Introduction
Intrahepatic cholestasis of pregnancy (ICP) is a
liver disorder specific to pregnancy, characterized by maternal
pruritus in the third trimester of gestation and increased levels
of serum bile acid. Although the symptoms disappear quickly in
mothers following delivery, the high level of bile acid is
detrimental to the development of fetal organs and the functions of
the umbilical cord and placenta, and may result in preterm labor
and elevated prenatal mortality (1–3). The
etiology of ICP is complex and has yet to be fully elucidated;
however, a recent study suggested that ICP may be associated with
the immunity imbalance in pregnant patients (4).
Numerous studies have investigated the mechanisms of
maternal-fetal immune tolerance. The placenta functions as an
immunological barrier between the mother and the fetus, which
protects the fetus from maternal immune rejection (5). Human leukocyte antigen G (HLA-G)
belongs to the HLA nonclassical class I heavy chain paralogues and
mediates the protection from the deleterious effects of natural
killer cells, cytotoxic T lymphocytes, macrophages and mononuclear
cells (6–8). HLA-G is highly expressed in the
placental trophoblast cells and thus has an important role in the
regulation of immune tolerance in pregnancy and the maintenance of
gestation (6–8). In a previous study, we demonstrated
that the expression of HLA-G protein was significantly reduced in
the extravillous trophoblasts of patients with ICP, whilst tumor
necrosis factor-α (TNF-α) and TNF-α/interleukin-4 (IL-4) were
significantly elevated in patients with ICP (9). Therefore, it may be predicted that
the downregulation of HLA-G and the imbalance of T helper 1
(Th1)/Th2 cytokines may be associated with the occurrence of
ICP.
MicroRNAs (miRNAs) are a class of cellular RNAs that
affect the translation or stability of target messenger RNAs.
Active, mature miRNAs are 17–24 bases long, and are single-stranded
RNA molecules expressed in eukaryotic cells, which are highly
conserved across species (10–12).
miRNAs have an important role in a number of physiological and
pathological processes. Numerous studies have demonstrated that
miRNAs have a key role in the differentiation of immune cells and
regulation of immune responses (13,14).
miR-148 is an important miRNA associated with immunity, and has a
role in the regulation of immune balance, the innate immune
response of dendritic cells, antigen presentation and inhibition of
the production of numerous inflammation-associated cytokines
(15,16). It has been demonstrated that
miR-148a and miR-152 directly downregulate HLA-G expression by
binding to the 3′ untranslated region (UTR), and are expressed at
low levels in the placenta compared with other healthy tissues
(17). These findings are
consistent with the specific high expression levels of HLA-G
observed in the placenta. However, the expression pattern of miRNAs
and their functions in the placenta of patients with ICP remain
unclear.
In the present study, the expression levels of HLA-G
and miR-148a in the placenta of patients with ICP and healthy
subjects were investigated, as well as the levels of miRNA-18a and
bile acids in the peripheral blood. The correlation between HLA-G
or miR-148a expression and serum total bile acid (TBA) levels was
also analyzed. This study may provide new evidence for
understanding the pathogenesis of ICP.
Materials and methods
Patients and sample collection
Between December 2011 and January 2013, 37 patients
underwent a cesarean section at the Department of Obstetrics,
Second Xiangya Hospital, Central South University (Changsha,
China). These included 20 patients with ICP (mean age, 28.40±4.057
years; gestational week at delivery, 37.44±0.891) and 17 healthy
subjects (mean age, 28.82±4.812 years; gestational week at
delivery, 37.81±0.570). The enrollment criteria for patients with
ICP were as follows: pruritus and jaundice in the third trimester
of pregnancy; no signs of chronic liver disease, skin disease or
symptomatic cholelithiasis; elevated levels of aminotransferases
and TBAs; and normal cholestasis following delivery. The healthy
subjects did not have any history of gallstones or cholecystopathy,
pruritus, drug consumption, hepatitis or any other diseases
associated with hepatobiliary function. None of the patients in the
present study received immune and hormone therapy during
pregnancy.
Fresh placenta tissues were obtained during the
cesarean section. The tissues were immediately frozen in liquid
nitrogen and then stored at −80°C for RNA and protein isolation.
