Introduction
Budd-Chiari syndrome (BCS) is a diverse group of
conditions associated with obstructions of hepatic venous outflow
at the level of the large hepatic vein (HV) or the extrahepatic
segment of the inferior vena cava (IVC). All forms of BCS can lead
to serious hemodynamic consequences and severe liver damage from
intense centrilobular congestion of the liver, with ischemia,
pressure necrosis and loss of parenchymal cells in the center of
the liver lobule. This may be life threatening if not diagnosed and
treated promptly (1,2). The most important goal in patients
who refer with the suspicion of having BCS is assessment of the
patency, size of the HVs and IVCs, as well as the collaterals. The
obstructive process in the HVs, IVCs and the collaterals may have
diverse appearances. The diagnosis of BCS predominantly depends on
sonographic examination and digital subtraction angiography (DSA).
However, the limitations of sonography include restriction from
body habitus, intestinal gas or excessive ascites, failure to
demonstrate patent veins within a congested or conversely, shrunken
cirrhotic liver, failure to demonstrate retroperitoneal collaterals
unless they are extremely dilated, and operator-dependency. DSA may
result in vascular injury. The application of computed tomography
angiography (CTA) through the use of a 64-slice spiral CT provides
a noninvasive and accurate method of diagnosing BCS and manifesting
the collateral circulations. The aim of this study was to
illustrate the CTA characteristics of collateral circulations in
BCS.
Materials and methods
Ethical approval
This study was approved by the Ethics Committee of
the Provincial Hospital Affiliated to Shandong University (Jinan,
China), and written informed consent was obtained from all
patients.
Patients
A total of 80 patients with suspected BCS following
ultrasonographic examination underwent CT examination between April
2005 and December 2012 in the Provincial Hospital Affiliated to
Shandong University. The patient cohort consisted of 42 males and
38 females (age range, 19–51 years; mean age, 42.58±8.92 years).
The delay from the appearance of the first clinical symptoms to
diagnosis ranged between 6 months and 24 years. The main clinical
symptoms were right upper abdominal distention and edema of both
lower extremities. Patients in whom BCS was secondary to
oncothlipsis or malignant cell embolism were excluded.
CT examination
All CT scans were performed with a 64-detector row
CT scanner (Lightspeed® VCT; GE Healthcare, Waukesha,
WI, USA) by intravenously injecting 80 ml iopromide
(Ultravist® 300, 740 mg/ml) at a rate of 4 ml/sec,
following 20 ml normal sodium reinjection to make optimal use of
the diagnostic opacity. A dual-phase spiral CT protocol (arterial-
and venous-phase) was performed. Arterial-phase imaging was
performed by using bolus tracking. The data acquisition was
initiated 10 sec after reaching 80 HU in the region of interest,
which was positioned in the aorta at the level of the celiac
artery. The portal and hepatic venous-phase acquisition times were
30 and 50 sec, respectively, after the arterial phase. The average
scanning delay was 20–25 sec for the arterial phase, 55–60 sec for
the portal venous phase and 75–80 sec for the hepatic venous phase
following a bolus injection of contrast agent.
The CT parameters were set as follows: Voltage, 120
kV; tube current, 320 mAsec; collimation, 40 mm; pitch, 0.984; and
reconstruction interval, 0.5 mm. Image reformations were conducted
on a separate workstation (Syngo Volume Perfusion CT Body; Siemens
Healthcare, Erlangen, Germany). Volume rendering, maximum intensity
projection and multiple planar reconstruction images were acquired.
Two readers (both with >10 years vascular CT experience)
retrospectively evaluated the data.
Results
Obstructed HVs and IVCs, as well as the intra- and
extrahepatic collaterals, were found in each of the patients. Based
on the CTA features, collateral pathways were divided into three
groups: Intrahepatic, extrahepatic and portosystemic collateral
pathways.
Intrahepatic collateral circulations
General manifestations
CTA manifestations were consistent with the
ultrasonic features described in our previous study (3). Blood from the obstructed HVs was
drained to the IVC, right atrium, paraumbilical veins or inferior
phrenic veins through various numbers of communicating branches of
different diameters, and was then distributed to the corresponding
systemic venous system. The intrahepatic collateral circulations
were further classified as one of the following six types.
