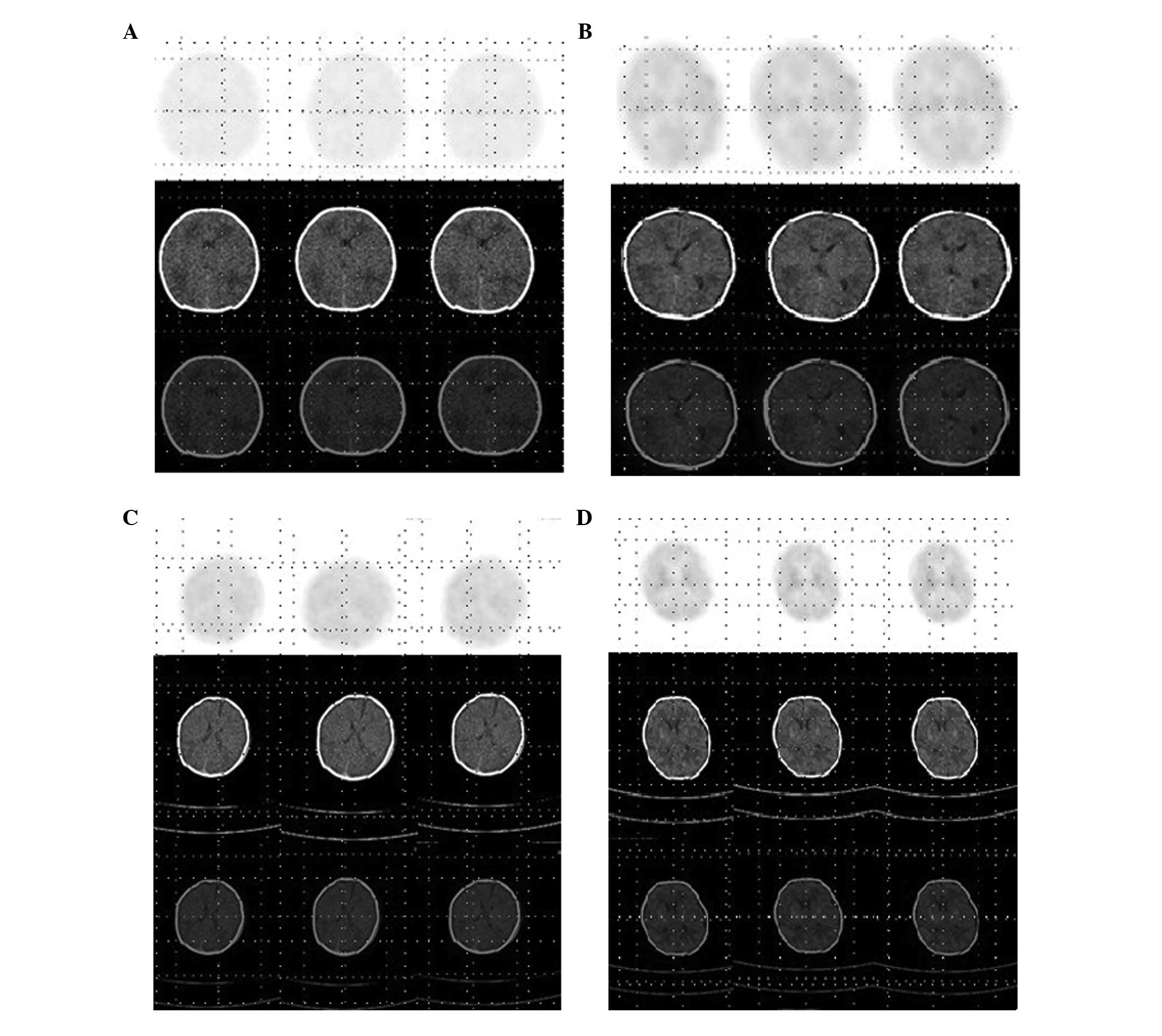
Introduction
Hypoxic-ischaemic encephalopathy (HIE) is an anoxia-
and ischaemia-associated secondary cerebral injury caused by
various factors. It is a significant complication of neonatal
asphyxia. HIE is also a leading cause of neonatal fatality and
disability in children; therefore, it remains the focus for
investigation in numerous studies (1). The pathogenesis of HIE remains
unclear although, based on related studies, it has been considered
as a multi-mechanism combination of anoxia- and ischaemia-induced
energy exhaustion, reperfusion and oxidative stress, the toxic
effects of excitatory amino acids (EAAs), inflammatory injury
(2) and cell apoptosis (3). The morbidity rate of HIE is generally
accepted to be between 2 and 9% (4,5). In
addition to the classic symptomatic and supportive therapies, mild
hypothermia therapy is the only specific neuroprotective procedure
recommended for the treatment of HIE among the various treatment
methods (6).