Blood samples from patients with ICP were collected prior to drug
treatment, while blood samples from normal pregnant women were
collected following overnight fasting. The serum was obtained by
centrifugation and stored at −20°C until further analysis. The
serum TBA was measured using an enzymatic cycling assay. Briefly,
the cells are lysed and the total protein is collected. The protein
is then centrifuged for 10 min by 15,294 × g at 4°C. Bile acid is
specifically oxidized by 3 alpha-hydroxy steroid dehydrogenase and
Thio-NAD+, and then 3-ketosteroid and Thio-NADH are generated. In
addition, with the presence of 3 alpha-hydroxy steroid
dehydrogenase and NADH, the 3-ketosteroids transfer into bile acid
and NAD+. This procedure is repeated multiple times to amplify the
amount of bile acids, determine the absorbance of Thio-NADH
generated and obtain the value of bile acid.
Informed consent was obtained from all subjects and
the protocol was approved by the Ethics Committee of the Second
Xiangya Hospital. All data were recorded in a computerized
database.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was extracted from frozen placenta tissues
using TRIzol® reagent (Invitrogen Life Technologies,
Grand Island, NY, USA) in accordance with the manufacturer’s
instructions. qPCR was performed using the SYBR® Green
Real-time PCR Master Mix (Toyobo, Osaka, Japan). The primers of
HLA-G were as follows: forward, 5′-CCACCACCCTGTCTTTGACTAT-3′ and
reverse, 5′-GTGTATCTCTGCTCCTCTCCAG-3′. GAPDH was used as a control
using the following primers: forward, 5′-GCACCGTCAAGGCTGAGAAC-3′
and reverse, 5′-TGGTGAAGACGCCAGTGGA-3′. The results were analyzed
using the Ct (2−ΔCt) method using the following formula:
ΔCt = (CtHLA-G − CtGAPDH).
In order to analyze miR-148a and RNU6B expression
(U6 snRNA was used as a reference gene), a two-step qPCR with
specific primers for miR-148a and RNU6B was performed in accordance
with the manufacturer’s instructions. The primers were designed by
Applied Biosystems (Foster City, CA, USA). The qPCR was performed
using a PRISM 7300 Sequence Detection system (Applied Biosystems),
with a 25-μl reaction system containing 10 μl PCR Master Mix
(Ambion, Austin, TX, USA) and 1.33 μl reverse transcription
product, and each sample was analyzed in triplicate. PCR was
performed at 95°C for 10 min, followed by 40 cycles of 95°C for 15
sec and 60°C for 60 sec. The results represent three independent
assays. Relative expression of miR-148a in ICP tissues was
calculated using the comparative Ct (2−ΔCt) method,
using U6 small nuclear RNA (Ambion) as the endogenous control.
Western blot analysis
Total proteins were extracted and separated using
10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
then transferred onto polyvinylidene fluoride membranes (Millipore,
Bedford, MA, USA). The membranes were incubated with anti-human
HLA-G antibody (Abcam, Cambridge, MA, USA) at 4°C overnight, and
then incubated with horseradish peroxidase-conjugated secondary
antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1 h at
room temperature prior to detection by chemiluminescence
(SuperSignal West Pico Chemiluminescent Substrate; Thermo
Scientific, Rockford, IL, USA). β-actin antibody (Santa Cruz
Biotechnology) was used as a loading control. The intensity of
bands was quantified using Quantity One software (Bio-Rad
Laboratories Ltd., Hercules, CA, USA), and the relative intensity
of the protein bands was determined against β-actin.