HV-accessory HV collaterals
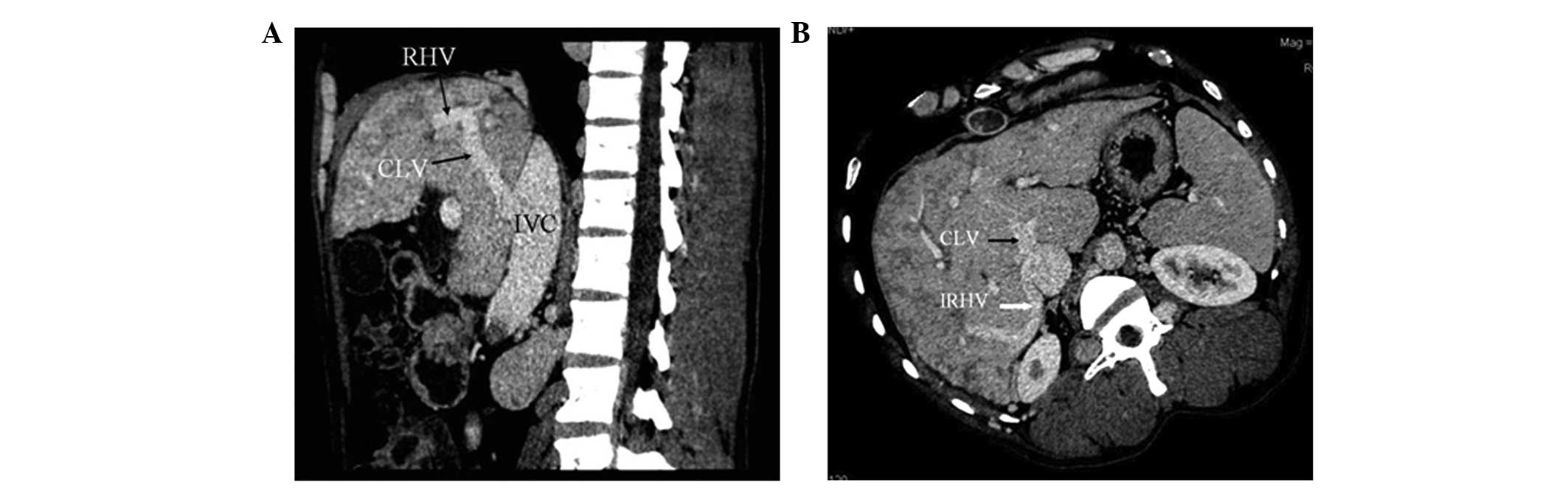
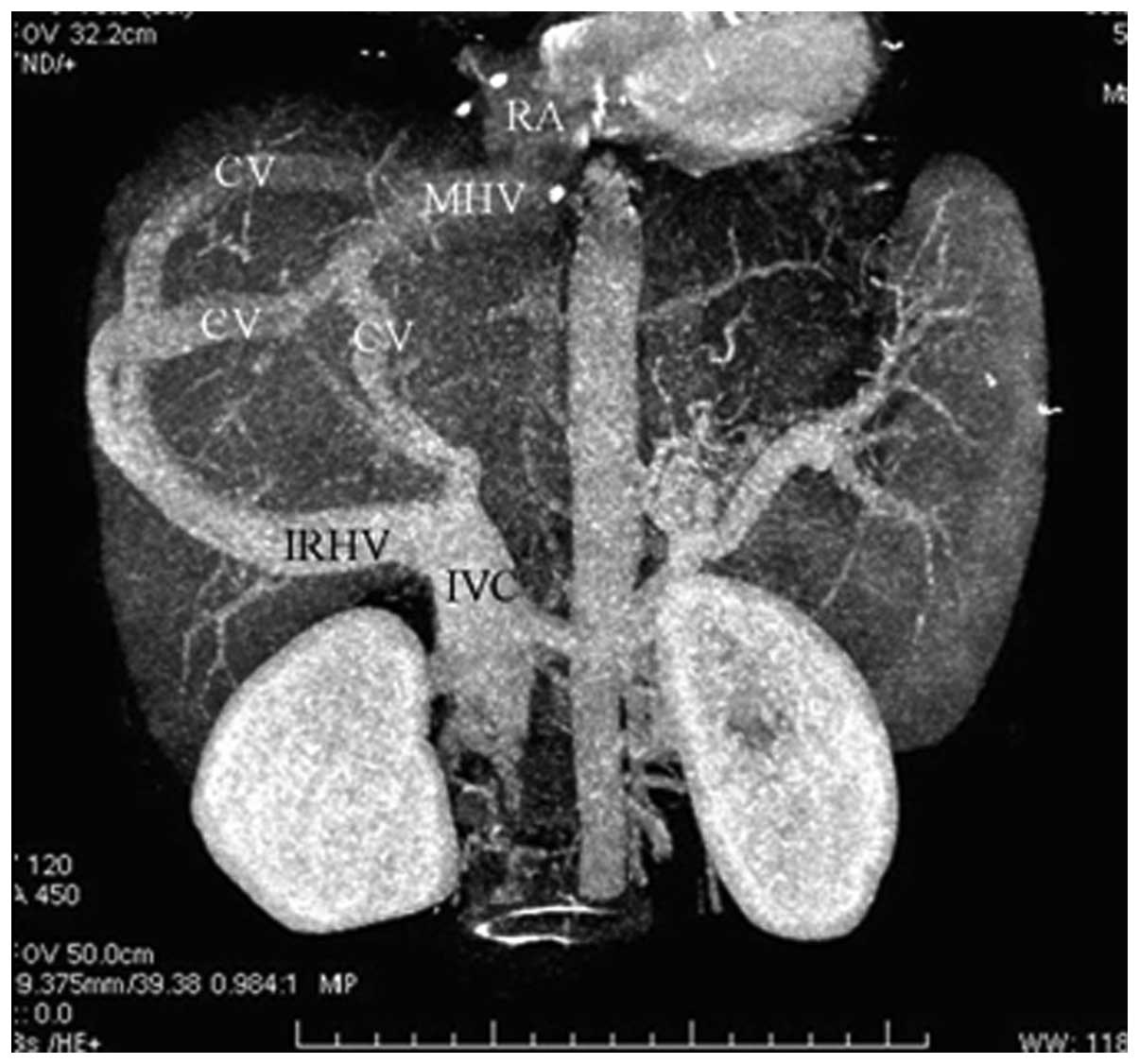
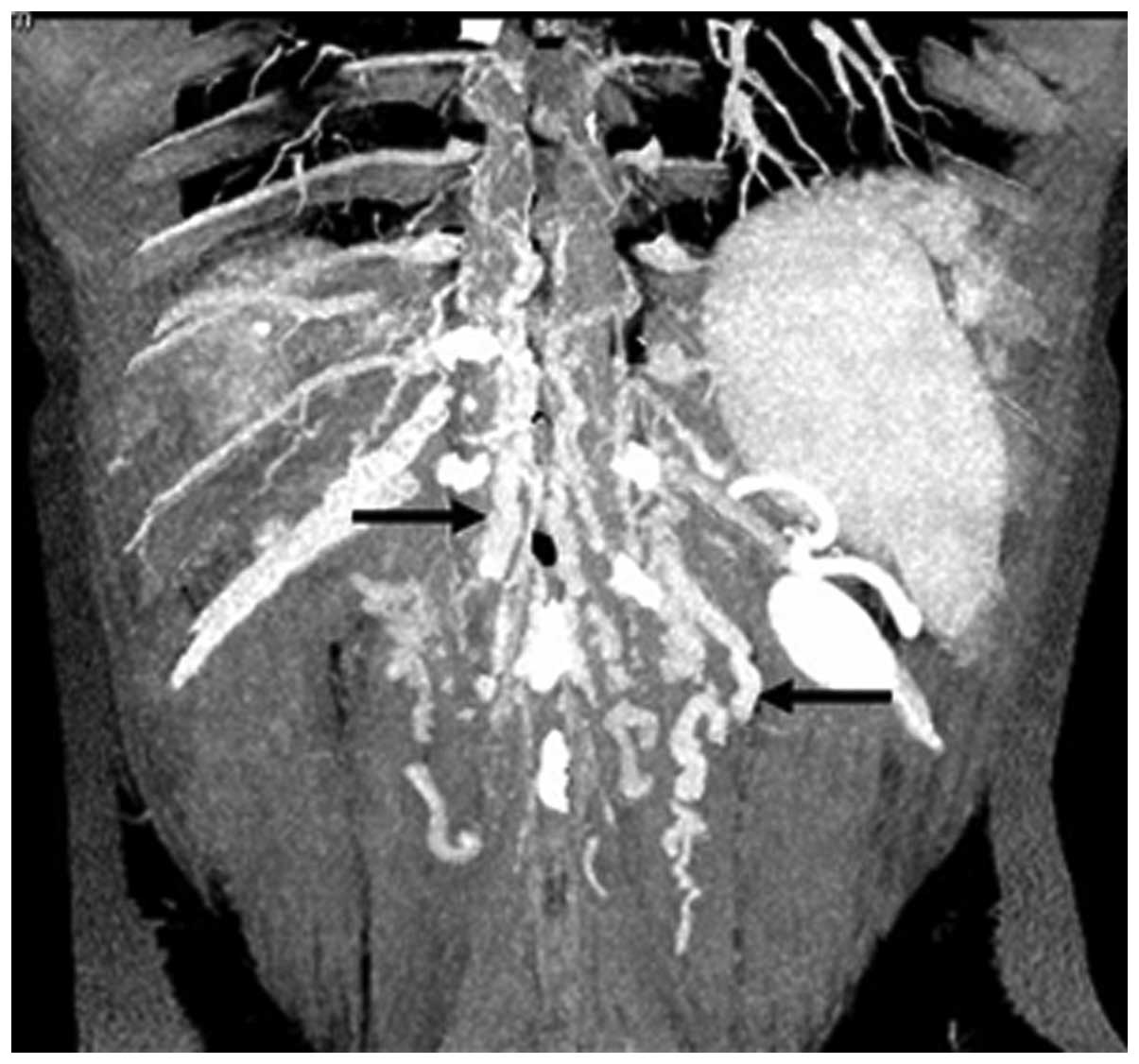
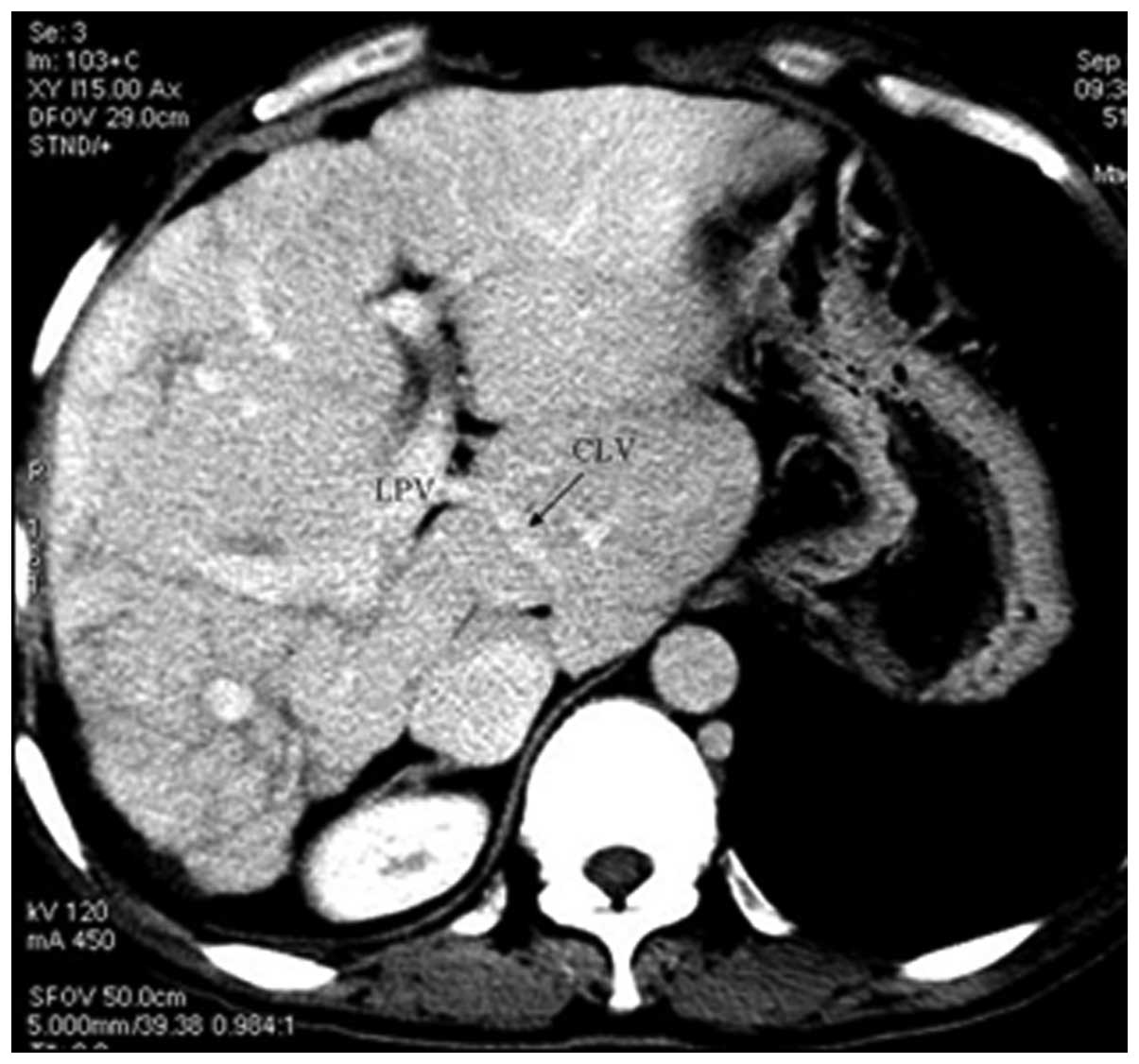
In 51 patients (63.8%), CTA showed that blood from
the occluded HVs was drained into the IVC through the dilated
accessory HVs (Fig. 1). The
accessory HVs included the caudate lobe veins and inferior right
HVs. Caudate lobe veins are the largest veins draining from the
caudate (Spiegel’s) lobe and flowing directly into the retrohepatic
segment of the IVC (4–6). Inferior right HVs are predominantly
in segment VI and in the transverse section; these veins are
located at the posterior interior side of the posterior right
branch of the portal vein and situated within the hepatic
parenchyma in the renal impression. The inferior right HVs enter
into the IVC at the level of the first portal hilum (7).
