNP and creatinine
following ESP treatment. |
Table IV
Comparison of BNP and creatinine
following ESP treatment.
| First author
(year) | No. of patients
(ESP/Placebo) | Baseline BNP
(pg/ml) | BNP at end
(pg/ml) | Baseline creatinine
(mg/dl) | Creatinine at end
(mg/dl) |
|---|
|
|
|
|
|---|
| ESP | Placebo | ESP | Placebo | ESP | Placebo | ESP | Placebo |
|---|
| Palazzuoli
(2006) | 38 (20/18) | 568 | 585 | 271 | 496 | 2.5 | 2.4 | 1.8 | 2.2 |
| Parissis
(2008) | 32 (21/11) | 1102 | 788 | 661 | 1212 | | | | |
| Kourea (2008) | 41 (21/20) | 829 | 725 | 517 | 1040 | | | | |
| Parissis
(2009) | 30 (15/15) | 1105 | 988 | 669 | 1202 | | | | |
| Palazzuoli
(2009) | 58 (29/29) | 702 | 690 | 422 | 535 | 2.3 | 2.4 | 2.2 | 2.4 |
| Palazzuoli
(2011) | 42 (13 α-EPO+14
β-EPO/25) | 512/659 | 610 | 335/449 | 582 | 2.3/2.3 | 2.3 | 2.0/2.2 | 2.3 |
| Silverberg
(2001) | 32(16/16) | | | | | 1.7 | 1.4 | 1.7 | 1.8 |
| Van Veldhuisen
(2007) | 165 (56 WD+54
FD/55) | | | | | 1.4 | 1.5 | 1.3 | 1.6 |
 | Table VComparison of heart function
following ESP treatment. |
Table V
Comparison of heart function
following ESP treatment.
| First author
(year) | No. of patients
(ESP/Placebo) | Baseline NYHA
class | NYHA class at
end | Baseline LVEF
(%) | LVEF at end
(%) |
|---|
|
|
|
|
|---|
| ESP | Placebo | ESP | Placebo | ESP | Placebo | ESP | Placebo |
|---|
| Palazzuoli
(2006) | 38 (20/18) | 3.5 | 3.4 | 2.8 | 3.6 | | | | |
| Parissis
(2008) | 32 (21/11) | 2.8 | 2.7 | 2.1 | 3.2 | 26 | 28 | 31 | 25 |
| Palazzuoli
(2009) | 58 (29/29) | 3.35 | 3.32 | 2.77 | 3.28 | 30.1 | 30.9 | 32.3 | 30.9 |
| Palazzuoli
(2011) | 42 (13 α-EPO/14
β-EPO/25) | 3.4/3.5 | 3.3 | 2.7/2.8 | 3.2 | | | | |
| Van Veldhuisen
(2007) | 165 (56 WD+54
FD/55) | | | | | 29 | 27 | 28.98 | 28.27 |
| Kourea (2008) | 41 (21/20) | | | | | 26 | 28 | 32 | 28 |
| Parissis
(2009) | 30 (15/15) | | | | | 28 | 27 | 33 | 28 |
Discussion
EPO receptors are expressed in both hematopoietic
and nonhematopoietic cells. ESPs, which are clinically used
exclusively for erythropoiesis in patients with anemia, demonstrate
potential in the treatment of pathological conditions of
nonhematopoietic organs, including the brain and heart (3). Further investigations have
demonstrated that ESP-mediated cardioprotection is mainly achieved
through reducing apoptosis, increasing neovascularization,
mobilizing endothelial progenitor cells and inducing angiogenesis
through the phosphorylation and activation of signaling pathways
(7), including Janus kinase 1/2,
signal transducer and activator of transcription 3 (STAT3), STAT5
and phosphoinositide-3-kinase (25).
Patients with CHF together with anemia experience
higher rates of hospitalization than those without anemia. A prior
meta-analysis by van der Meer et al (26) of seven prospective, randomized,
placebo-controlled trials of the use of ESPs in CHF enrolling 650
patients suggested a statistically significant decrease of 41% in
hospitalizations due to HF in ESP-treated patients compared with
controls. A similar result was noted in the present study, and was
consistent with a pooled, patient-level analysis of three
randomized trials of darbepoetin α that enrolled 516 subjects with
HF (27).