Statistical analysis
The SPSS software package, version 13.0 (SPSS, Inc.,
Chicago, IL, USA) was used for statistical analysis. All data are
represented as the mean ± standard error (SE). One-way analysis of
variance (one-way ANOVA) was used for statistical analysis. The
correlation analyses were evaluated by linear regression using
GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
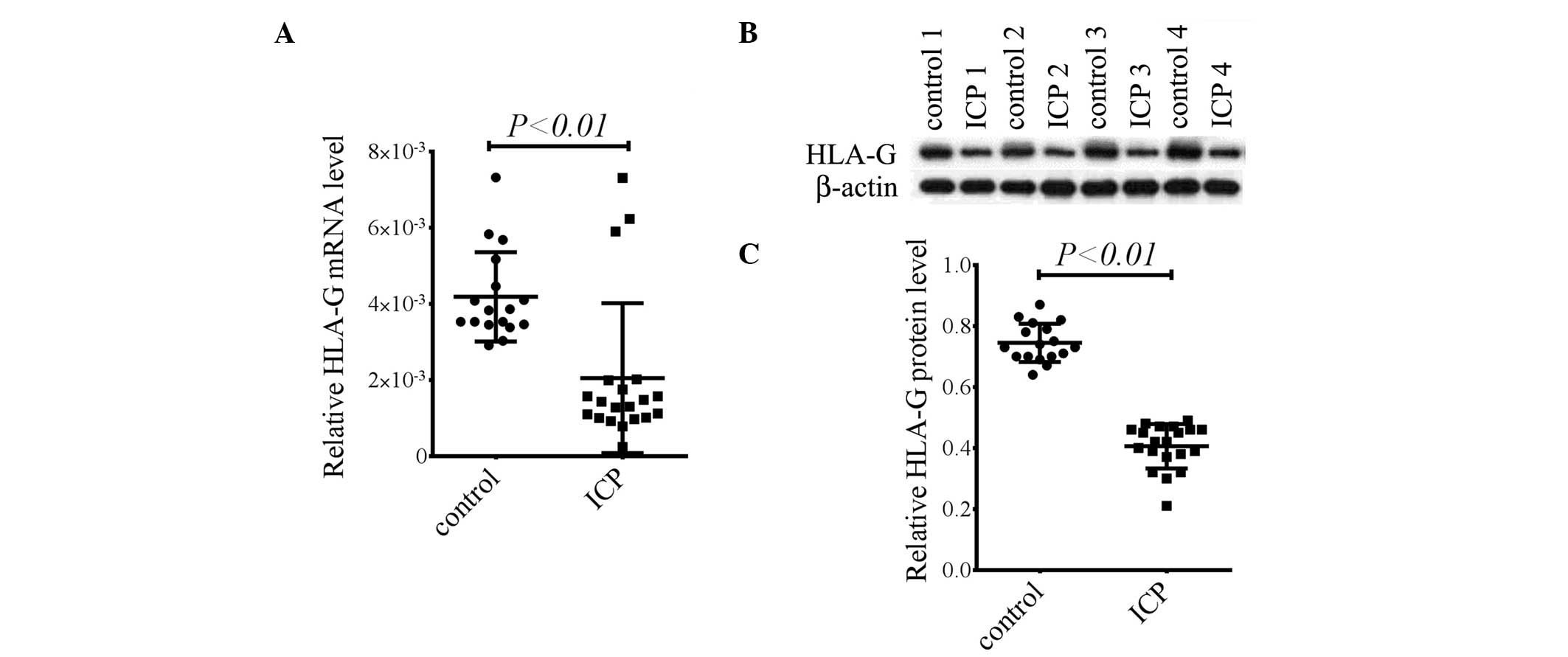
HLA-G mRNA and protein are significantly
reduced in the placenta in patients with ICP
Patients with ICP were verified by determining the
levels of serum TBA, alanine transaminase (ALT) and aspartate
transaminase (AST; data not shown). To investigate the possible
role of HLA-G in patients with ICP, the mRNA expression levels of
HLA-G in the placentas of patients with ICP and healthy pregnant
females were analyzed. The HLA-G mRNA expression levels in the
placenta were significantly reduced in patients with ICP compared
with those in normal pregnant females (P<0.01, Fig. 1A). Consistent with the mRNA levels,
HLA-G protein expression levels were also markedly decreased in the
placentas of patients with ICP (P<0.01, Fig. 1B and C). These results indicate
HLA-G may be involved in the pathogenesis of ICP.