Mild hypothermia therapy is considered to be an
important treatment that is able to improve the prognosis of HIE
and greatly decrease the mortality rate and morbidity of the
impairments or disabilities in infants with full-term HIE. Although
the main side-effects of mild hypothermia therapy have been
demonstrated to involve increased morbidity due to arrhythmia and
thrombocytopenia (6), mild
hypothermia has been deemed to be safe and viable as a treatment
option, and its mechanism of action is nonspecific,
multi-channelled, and multi-targeted. Aberrant energy metabolism in
brain cells underlies HIE pathogenesis (2,3). The
level and duration of aberrant energy metabolism may affect not
only the short-term but also the long-term prognosis of patients.
Mild hypothermia protects the brain cells through multiple
mechanisms (7), whereas the
effects of mild hypothermia on the brain energy metabolism of
individuals with HIE remains unclear. The development of positron
emission tomography/computed tomography (PET/CT), one of the most
advanced medical techniques (8),
provides the leading medical imaging invention worldwide. It is
possible that imaging with 18F-fluorodeoxyglucose
(18F-FDG) may directly reflect the state of local
cellular energy metabolism. PET/CT scans have been widely applied
in adults and children but are very rarely used in newborns
(9).
Based on the aforementioned knowledge, the present
study used 18F-FDG PET/CT scanning to evaluate and
analyse the changes in the energy metabolism of the brain cells of
patients with neonatal HIE following mild hypothermia therapy.
Subjects and methods
Subjects
There were 305 cases of HIE in the Neonatal
Department of The Affiliated Hospital of Luzhou Medical College
(Luzhou, China) from May 2012 to September 2013. Of these, 63
patients were selected for inclusion in this study.
The inclusion criteria were as follows: i) born
within 6 h of labour following a ≥36-week gestation period; ii) met
the diagnostic criteria for medium or severe HIE (10); iii) not treated with high-dose
anti-spasmodic drugs and did not have congenital malformation,
intracranial hemorrhage, scalp injury or related conditions and iv)
an informed consent form was provided by the newborn’s legal
guardian or family member.
In total, 37/63 patients received mild hypothermia
therapy. Among them, 24 were male and 13 were female. The mean
gestational age at birth was 37.55±4.21 weeks (range, 36–41±5
±days). The mean body mass at birth was 3,390.27±573.08 g (range,
2.55–4.75 kg) and the mean age at hospital admission was 4.03±1.28
h (range, 1.7–5.7 h). The other 26 cases, 10 male and 16 female,
who did not undergo mild hypothermia therapy, had a mean
gestational age of 38.12±1.21 weeks (range, 36±3–40 ±5 days), mean
body mass at birth of 3,393.07±473.41 g (range, 2.85–4.17 kg) and a
mean age at hospital admission of 4.22±1.05 h (range 1.5–5.2 h).
The general data for the two groups are summarised in Table I. In addition, 6 newborns that were
hospitalized for monitoring according to their guardian’s request
but were diagnosed as normal constituted a control group. The
newborns were equal in gender, with a mean gestational age of
40.12±0.23 weeks (range, 39+3–40+4 weeks), a mean body mass at
birth of 3250.15±335.50 g (range, 2,950–3,520 g) and a mean age at
hospital admission of 4.02±0.15 h (range, 2.5–5.5 h).
 | Table IComparison of general patient
information. |
Table I
Comparison of general patient
information.
