Introduction
The blood-brain barrier (BBB) plays an important
role in maintaining a stable environment in normal brain and spinal
cord function. Changes in BBB permeability have been described in
several pathological conditions, including poisoning, immune
insults and irradiation, as well as in selected neurological
disorders, such as stroke, traumatic brain injury and spinal cord
injury (1), where the parenchyma
of the brain or spinal cord is severely damaged (2). Additionally, there have been studies
demonstrating that disruption of the BBB can occur in certain
depressive disorders (3,4), including 5-hydroxytryptamine
(5-HT)-related diseases. For example, abnormal levels of 5-HT have
been demonstrated to result in neuronal malfunction, and a genetics
study showed that mice with insufficient 5-HT exhibited anxiety and
aggressive behavior (5). A number
of studies have also reported that antibodies against 5-HT
(6), inhibitors of 5-HT synthesis
(7) and 5-HT-modulating compounds
(8) can influence the permeability
of the BBB. 5-HT is synthesized from L-tryptophan in two steps
which are catalyzed by tryptophan hydroxylase (TPH). Thus, TPH is
able to regulate 5-HT in the peripheral tissues and central nervous
system. The genes encoding Tph1 and Tph2 are located on chromosomes
7B5 and 10D1 in the mouse (9).
TPH1 is mainly expressed and synthesized in the periphery (10), but TPH2 is preferentially
synthesized in the brain. However, the effect of tryptophan
hydroxylase 2 (TPH2), a rate-limiting enzyme of 5-HT biosynthesis,
on the integrity of the BBB remains unclear. Therefore, the present
experiment investigated the effect of TPH2 on BBB disruption. BBB
permeability was evaluated by Evans blue (EB) staining in
TPH2-knockout mice.
Materials and methods
Materials
EB (E2129-1G) was purchased from Sigma-Aldrich (St.
Louis, MO, USA). PL2000 DNA marker (D501A; 2,000 bp) was purchased
from Takara Biotechnology, Co., Ltd. (Dalian, China). Anti-TPH2
antibody (PA1-778) was purchased from Thermo Fisher Scientific,
Inc. (Waltham, MA, USA). Fluorescein isothiocyanate (FITC)-albumin
(A9771) was purchased from Sigma-Aldrich. Wild-type (C57BL/6) mice
were crossed with heterozygous TPH2-flox mice and their offspring
generated homozygous TPH2-knockout mice.
Animals and treatment
All animal protocols used in this study were
approved by the Animal Committee of Tongji University School of
Medicine (TJmed-010–10; Shanghai, China) (11). Adult (12 weeks old, weighing ~25 g)
TPH2-knockout (n=6) and wild-type (n=6) mice were used for this
study. For immunocytochemistry (n=3 from each group) (12), mice were anesthetized with
pentobarbital [50 mg/kg, intraperitoneal (i.p.)] prior to
undergoing transcardial perfusion with 4% paraformaldehyde. The
brain was removed and placed in a 10% sucrose solution overnight.
The following day, the brain was placed in a 20% sucrose solution
for 2 h and then transferred into a 30% sucrose solution. The brain
was subsequently sectioned on a microtome at a thickness of 40 μm.
The sections through the brainstem were collected in a cell culture
plate containing cryoprotectant [30% glycerol, 30% ethylene glycol
and 40 μm phosphate-buffered saline (PBS)] (13). Serial sections were collected and
placed individually into each of the six wells. This sectioning
protocol resulted in six series of sections in total (~40
sections/series) through the brainstem that were 240 μm apart (40
μm × 6). Two wells from each animal were assayed for TPH2
expression.
Immunocytochemistry
Brain sections (40-mm) were incubated with primary
antibody (anti-TPH2; 1:1,000) (14) at 4°C overnight. Subsequent to
washing in PBS, the sections were incubated with
rhodamine-conjugated affinity pure goat anti-rabbit immunoglobulin
G (IgG) (Heavy and Light chain; 1:100; Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA) secondary antibody for 2 h
at room temperature and then washed in PBS. No immunostaining
signals were observed when the primary antibody was omitted or
replaced with normal IgG. Stained sections were observed and
scanned under a fluorescence microscope (Olympus BX53; Olympus,
Tokyo, Japan).