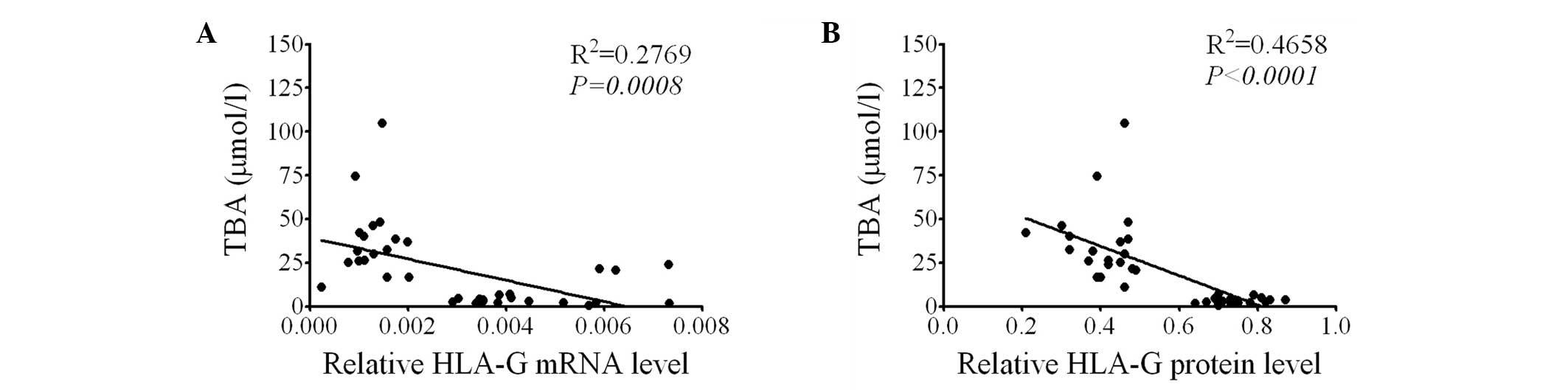
HLA-G expression is negatively correlated
with the serum TBA level
In order to determine the association between HLA-G
expression and ICP, using the data from ICP patients and healthy
pregnant females, the correlation between placental HLA-G
expression and serum TBA level was determined. It was observed that
the HLA-G mRNA level in the placenta was negatively correlated with
the serum TBA level (P=0.008, Fig.
2A). Furthermore, a negative correlation was observed between
the placental HLA-G protein levels and the serum TBA level
(P<0.0001, Fig. 2B).
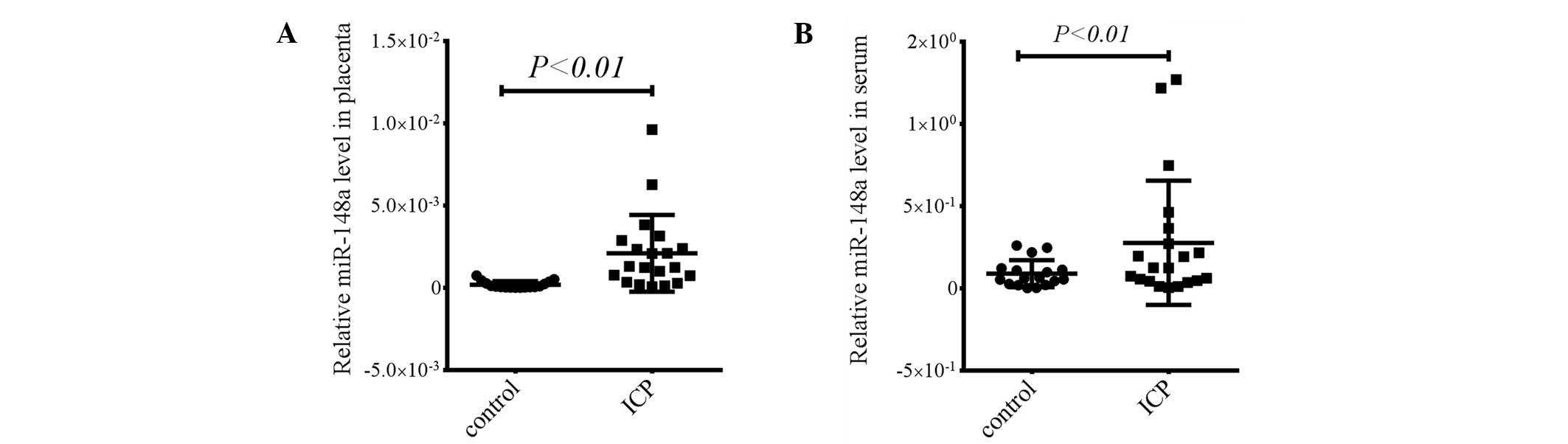
miR-148a levels are elevated in the
placenta and serum in patients with ICP
It has previously been demonstrated that miR-148a is
involved in the regulation of immune response and downregulates
HLA-G expression directly (16,17).