| Group | Gestational age
(weeks) | Body mass at birth
(g) | Gender
(male/female) | Admission age
(h) |
|---|
| Mild hypothermia
(n=37) | 37.55±4.21 | 3390.27±573.08 | 24/13 | 4.03±1.28 |
| Conventional
(n=26) | 38.12±1.21 | 3393.07±473.41 | 10/16 | 4.22±1.01 |
| Statistical
values | Z=0.52 | Z=0.41 |
χ2=4.32 | Z=0.60 |
| P-value | 0.68 | 0.50 | 0.30 | 0.68 |
This study was approved by the Ethics Committee of
the Affiliated Hospital of Luzhou Medical College. Informed consent
was obtained from the patients’ legal guardians.
Grouping
Newborn patients were randomly divided into mild
hypothermia therapy (n=37) and conventional therapy (n=26) groups
using the coin-toss method. The conventional therapy group received
a ‘three-supportive and two-symptomatic’ treatment. In this
treatment: aeration and oxygenation were properly maintained to
avoid hypoxemia, hyperoxia, hypercapnia or hypocapnia; brain blood
perfusion was maintained to avoid severe blood pressure
fluctuation; a correct blood glucose level was maintained; liquid
intake was properly controlled to prevent encephaloedema and;
phenobarbital was administered to prevent convulsions. In the mild
hypothermia group, mild hypothermia therapy was performed in
addition to conventional therapy. 18F-FDG PET/CT
examination was performed at the time of hospital admission and
seven days following treatment.
Mild hypothermia therapy
The Olympic Cool-Cap 004204A, (Natus Medical Inc.,
Seattle, Washington, USA) a mild hypothermia therapeutic apparatus
with a cool-cap system, was used in the current study. The cap was
operated strictly according to the manual and moderate-sized cool
caps were used to cover the heads of the newborns. Special
temperature probes were placed at the rectum, bregma and hepatic
regions, and radiation board probes for emergency treatment were
placed at the brain and abdomen, according to the manufacturers’
instructions. The therapeutic apparatus was started, the
temperature of the cool-cap was set and the temperatures of the
different body sites were monitored. When the body temperature
reached a reasonable range for mild hypothermia therapy (≤35.5°C),
the settings were changed to maintain treatment. Specifically, the
rectal temperature was maintained at 34–35°C and the bregma
temperature at 20–25°C. If the infant’s temperature did not
decrease to the expected level, the power to the radiation board
was shut off, and further mild hypothermia therapy was induced. In
these cases, the temperature usually reached the expected level of
35.5°C in 1–2 h. Subsequently, the radiation board was powered on
and the mild hypothermia therapy was continued. Based on the
dynamic information on the display screen of the therapeutic
apparatus, the newborn patient’s skin was examined every 4 h and
the therapy was paused at 12-h intervals to enable the operator to
check the scalp and to adjust the contact site of the cool-cap as
required. The therapeutic apparatus was set to begin an automatic
rewarming program following the 72 h treatment period. The
temperature of the radiation board was raised according to the
display information until the patient’s rectal temperature
increased by 0.5°C. This benchmark represented the end of rewarming
and the completion of mild hypothermia therapy (11).
Monitoring index
The glucose metabolic rates in the neonatal cerebral
tissue were assessed at the time of hospitalisation and seven days
following treatment using 18F-FDG PET/CT imaging.
18F-FDG was intravenously administered at a dosage of
3.7 MBq/kg and scanning was performed 40–60 min following the
patient entering deep sleep. Semi-quantitative analysis was
performed based on the standardised uptake values (SUVs) in the
regions with abnormal glucose metabolic rates.
A radiologist with >10 years experience and a
similarly senior doctor of nuclear medicine jointly read the films.
A cross-section was selected and the maximum SUV value on that
plane was read. The sections that crossed the site on the first
plane with the maximum SUV value were then compared to obtain the
overall maximum SUV value. The maximum SUV values of the brain
regions, including the thalamus, basal ganglia, sensorimotor area,
parietal lobe, occipital lobe and cerebellar cortex, were
calculated using this method.