In the present pooled analysis of patients with HF,
it was found that the anemia of patients with CHF improved with ESP
treatment. The majority of the changes in the anemic condition
occurred together with increases in Hb and Hct; however, the most
significant improvements were observed after one year. A moderate
reduction in mortality risk was also observed for patients with CHF
treated with ESPs, compared with those treated with placebo. This
may be due in part to the improved oxygenation and reduced
oxidative stress caused by the improvement in the anemia or due to
the direct effects of the drug. Another meta-analysis including
>150,000 patients with CHF identified that low Hb levels
increased the risk of all-cause mortality over a six-month to
five-year follow-up period, irrespective of whether CHF was due to
systolic or diastolic dysfunction (28). Other research, however, has
reported contradictory results (26). Differences may be due to skews in
data caused by patients with a poor initial response to the ESP,
who went on to receive higher doses of the drug and had increased
rates of cardiovascular events and all-cause mortality. The present
study found that patients who received ESP treatment exhibited a
statistically significant improvement in exercise capacity and
confirmed a previous report, according to which subcutaneous EPO
administration improves exercise capacity, quality of life and LVEF
in patients with CHF and anemia (29).
BNP levels are a reflection of ventricular
stretching and are accepted as an effective marker of the presence
and severity of CHF, since high levels of BNP are independent
predictors of adverse clinical outcomes in CHF (22). Neurohormonal and metabolic effects
induced by anemia can result in direct myocardial toxicity,
myocardial hypertrophy, and salt and water retention. A reduction
in BNP levels in patients with CHF treated with ESPs was observed
in the present study. This decrease in BNP levels could be
associated with improvements in a number of aspects, including
improvements in cardiac function as a result of increased oxygen
supply to the heart, reduced load caused by the prevention of
anemia-induced tachycardia and increased stroke volume, reduction
in plasma volume and a reduction in the activity of the sympathetic
and renin angiotensin-aldosterone system, which occurs in anemia
(3,30). In the present study, the
improvement in renal function in the treated group was reflected by
the improvement in creatinine level. Since renal failure is one of
the primary negative prognostic factors for mortality and morbidity
in CHF, the preservation of renal function by reversal of anemia is
another important contribution of ESP treatment for improved
prognosis in CHF (5). In this
context, the data demonstrated a trend towards a reduction in
cardiac adverse events and a significant improvement in NYHA class
and LVEF without any other potential adverse effects.
In conclusion, treatment of patients with
symptomatic CHF and anemia with ESPs results in significant
improvements in hospitalization rate, Hb, Hct and BNP levels,
mortality, exercise capacity, renal function, NYHA class and LVEF.
These results support the instigation of larger trials
investigating the safety and efficacy of ESPs for the treatment of
anemia in anemic patients with symptomatic CHF.
References
|
1
|
Tang YD and Katz SD: Anemia in chronic
heart failure: prevalence, etiology, clinical correlates, and
treatment options. Circulation. 113:2454–2461. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Felker GM, Adams KF Jr, Gattis WA and
O’Connor CM: Anemia as a risk factor and therapeutic target in
heart failure. J Am Coll Cardiol. 44:959–966. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van der Meer P, Voors AA, Lipsic E, van
Gilst WH and van Veldhuisen DJ: Erythropoietin in cardiovascular
diseases. Eur Heart J. 25:285–291. 2004.