Furthermore, HLA-G is highly expressed in the trophoblast cells in
the placenta (6). Therefore, the
present study investigated the expression of miR-148a in the
placenta. The miR-148a levels in the placenta were significantly
elevated in the patients with ICP compared with those in healthy
pregnant females (P<0.01, Fig.
3A). In addition, miR-148a levels were measured in the serum
and it was found that miR-148a levels were also upregulated in the
serum of patients with ICP (P<0.01, Fig. 3B). These results suggest that the
elevated miR-148a levels may inhibit HLA-G expression in the
placenta of patients with ICP.
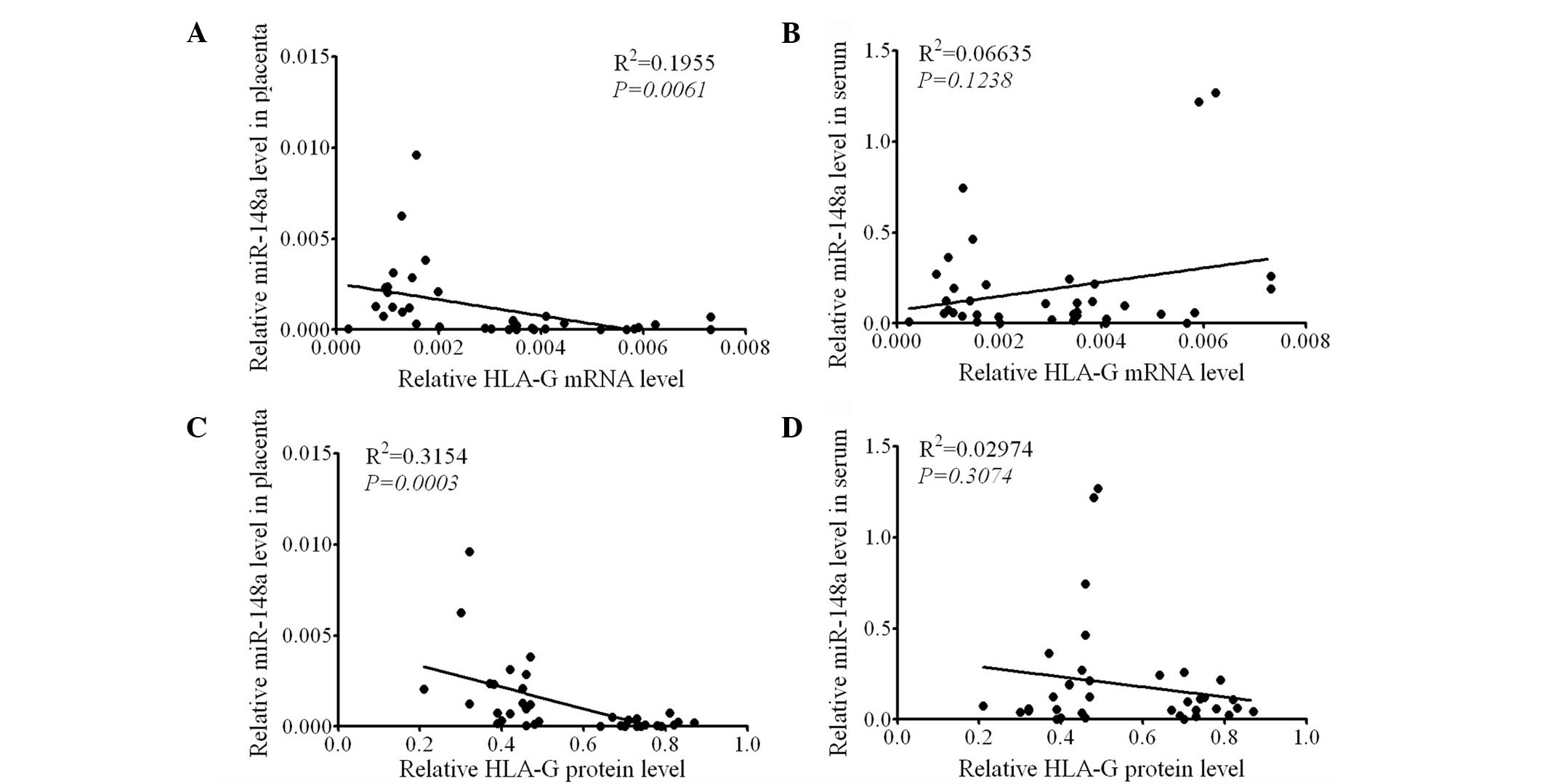
HLA-G expression is negatively correlated
with miR-148a expression levels in the placenta
To verify the association between HLA-G expression
and miR-148a levels, a correlation analysis using linear regression
was performed. In all subjects it was found that the mRNA
expression levels of HLA-G in the placenta were negatively
correlated with the miR-148a levels in the placenta (P=0.0061,
Fig. 4A), but not in the serum
(P=0.1238, Fig. 4B). Furthermore,
HLA-G protein expression levels in the placenta were negatively
correlated with the miR-148a level in the placenta (P=0.0003,
Fig. 4C), but not in the serum
(P=0.3074, Fig. 4D). These results
further support the hypothesis that miR-148a in the placenta is a
negative regulator of HLA-G expression.