PET scanner and photographic
developer
A Gemini TF/T16 PET/CT scanner (Philips, Best, The
Netherlands) was used to carry out the imaging. Image acquisition
was performed over a 10-min interval using 3-dimensional volume
scanning. CT image reconstruction was performed using filtered
backprojection with a 512×512 pixel matrix and 2.5-fold
magnification. 18F-FDG was prepared by the PET Center at
The Affiliated Hospital of Luzhou Medical College The product was
clear and transparent with pH 7.3, a radiochemical purity >95%
and a radioactive half-life of 109 min. Bacterial cultures and
pyrogen tests presented negative results.
Statistical analysis
SPSS software, version 9.0 (SPSS, Inc., Chicago, IL,
USA) was used to carry out the statistical analyses in the current
study. Measurement data are presented as the mean ± standard
deviation (SD); enumeration data are presented as percentages.
Non-parametric and the χ2 tests were used to compare
general information. Variance analysis and the rank-sum test were
used for the analysis of differences in 18F-FDG PET/CT
data between groups. The change in glucose metabolism rate as an
effect of therapy was analysed using a paired-sample Student’s
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
General characteristics of the study
subjects
There were no differences between the two groups of
patients with HIE in terms of gestational age, body mass at birth
and age at hospitalisation as determined using a non-parametric
test for two independent samples. There was also no difference in
gender ratio, as determined by the χ2 test (P>0.05;
Table I).
Comparison of the glucose metabolic rates
between groups
According to the results, the mild hypothermia and
conventional therapy treatments significantly improved the rate of
glucose metabolism (Table II and
Fig. 1; P<0.001). There were no
significant differences in the metabolic rate of each brain region,
thalamus, basal ganglia, frontal lobe, parietal lobe and occipital
lobe, between the two groups prior to treatment (Z=1.04, 0.13,
0.80, 1.10, 1.03; P=0.30, 0.90, 0.42, 0.28, 0.31; P>0.05). In
terms of the level of improvement, mild hypothermia therapy had
more promising effects on glucose metabolism rate than conventional
therapy, with statistical significance (Z=4.46, 4.46, 4.46, 4.46,
4.47; P<0.0001).
 | Table IIComparison of the rates glucose
metabolic as assessed by 18F-fluorodeoxyglucose positron
emission computed tomography (18F-FDG PET/CT). |
Table II
Comparison of the rates glucose
metabolic as assessed by 18F-fluorodeoxyglucose positron
emission computed tomography (18F-FDG PET/CT).
| Group (n) | Time point | Normal metabolic
rate, n (%) | Slightly decreased
metabolic rate, n (%) | Markedly decreased
metabolic rate, n (%) | Increased metabolic
rate, n (%) | Comparison between
pre- and post-treatment |
|---|
| Mild hypothermia
(37) | Pre-treatment | 10 (27.03) | 9 (24.32) | 17 (45.95) | 1 (2.70) | χ2=29.20,
P<0.001 |
| Post-treatment | 15 (40.54) | 20 (54.05) | 0 (0.00) | 2 (5.40) | |
| Conventional
(26) | Pre-treatment | 3 (11.54) | 7 (26.92) | 15 (57.69) | 1 (3.85) |
χ2=8.42
P=0.003 |
| Post-treatment | 6 (23.07) | 13 (50.00) | 5 (19.23) | 2 (7.69) | |
| Control (6) | Day 1 | 6 (100.0) | 0 (0.00) | 0 (0.00) | 0 (0.00) | - |
| Day 7 | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | - |
Discussion
Primary and secondary energy failure are significant
constituents of the pathogenesis of neonatal HIE. Furthermore, a
previous study observed a decreased rate of glucose metabolism in
association with HIE (12). The
present study demonstrated that the reduced rate of glucose
metabolism was improved using conventional and mild hypothermia
treatment approaches. Compared with the effects of conventional
therapy, mild hypothermia therapy was more efficacious at improving
the rate of glucose metabolism.
The pathogenesis of neonatal HIE remains unclear.
Potential causes include the combined action of various factors,
including primary and secondary energy exhaustion, the toxic effect
of EAAs and the inflow of Ca2+ ions. Recently,
additional factors have been discovered. Oxygen radicals, generated
in brain injury-associated ischaemia reperfusion, cause cell death
and further injuries to brain tissue (2,3).