EB extravasation
The BBB permeability was measured using EB (14). In brief, TPH2-knockout mice and
their age-matched controls were weighed and injected (i.p.) with 50
μg/g EB dye in PBS. Twelve hours after injection, the mice were
anesthetized and perfused with PBS for 5 min. Following perfusion,
the brains were dissected and the olfactory bulbs and cerebella
were removed. For the inspection of EB extravasation, the brains
were placed in PBS containing 30% sucrose overnight, and 40-μm
coronal sections were then cut on a cryostat and mounted onto
gelatin-coated glass slides. All mice used in this study exhibited
high levels of EB dye in the liver.
FITC-albumin leakage assay
The permeability of the BBB was analyzed using an
FITC-albumin leakage assay, as previously described (15). Animals were injected intravenously
with 100 mg/kg FITC-albumin 1.5 h after lipopolysaccharide
injection (i.p.). At 2.5 h after the FITC-albumin injection, the
mice were anesthetized and the brains were harvested. The left
hemi-brains were fixed with paraformaldehyde and cut using a
cryostat at a 40-μm thickness for histological analysis. The right
hemi-brains were homogenized in five volumes (wt/vol) of cold PBS
with a Teflon-glass homogenizer (Thomas Scientific, Swedesboro, NJ,
USA). The samples were centrifuged at 10,000 × g for 30 min. The
supernatant was collected and the optical densities of the
homogenates supernatant were read at 488 nm excitation and 525 nm
emission with a fluorescent plate reader (Ascent, Thermo
Scientific, Waltham, MA, USA).
Polymerase chain reaction (PCR)
The toes from all mice were removed according to toe
numbering scheme (http://research.fhcrc.org/fero/en/fero-lab-protocols/mouse-toe-identification.html)
at the postnatal stage P7 and immediately frozen on dry ice. DNA
was extracted using protocols provided by the Dr Yu-Qiang Ding of
Tongji University. The extracted DNA was stored at −20°C until
required. TPH2 was analyzed using a PCR detection system (Biometra
070–851; Analytik Jena, Jena, Germany). The TPH2 or Cre gene
fragments were amplified using previously described primers
(11). The 10 μl total reaction
mixture contained 1 μl genomic DNA, 1 μl of each primer, 3.5 μl
2xTaq PCR MasterMix (Tiangen Biotech, Co., Ltd., Beijing, China)
and 3.5 μl ddH2O. The reaction mixture was initially
denatured at 94°C for 2 min, followed by 30 cycles at 94°C for 30
sec, 58–60°C for 30 sec and 72°C for 45 sec. The PCR was completed
by a final extension cycle at 72°C for 5 min. Successful
amplification of the fragments was confirmed by detection of a 213
or 384 bp band for Tph2, or 400 bp strand for Cre on a 1.5% agarose
gel.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Two-group comparisons were performed by the Student’s
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results and Discussion
Disturbances in the BBB are becoming a common
denominator in depressive disorders (3,4). A
dysfunctional BBB can lead to the leakage of various neurotoxic
substances into the brain, resulting in neuronal damage (14) and brain dysfunction. In the present
study, it was demonstrated using EB staining that BBB impairment
does not occur in TPH2-knockout mice.
In order to ensure that the TPH2 gene was knocked
out in the experiment, PCR and immunocytochemistry methods were
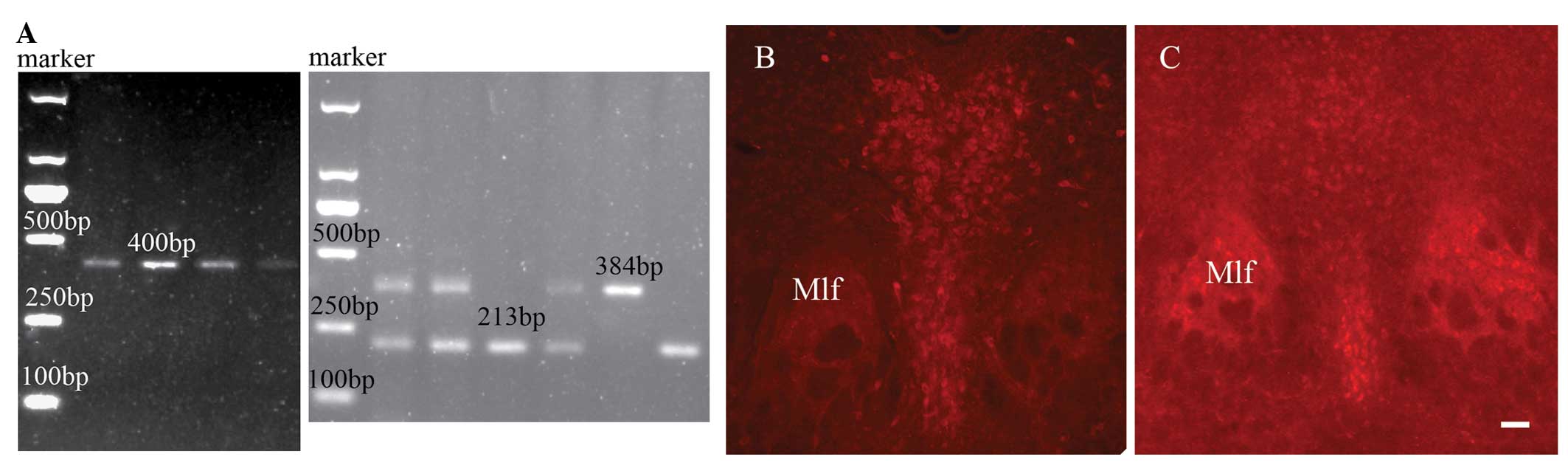
performed, as illustrated in Fig.