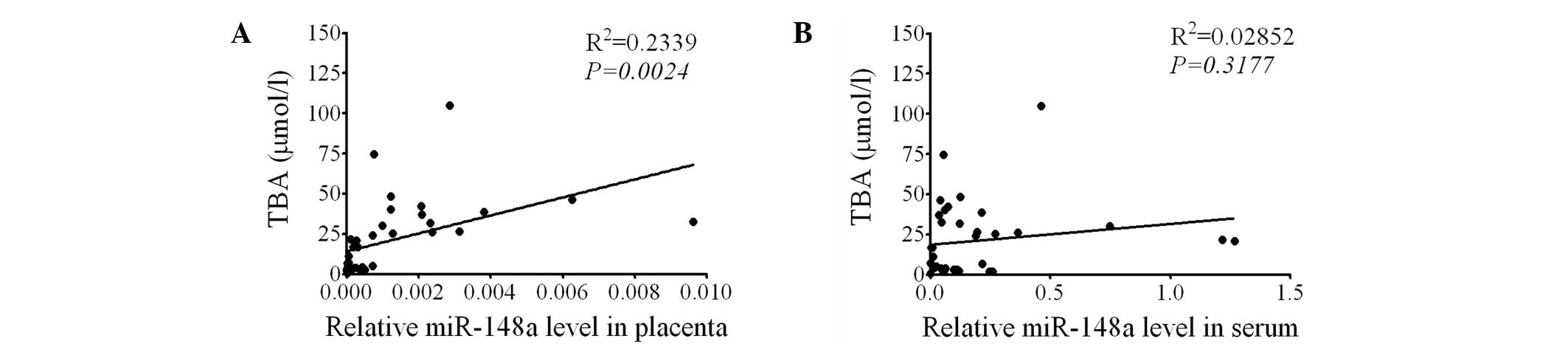
miR-148a levels in the placenta are
positively correlated with serum TBA levels
Since HLA-G expression was found to be negatively
correlated with serum TBA levels and placental miR-148a levels, the
correlation between miR-148a levels and serum TBA was then
investigated. The results demonstrated that the miR-148a levels in
the placenta were positively correlated with serum TBA levels
(P=0.0024, Fig. 5A), while serum
miR-148a was not correlated with serum TBA levels (P=0.3177,
Fig. 5B). These results indicate
that the miR-148a in the placenta may be involved in the regulation
of TBA levels.
Discussion
ICP is a common pregnancy-associated liver disorder,
which is associated with a higher frequency of fetal distress,
preterm delivery and sudden intrauterine fetal mortality (1–3). ICP
is characterized by pruritus starting from the second or third
trimester of pregnancy, which disappears following delivery. The
most sensitive laboratory abnormality in ICP is an increase of
serum TBA levels (2,3). In the present study, serum TBA levels
were found to be significantly elevated in patients with ICP, which
indicates that patients with ICP were correctly identified. ALT and
AST are two important indices for evaluating liver cell damage
(18). Although it has not yet
been demonstrated that the etiology of ICP is associated with
changes in ALT and AST levels, the elevated ALT and AST levels
observed in patients with ICP indicate the adverse effect of ICP on
liver cells.