Oxidative stress is a major factor in the
development of reperfusion injury (13). Furthermore, inflammatory injury may
also partly contribute to the occurrence of HIE. The cellular
contents released as a consequence of the death and lysis of brain
cells initiates the production of several pre-inflammatory and
inflammatory cytokines, including interleukin (IL)-1β, IL-6, IL-8
and tumour necrosis factor (TNF)-α (2). IL-6 promotes the accumulation of
neutrophils at the site of injury, which increases the permeability
of endotheliocytes and aggravates cellular damage in the brain.
Furthermore, TNF-α induces the degradation of the basement
membrane, inhibits myelinogenesis and triggers thrombus and
haemorrhage, which results in pathological lesions and cell death.
Various apoptosis-promoting factors, including abnormal energy
metabolism, calcium overload, excessive quantities of oxygen
radicals and impairments in DNA replication may contribute to the
development of ischaemia and anoxia by triggering the release of
cytochrome c. This activates caspase 3 which initiates the
apoptosis of neurons under a caspase 3-mediated pathway (3). Disorders of energy metabolism are
major factors in the various forms of HIE pathogenesis.
Mild hypothermia therapy protects brain cells
through various mechanisms, including the inhibition of neuronal
apoptosis, which may be attributed to reduced ATP consumption,
energy expenditure, the accumulation of excitatory
neurotransmitters, oxygen radical generation, cytochrome c
release and caspase 3 activation. Decreased Ca2+ inflow
protects the brain cells and prevents the destruction of structural
proteins. According to a previous study, neuronal apoptosis
associated with abnormal metabolism, oxygen radicals,
pro-inflammatory factors and the excessive release of EAAs usually
peaks at 72 h following a stimulus (14). Since 2005, randomised controlled
trials (RCTs) for systemic and selective local mild hypothermia
therapy have been performed in international centres in the USA,
UK, New Zealand, Canada and other countries. These have verified
the safety and validity of mild hypothermia therapy for neonatal
HIE (15–17).
PET/CT has become a popular procedure for the early
diagnosis of various tumours. It is especially effective for
distinguishing between benign and malignant states. The method has
also been applied in cardiovascular and nervous system diseases,
including in the diagnosis and monitoring of coronary heart
conditions, the assessment of myocardium viability, the
localisation of epileptogenic foci and the early differential
diagnosis of Alzheimer’s and Parkinson’s diseases (18). Due to restrictions based on safety
considerations, PET/CT was rarely applied in paediatrics in the
past (19). In recent years, there
have been numerous studies conducted on the application of PET/CT
in paediatrics and neonatology (20–22).
Therefore, it is reasonable to speculate that PET/CT may
potentially be valuable in various other medical applications,
including for the diagnosis, assessment of treatment and monitoring
of prognosis in neonatal HIE (12). 18F-FDG is the most
commonly used radioactive tracer worldwide. An isomer of glucose,
it is usually absorbed and transported into the brain. The
retention of FDG-6-phosphate (FDG-6-P), a metabolic end-product of
18F-FDG, in the brain reflects the utilisation and
metabolism of glucose in brain cells (23). A local reduction in the level of
blood in HIE results in the reduced utilisation of glucose and a
failure to supply energy. Subsequently, histopathological changes
occur in the neurons, which may account for the damage in brain
tissue that occurs in the disease. PET scanning and localisation
are currently used to demonstrate the extent of hypoxic damage in
adult brains. However, contrary to the stable metabolic conditions
observed in adults, glucose metabolism in newborns is substantially
different, with physiological decreases in the frontal lobe
(24) and increased glucose intake
in the cortex and thalamus. The even distribution of glucose
revealed in PET scans has demonstrated that glucose metabolism is
stable in newborns following a meal. Given their accuracy in
localisation, PET scans may be used to reveal early-stage
abnormalities that are not detectable by CT scans or MRI
examinations. Furthermore, the capability of PET in the assessment
of neurogenesis may enhance and supplement current therapeutic
schedules and early interventions.