1. Mice were genotyped by PCR with primers against Cre
(forward, TCG ATG CAA CGA GTG ATGAG; reverse, TCC ATG AGT GAA CGA
ACC TG), resulting in a 400-bp product, as well as against
TPH2-flox (forward, CAG GTA GAG AGC CAA TCA AAG AGTG; reverse, CTG
GGC TGG CCG ATA GTA ACAC), resulting in 213-bp wild-type and 384-bp
heterozygote products. All mice carrying the Cre gene were found to
be viable, without any evident abnormalities. Fig. 1A shows the DNA detection results of
the wild-type and homozygous TPH2-knockout mice (Cre, 400 bp;
wild-type, 213 bp; and heterozygous TPH2-knockout, 384 bp).
TPH2-positive neurons were observed in the wild-type mice (Fig. 1B), but not in the knockout mice
(Fig. 1C). Thus, the results
showed that all the knockout mice used in the study exhibited TPH2
gene absence.
As shown in Fig. 2A and
B, no EB stain could be observed in the whole brain in the
TPH2-knockout or wild-type groups after 12 h EB i.p. injection.
Similarly, no differences were observed in the tissue sections
between the two groups (Fig. 3).
Notably, when the mouse abdominal spaces were open, it was observed
that the lung, heart, kidney and liver were all stained blue by EB.
Furthermore, in order to verify the effect of the TPH2 gene on the
BBB integrity, the permeability of the BBB was examined using the
FITC-albumin leakage assay. FITC-labeled albumin was injected from
the tail vein, and leakage of dye into the brain parenchyma was
measured as an index of BBB permeability. FITC-albumin was
restricted to the inside of the brain blood vessels and no
significant signals were detected in the brain parenchyma in the
two genotypes following PBS injection (data not shown).
Quantification of the FITC-albumin leakage revealed that the
severity of the BBB breakdown was not significantly different in
the TPH2-knockout and wild-type mice. Therefore, it was speculated
that the knockout of the TPH2 gene had no effect on the BBB. By
contrast, BBB permeability has been shown to be influenced by the
elevation of circulating 5-HT levels (16), neutralization of endogenous 5-HT
activity and/or the blocking of its receptors (6,17).
Therefore, the data on the effect of serotonin on BBB permeability
are contradictory. This may be due to differences in the species
used, the dose regimen applied or the animal model.
The BBB is known to change in major depressive
disorder (MDD), which is a severe psychiatric syndrome with a high
prevalence and socioeconomic impact (18). MDD (lifetime prevalence 13–16%) is
also a complex combination of disturbances in cognition, behaviour
and physical functioning. MDD is the third leading cause of global
disease and a leading cause of disability worldwide as depression
is clinically and aetiologically heterogeneous (19). However, the underlying
pathophysiology of MDD has yet to be fully elucidated. Despite
this, it has been indicated that a disturbance in central 5-HT
activity is a key factor (18). In
the present study, when TPH2 was knocked out and the 5-HT neurons
were lost, abnormal behavior was observed, but no difference was
identified in the EB staining. Thus, we hypothesized that BBB
permeability in 5-HT-related MDD is influenced by a number of
factors (4).
In conclusion, the 5-HT system offers numerous
possibilities to develop novel treatments for MDD. Understanding
the association between TPH2 (a 5-HT synthesis rate-limiting enzyme
in the central nervous system) and BBB permeability may be
beneficial for the identification of novel therapeutic and
preventative approaches in MDD.
Acknowledgements
This study was supported by the Zhejiang Provincial
Natural Science Foundation of China (no. LY13H090007) and the
Scientific Research Foundation of Wenzhou Medical College
(QTJ12007).