|
|
4
|
Anand I, McMurray JJ, Whitmore J, Warren
M, Pham A, McCamish MA and Burton PB: Anemia and its relationship
to clinical outcome in heart failure. Circulation. 110:149–154.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Westenbrink BD, Visser FW, Voors AA,
Smilde TD, Lipsic E, Navis G, Hillege HL, van Gilst WH and van
Veldhuisen DJ: Anaemia in chronic heart failure is not only related
to impaired renal perfusion and blunted erythropoietin production,
but to fluid retention as well. Eur Heart J. 28:166–171. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Felker GM, Gattis WA, Leimberger JD, Adams
KF, Cuffe MS, Gheorghiade M and O’Connor CM: Usefulness of anemia
as a predictor of death and rehospitalization in patients with
decompensated heart failure. Am J Cardiol. 92:625–628. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kosiborod M, Smith GL, Radford MJ, Foody
JM and Krumholz HM: The prognostic importance of anemia in patients
with heart failure. Am J Med. 114:112–119. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mozaffarian D, Nye R and Levy WC: Anemia
predicts mortality in severe heart failure: the prospective
randomized amlodipine survival evaluation (PRAISE). J Am Coll
Cardiol. 41:1933–1939. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Silverberg DS, Wexler D, Blum M, Keren G,
Sheps D, Leibovitch E, Brosh D, Laniado S, Schwartz D, Yachnin T,
Shapira I, Gavish D, Baruch R, Koifman B, Kaplan C, Steinbruch S
and Iaina A: The use of subcutaneous erythropoietin and intravenous
iron for the treatment of the anemia of severe, resistant
congestive heart failure improves cardiac and renal function and
functional cardiac class, and markedly reduces hospitalizations. J
Am Coll Cardiol. 35:1737–1744. 2000. View Article : Google Scholar
|
|
10
|
Silverberg DS, Wexler D, Sheps D, Blum M,
Keren G, Baruch R, Schwartz D, Yachnin T, Steinbruch S, Shapira I,
Laniado S and Iaina A: The effect of correction of mild anemia in
severe, resistant congestive heart failure using subcutaneous
erythropoietin and intravenous iron: a randomized controlled study.
J Am Coll Cardiol. 37:1775–1780. 2001. View Article : Google Scholar
|
|
11
|
Ruifrok WP, de Boer RA, Westenbrink BD,
van Veldhuisen DJ and van Gilst WH: Erythropoietin in cardiac
disease: new features of an old drug. Eur J Pharmacol. 585:270–277.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Swedberg K, Young JB, Anand IS, Cheng S,
Desai AS, Diaz R, Maggioni AP, McMurray JJ, O’Connor C, Pfeffer MA,
Solomon SD, Sun Y, Tendera M and van Veldhuisen DJ; RED-HF
Committees; RED-HF Investigators. Treatment of anemia with
darbepoetin-alfa in systolic heart failure. N Engl J Med.
368:1210–1219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cleland JG, Sullivan JT, Ball S, Horowitz
JD, Agoram B, Rosser D, Yates W, Tin L, Fuentealba P and Burton PB:
Once-monthly administration of darbepoetin α for the treatment of
patients with chronic heart failure and anemia: a pharmacokinetic
and pharmacodynamic investigation. J Cardiovasc Pharmacol.
46:155–161. 2005.
|
|
14
|
Kourea K, Parissis JT, Farmakis D,
Paraskevaidis I, Panou F, Filippatos G and Kremastinos DT: Effects
of darbepoetin alfa on quality of life and emotional stress in
anemic patients with chronic heart failure. Eur J Cardiovasc Prev
Rehabil. 15:365–369. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cosyns B, Velez-Roa S, Droogmans S,
Pierard LA and Lancellotti P: Effects of erythropoietin
administration on mitral regurgitation and left ventricular
remodeling in heart failure patients. Int J Cardiol. 138:306–307.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Parissis JT, Kourea K, Panou F, Farmakis
D, Paraskevaidis I, Ikonomidis I, Filippatos G and Kremastinos DT:
Effects of darbepoetin alfa on right and left ventricular systolic
and diastolic function in anemic patients with chronic heart
failure secondary to ischemic or idiopathic dilated cardiomyopathy.
Am Heart J. 155:7512008. View Article : Google Scholar
|
|
17
|
Palazzuoli A, Silverberg D, Iovine F,
Capobianco S, Giannotti G, Calabrò A, Campagna SM and Nuti R:
Erythropoietin improves anemia exercise tolerance and renal
function and reduces B-type natriuretic peptide and hospitalization
in patients with heart failure and anemia. Am Heart J.