The conventional ‘three-supportive and
two-symptomatic’ therapy is beneficial for the improvement of
clinical symptoms and energy metabolism. However, the present study
indicated that the application of mild hypothermia therapy in
addition to a conventional therapeutic strategy may achieve
improved treatment results. The effect of mild hypothermia therapy
on HIE was definitively identified. Although conventional therapy
improved the rate of glucose metabolism, a lower-than-average
cerebral glucose metabolism rate remained in the group. By
contrast, the mild hypothermia therapy group revealed greater
clinical effects and this difference was statistically significant.
The pre-treatment data concerning the glucose metabolism rate in
the cerebral regions revealed no significant differences between
the conventional and mild hypothermia therapy groups. The
post-treatment data indicated that the level of improvement with
mild hypothermia therapy was significantly higher when compared
with that of conventional therapy. In terms of safety,
thrombocytopoenia occurred in one case and the patient recovered
following several days without special treatment. Scalp oedema was
observed in 23 cases, although these patients returned to normal in
3–5 days following the cessation of treatment. No other evident
therapy-associated adverse effects were observed in the patients
during the hospitalisation period.
The present study has certain limitations. Firstly,
although a short-term therapeutic effect was obtained, the
long-term prognosis remains to be further investigated with more
information and study. It is widely accepted that the time window
for mild hypothermia therapy is within 6 h of HIE onset (25,26),
whereas there have been no studies, to the best of our knowledge,
on the definitive effects of mild hypothermia therapy for cases in
which therapy was initiated >6 h following HIE onset. Therefore,
in the present study, treatment was not declined for patients who
had missed the normal time window for treatment; the related
materials and information were collected for further study.
Furthermore, the potential adverse effects should be investigated.
The procedure should be performed according to specifications to
avoid related complications, including scalp oedema. The
thrombocyte count, diffused intravascular coagulation indices,
blood pressure and body-wide circulation should also be closely
monitored.
Compared with regular CT, which only displays
low-density images, the advantage of 18F-FDG PET/CT is
that it is able to provide more information for energy metabolic
analysis in brain tissue. The range of changes in glucose
metabolism may help to elucidate the functional conditions of brain
cells and to assess injury levels and therapeutic effects more
accurately. During the examination process, the radiation capture
time and the 18F-FDG injection should be administered
under strict dosage control.
References
|
1
|
van Laerhoven H, de Haan TR, Offringa M,
et al: Prognostic tests in term neonates with hypoxic-ischemic
encephalopathy: a systematic review. Pediatrics. 131:88–98.
2013.PubMed/NCBI
|
|
2
|
Mao M, Hua Y, Jiang X, et al: Expression
of tumor necrosis factor alpha and neuronal apoptosis in the
developing rat brain after neonatal stroke. Neurosci Lett.
403:227–232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
West T, Atzeva M and Holtzman DM:
Pomegranate polyphenols and resveratrol protect the neonatal brain
against hypoxic-ischemic injury. Dev Neurosci. 29:363–372. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Futrakul S, Praisuwanna P and Thaitumyanon
P: Risk factors for hypoxic-ischemic encephalopathy in asphyxiated
newborn infants. J Med Assoc Thai. 89:322–328. 2006.PubMed/NCBI
|
|
5
|
Glass HC and Ferriero DM: Treatment of
hypoxic-ischemic encephalopathy in newborns. Curr Treat Options
Neurol. 9:414–423. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Key Laboratory of Neonatal Diseases;
Ministry of Health; Children’s Hospital of Fudan University;
Editorial Board of Chinese Journal of Evidence-based Pediatrics;
China GRADE Centre. Guideline of evidence-based treatment for
hypoxic-ischemic encephalopathy in full-term infants (standard
version, 2011). Chinese Journal of Evidence-Based Pediatrics.
6:327–335. 2011.(In Chinese).
|
|
7
|
Polderman KH: Induced hypothermia and
fever control for prevention and treatment of neurological
injuries. Lancet. 371:1955–1969. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yao Z: New products and applications
advantages of PET/CT. China Medical Devices Information. 17(4):
13–16. 2011.(In Chinese).
|
|
9
|
Schirmer M, Calamia KT, Wenger M, et al:
18F-fluorodeoxyglucose positron emission tomography: a
new explorative perspective. Exp Gerontol. 38:463–470. 2003.