The exact cause of ICP has yet to be elucidated;
however, a number of studies have demonstrated that ICP is
associated with the immunity imbalance in the placenta (4,19).
The nonclassical HLA-G molecule is a trophoblast-specific molecule
present in almost every pregnancy. Furthermore, HLA-G appears to be
responsible for the reprogramming of local maternal immune response
at the maternal-fetal interface (6,20).
One study demonstrated that mixed purified HLA-G has a dual role in
the first trimester placenta; low HLA-G results in an augmentation
of the allocytotoxic T-lymphocyte response and induction of a Th1
cytokine response, whilst high HLA-G suppresses the
allo-T-lymphocyte response and induces a Th2-type cytokine response
(4). Furthermore, another study
observed that the decreased expression of HLA-G in the extravillous
trophoblasts of patients with ICP may cause a shift of Th1 cytokine
response (21). In the present
study, it was also found that the mRNA and protein expression
levels of HLA-G were significantly reduced in the placentas of
patients with ICP. These results are consistent with previous
findings, and indicate that reduced expression of HLA-G may cause
the imbalance of Th1/Th2 cytokine response and thus is associated
with the pathogenesis of ICP. To verify this hypothesis, the
correlation between HLA-G expression levels in the placenta and
serum TBA levels was investigated in the present study. In all 37
subjects, the expression (mRNA and protein) of HLA-G in the
placenta was negatively correlated with serum TBA levels, although
this correlation was not observed in the ICP group or healthy
pregnant females group. The reason for this difference is that the
variance of HLA-G expression or serum TBA level was small in each
group. Thus these data further confirm that HLA-G is involved in
the pathogenesis of ICP. The downregulation of HLA-G expression in
the placenta is another diagnostic marker for patients with ICP. In
addition, since soluble HLA-G induces a shift in Th1/Th2 cytokine
responses, HLA-G may be considered as a novel therapeutic strategy
for the treatment of patients with ICP.
miRNAs are a class of small RNA molecules that
regulate gene expression and are involved in a number of
physiological and pathological processes, including developmental
processes and immune responses. In addition, they are involved in a
number of pathologies, including cancer and autoimmunity. miRNAs
negatively regulate gene expression post-transcriptionally by
promoting the degradation or inhibiting the translational of target
mRNAs (10–14). However, to the best of our
knowledge, the roles of miRNAs in the pathogenesis of ICP have not
yet been investigated. In the present study it was found that the
miR-148a levels were markedly elevated in the placenta and
peripheral blood of patients with ICP. This suggests that miR-148a
may have a role in the pathogenesis of ICP, and this is the first
report regarding the potential role of miRNA in ICP. A previous
study demonstrated that miR-148a negatively regulates HLA-G
expression by binding to the 3′UTR (17). In the present study, it was
demonstrated that the mRNA and protein expression levels of HLA-G
in the placenta were negatively correlated with the miR-148a levels
in the placenta but not in the peripheral blood, and this finding
further confirms that miR-148a is a negative regulator of HLA-G
expression. Since HLA-G expression in the placenta was negatively
correlated with serum TBA levels, the association between miR-148a
level and serum TBA levels was also determined. It was found that
the miR-148a level in the placenta, but not in the peripheral
blood, was positively correlated with serum TBA levels. This
suggests that miR-148a may be associated with the changes in serum
TBA levels, and HLA-G may also be involved in this association.
In conclusion, in the present study, it was found
that HLA-G was reduced in the placenta of patients with ICP and was
negatively correlated with serum TBA levels. In addition, it was
demonstrated for the first time, to the best of our knowledge, that
miR-148a is upregulated in the placenta and peripheral blood of
patients with ICP. The miR-148a levels in the placenta were
negatively correlated with HLA-G expression, but positively
correlated with serum TBA levels. Therefore, miR-148a is a negative
regulator of HLA-G expression and may regulate serum TBA levels via
the inhibition of HLA-G expression. HLA-G and miR-148a may be
associated with the pathogenesis of ICP; however, the exact
mechanism required further investigation.