References
|
1
|
Xu CJ, Xu L, Huang LD, et al: Combined NgR
vaccination and neural stem cell transplantation promote functional
recovery after spinal cord injury in adult rats. Neuropathol Appl
Neurobiol. 37:135–155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ovadia H, Abramsky O, Feldman S and
Weidenfeld J: Evaluation of the effect of stress on the blood-brain
barrier: critical role of the brain perfusion time. Brain Res.
905:21–25. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Niklasson F and Agren H: Brain energy
metabolism and blood-brain barrier permeability in depressive
patients: analyses of creatine, creatinine, urate, and albumin in
CSF and blood. Biol Psychiatry. 19:1183–1206. 1984.PubMed/NCBI
|
|
4
|
Najjar S, Pearlman DM, Devinsky O, Najjar
A and Zagzag D: Neurovascular unit dysfunction with blood-brain
barrier hyperpermeability contributes to major depressive disorder:
a review of clinical and experimental evidence. J
Neuroinflammation. 10:1422013. View Article : Google Scholar
|
|
5
|
Ding YQ, Marklund U, Yuan W, et al: Lmx1b
is essential for the development of serotonergic neurons. Nat
Neurosci. 6:933–938. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sharma HS, Patnaik R, Patnaik S, Mohanty
S, Sharma A and Vannemreddy P: Antibodies to serotonin attenuate
closed head injury induced blood brain barrier disruption and brain
pathology. Ann NY Acad Sci. 1122:295–312. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sharma HS, Winkler T, Stålberg E, Mohanty
S and Westman J: p-Chlorophenylalanine, an inhibitor of serotonin
synthesis reduces blood-brain barrier permeability, cerebral blood
flow, edema formation and cell injury following trauma to the rat
brain. Acta Neurochir Suppl. 76:91–95. 2000.
|
|
8
|
Polo PA, Reis RO, Cedraz-Mercez PL, et al:
Behavioral and neuropharmacological evidence that serotonin crosses
the blood-brain barrier in Coturnix japonica (Galliformes;
Aves). Braz J Biol. 67:167–171. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gutknecht L, Kriegebaum C, Waider J,
Schmitt A and Lesch KP: Spatio-temporal expression of tryptophan
hydroxylase isoforms in murine and human brain: convergent data
from Tph2 knockout mice. Eur Neuropsychopharmacol. 19:266–282.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang X1, Beaulieu JM, Sotnikova TD,
Gainetdinov RR and Caron MG: Tryptophan hydroxylase-2 controls
brain serotonin synthesis. Science. 305:2172004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song NN, Xiu JB, Huang Y, et al: Adult
raphe-specific deletion of Lmx1b leads to central serotonin
deficiency. PLoS One. 6:e159982011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bach H, Arango V, Huang YY, Leong S, Mann
JJ and Underwood MD: Neuronal tryptophan hydroxylase expression in
BALB/cJ and C57Bl/6J mice. J Neurochem. 118:1067–1074. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Watson RE Jr, Wiegand SJ, Clough RW and
Hoffman GE: Use of cryoprotectant to maintain long-term peptide
immunoreactivity and tissue morphology. Peptides. 7:155–159. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dickstein DL, Biron KE, Ujiie M, Pfeifer
CG, Jeffries AR and Jefferies WA: Abeta peptide immunization
restores blood-brain barrier integrity in Alzheimer disease. FASEB
J. 20:426–433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takeda S, Sato N, Ikimura K, Nishino H,
Rakugi H and Morishita R: Increased blood-brain barrier
vulnerability to systemic inflammation in an Alzheimer disease
mouse model. Neurobiol Aging. 34:2064–2070. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sharma HS, Olsson Y and Dey PK: Changes in
blood-brain barrier and cerebral blood flow following elevation of
circulating serotonin level in anesthetized rats. Brain Res.
517:215–223. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sharma HS, Westman J, Navarro JC, Dey PK
and Nyberg F: Probable involvement of serotonin in the increased
permeability of the blood-brain barrier by forced swimming. An
experimental study using Evans blue and 131I-sodium
tracers in the rat. Behav Brain Res. 72:189–196. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Artigas F: Serotonin receptors involved in
antidepressant effects. Pharmacol Ther. 137:119–131. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao YJ, Du MY, Huang XQ, et al: Brain
grey matter abnormalities in medication-free patients with major
depressive disorder: a meta-analysis. Psychol Med. 1–11.
2014.PubMed/NCBI
|