View Article : Google Scholar
|
|
10
|
Shao XM: Nervous System Diseases; Section
7, Anoxic Ischemic Encephalopathy Practical Neonatology. 4th
edition. People’s Publishing House; Beijing, China: pp. 699–706.
2010, (In Chinese).
|
|
11
|
Key Laboratory of Neonatal Diseases;
Ministry of Health; Children’s Hospital of Fudan University;
Editorial Board of Chinese Journal of Evidence-based Pediatrics;
China GRADE Centre. Guideline of evidence-based treatment for
hypoxic-ischemic encephalopathy in full-term infants (simplify
version, 2011). Chinese Journal of Evidence-Based Pediatrics.
6:337–339. 2011.(In Chinese).
|
|
12
|
Shi Y, Zhao JN, Jin RB, et al: Clinical
significance of positron emission tomography in preterm, term
newborn infants and neonatal hypoxic-ischemic encephalopathy. Chin
J Clinicians. 6:59–65. 2012.
|
|
13
|
Deng X, Long J, Sun Q, et al: Oxidative
stress and cerebral injury in newborns. Chinese Journal of
Evidence-Based Paediatrics. 25:50–52. 2010.(In Chinese).
|
|
14
|
Chen JC, Shao XM, Liu MH, et al: Clinical
research for elective hypothermia for treatment of newborn
hypoxic-ischemic encephalopathy. Acta Medica Sinica. 15:18–19.
2002.(In Chinese).
|
|
15
|
Shankaran S, Pappas A, Laptook AR, et al:
NICHD Neonatal Research Network: Outcomes of safety and
effectiveness in a multicenter randomized, controlled trial of
whole-body hypothermia for neonatal hypoxic-ischemic
encephalopathy. Pediatrics. 122:e791–e798. 2008. View Article : Google Scholar
|
|
16
|
Rutherford M, Ramenghi LA, Edwards AD, et
al: Assessment of brain tissue injury after moderate hypothermia in
neonates with hypoxic-ischaemic encephalopathy: a nested substudy
of a randomised controlled trial. Lancet Neurol. 9:39–45. 2010.
View Article : Google Scholar
|
|
17
|
Gluckman PD, Wyatt JS, Azzopardi D, et al:
Selective head cooling with mild systemic hypothermia after
neonatal encephalopathy: multicentre randomised trial. Lancet.
365:663–670. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang RF, Li XF and Zhang CL: Progress and
clinical application of PET/CT. China Medical Devices Information.
13(7): 1–4. 142007.(In Chinese).
|
|
19
|
Heneweer C and Grimm J: Clinical
applications in molecular imaging. Pediatr Radiol. 41:199–207.
2011. View Article : Google Scholar
|
|
20
|
Arnoux JB, Verkarre V, Saint-Martin C, et
al: Congenital hyperinsulinism: current trends in diagnosis and
therapy. Orphanet J Rare Dis. 6:632011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kannan S and Chugani HT: Applications of
positron emission tomography in the newborn nursery. Semin
Perinatol. 34:39–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi Y, Jin R, Zhao J, et al: Cerebral
glucose metabolism in neonatal hypoxic-ischemic encephalopathy: a
study by 18F-fluorodeoxyglucose positron emission
tomography. Journal of the Third Military Medical University.
32:51–53. 2010.(In Chinese).
|
|
23
|
Chierichetti F and Pizzolato G:
18F-FDG-PET/CT. Q J Nucl Med Mol Imaging. 56:138–150.
2012.
|
|
24
|
Thorngren-Jerneck K, Ley D,
Hellström-Westas L, et al: Reduced postnatal cerebral glucose
metabolism measured by PET after asphyxia in near term fetal lambs.
J Neurosci Res. 66:844–850. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shao X: Therapeutic hypothermia for
neonatal hypoxic-ischemic encephalopathy. Chinese Journal of
Practical Pediatrics. 21:647–650. 2006.(In Chinese).
|
|
26
|
Ferriero DM: Timing is everything -
delaying therapy for delayed cell death. Dev Neurosci. 24:349–351.
2002. View Article : Google Scholar : PubMed/NCBI
|