Acknowledgements
This study was supported by a grant to Professor
Yiling Ding from Ethics Committee of the Second Xiangya Hospital
(no. KYLLSL-2012-055). This study was completed at Liver Cancer
Laboratory at Xiangya Hospital, Central South University. The
authors would like to thank Professor Lianyue Yang for technical
assistance, as well as Dr Ling Yu from the Second Xiangya Hospital
and Dr Hao Yang and Ruimin Chang from the Liver Cancer
Laboratory.
References
|
1
|
Pan C and Perumalswami PV:
Pregnancy-related liver diseases. Clin Liver Dis. 15:199–208. 2011.
View Article : Google Scholar
|
|
2
|
Williamson C, Miragoli M, Sheikh Abdul
Kadir S, et al: Bile acid signaling in fetal tissues: implications
for intrahepatic cholestasis of pregnancy. Dig Dis. 29:58–61. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Geenes V and Williamson C: Intrahepatic
cholestasis of pregnancy. World J Gastroenterol. 15:2049–2066.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yayi H, Danqing W, Shuyun L and Jicheng L:
Immunologic abnormality of intrahepatic cholestasis of pregnancy.
Am J Reprod Immunol. 63:267–273. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Christiansen OB: Reproductive immunology.
Mol Immunol. 55:8–15. 2013. View Article : Google Scholar
|
|
6
|
Hunt JS and Langat DL: HLA-G: a human
pregnancy-related immunemodulator. Cur Opin Pharmacol. 9:462–469.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
LeMaoult J, Caumartin J, Daouya M, et al:
Immune regulation by pretenders: cell-to-cell transfers of HLA-G
make effector T cells act as regulatory cells. Blood.
109:2040–2048. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Selmani Z, Naji A, Zidi I, et al: Human
leukocyte antigen-G5 secretion by human mesenchymal stem cells is
required to suppress T lymphocyte and natural killer function and
to induce
CD4+CD25highFoxp3+regulatory T
cells. Stem Cells. 26:212–222. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yi J and Ding Y: Expression of HLA-G
protein in placental tissues and its influence on Th1/Th2 cytokines
in peripheral blood in patients with intrahepatic cholestasis of
pregnancy. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 35:241–246.
2010.(In Chinese).
|
|
10
|
Bartel DP: MicroRNAs: target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morales Prieto DM and Markert UR:
MicroRNAs in pregnancy. J Reprod Immunol. 88:106–111.
2011.PubMed/NCBI
|
|
12
|
Nothnick WB: The role of micro-RNAs in the
female reproductive tract. Reproduction. 143:559–576. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Raisch J, Darfeuille-Michaud A and Nguyen
HT: Role of microRNAs in the immune system, inflammation and
cancer. World J Gastroenterol. 19:2985–2996. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu G and Abraham E: MicroRNAs in immune
response and macrophage polarization. Arterioscler Thromb Vasc
Biol. 33:170–177. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Y, Song YX and Wang ZN: The
MicroRNA-148/152 family: multi-faceted players. Mol Cancer.
12:432013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu X, Zhan Z, Xu L, et al:
MicroRNA-148/152 impair innate response and antigen presentation of
TLR-triggered dendritic cells by targeting CaMKIIα. J Immunol.
185:7244–7251. 2010.PubMed/NCBI
|
|
17
|
Manaster I, Goldman-Wohl D, Greenfield C,
et al: MiRNA-mediated control of HLA-G expression and function.
PLoS One. 7:e333952012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ozer J, Ratner M, Shaw M, Bailey W and
Schomaker S: The current state of serum biomarkers of
hepatotoxicity. Toxicology. 245:194–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ling B, Yao F, Zhou Y, et al:
Cell-mediated immunity imbalance in patients with intrahepatic
cholestasis of pregnancy. Cell Mol Immunol. 4:71–75.
2007.PubMed/NCBI
|
|
20
|
Rizzo R, Vercammen M, van de Velde H, Horn
PA and Rebmann V: The importance of HLA-G expression in embryos,
trophoblast cells, and embryonic stem cells. Cell Mol Life Sci.
68:341–352. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peng B, Liu SY, Chen Q, et al: Expression
of human leucocyte antigen G on human placenta and its gene
polymorphism in relation to intrahepatic cholestasis of pregnancy.
Zhonghua Fu Chan Ke Za Zhi. 42:443–437. 2007.(In Chinese).
|