152:10962006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ponikowski P, Anker SD, Szachniewicz J,
Okonko D, Ledwidge M, Zymlinski R, Ryan E, Wasserman SM, Baker N,
Rosser D, Rosen SD, Poole-Wilson PA, Banasiak W, Coats AJ and
McDonald K: Effect of darbepoetin alfa on exercise tolerance in
anemic patients with symptomatic chronic heart failure: a
randomized, double-blind, placebo-controlled trial. J Am Coll
Cardiol. 49:753–762. 2007. View Article : Google Scholar
|
|
19
|
van Veldhuisen DJ, Dickstein K,
Cohen-Solal A, Lok DJ, Wasserman SM, Baker N, Rosser D, Cleland JG
and Ponikowski P: Randomized, double-blind, placebo-controlled
study to evaluate the effect of two dosing regimens of darbepoetin
alfa in patients with heart failure and anaemia. Eur Heart J.
28:2208–2216. 2007.
|
|
20
|
Ghali JK, Anand IS, Abraham WT, Fonarow
GC, Greenberg B, Krum H, Massie BM, Wasserman SM, Trotman ML, Sun
Y, Knusel B and Armstrong P; Study of Anemia in Heart Failure Trial
(STAMINA-HeFT) Group. Randomized double-blind trial of darbepoetin
alfa in patients with symptomatic heart failure and anemia.
Circulation. 117:526–535. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Palazzuoli A, Silverberg DS, Calabrò A,
Spinelli T, Quatrini I, Campagna MS, Franci B and Nuti R: Beta
erythropoietin effects on ventricular remodeling, left and right
systolic function, pulmonary pressure, and hospitalizations in
patients affected with heart failure and anemia. J Cardiovasc
Pharmacol. 53:462–467. 2009. View Article : Google Scholar
|
|
22
|
Palazzuoli A, Quatrini I, Calabrò A,
Antonelli G, Caputo M, Campagna MS, Franci B and Nuti R: Anemia
correction by erythropoietin reduces BNP levels, hospitalization
rate, and NYHA class in patients with cardio-renal anemia syndrome.
Clin Exp Med. 11:43–48. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Parissis JT, Kourea K, Andreadou I,
Ikonomidis I, Markantonis S, Ioannidis K, Paraskevaidis I,
Iliodromitis E, Filippatos G and Kremastinos DT: Effects of
Darbepoetin Alfa on plasma mediators of oxidative and nitrosative
stress in anemic patients with chronic heart failure secondary to
ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol.
103:1134–1138. 2009. View Article : Google Scholar
|
|
24
|
Mancini DM, Katz SD, Lang CC, LaManca J,
Hudaihed A and Androne AS: Effect of erythropoietin on exercise
capacity in patients with moderate to severe chronic heart failure.
Circulation. 107:294–299. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chateauvieux S, Grigorakaki C, Morceau F,
Dicato M and Diederich M: Erythropoietin, erythropoiesis and
beyond. Biochem Pharmacol. 82:1291–1303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van der Meer P, Groenveld HF, Januzzi JL
Jr and van Veldhuisen DJ: Erythropoietin treatment in patients with
chronic heart failure: a meta-analysis. Heart. 95:1309–1314.
2009.PubMed/NCBI
|
|
27
|
Klapholz M, Abraham WT, Ghali JK,
Ponikowski P, Anker SD, Knusel B, Sun Y, Wasserman SM and van
Veldhuisen DJ: The safety and tolerability of darbepoetin alfa in
patients with anaemia and symptomatic heart failure. Eur J Heart
Fail. 11:1071–1077. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Groenveld HF, Januzzi JL, Damman K, van
Wijngaarden J, Hillege HL, van Veldhuisen DJ and van der Meer P:
Anemia and mortality in heart failure patients a systematic review
and meta-analysis. J Am Coll Cardiol. 52:818–827. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weiss G and Goodnough LT: Anemia of
chronic disease. N Engl J Med. 352:1011–1023. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Anand IS, Chandrashekhar Y, Ferrari R,
Poole-Wilson PA and Harris PC: Pathogenesis of oedema in chronic
severe anaemia: studies of body water and sodium, renal function,
haemodynamic variables, and plasma hormones. Br Heart J.
70:357–362. 1993. View Article : Google Scholar
|