HV-HV collaterals
In six cases (7.5%), each patient had at least one
patent HV (draining vein). The CTA manifestations were similar to
those for the HV-accessory HV collateral, with the exception that
the draining accessory HV was replaced by the patent HV. CTA showed
that the distal region of the occluded HVs connected to the IVC
through the draining veins. The lumina of the draining veins were
abnormally dilated.
HV-accessory HV plus HV
collaterals
In six cases (7.5%), CTA showed that the occluded
HVs connected to the IVC by means of patent and accessory HVs
(draining veins) simultaneously through communicating branches.
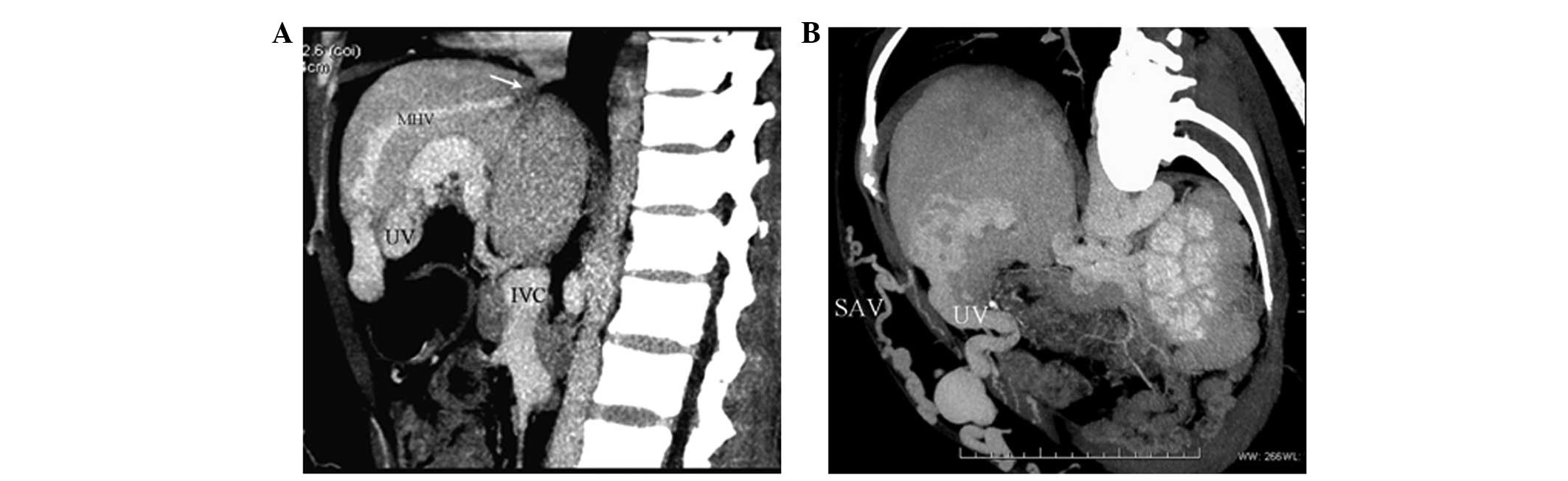
IVC-HV/accessory HV-HV-right atrium
collaterals
Segmental (occluded length >1.5 cm) or septal
(septum thickness ≤1.5 cm) IVC occlusion was detected in five cases
(6.3%). Reversed blood in the HV/accessory HV (inlet located below
the IVC occlusion) flowed through the web-like communicating
vessels, and then to the other HV (inlet located above the IVC
occlusion), prior to arriving at the right atrium (Fig. 2).
HV-umbilical vein collaterals
In four cases (5.0%), CTA showed segmental
obstructions of three HVs. Blood flow in the distal region of the
occluded HVs reversed to reopened paraumbilical veins through
communicating branches, anastomosing with portal venous flow. Blood
then entered the dilated paraumbilical vein and the anterior
abdominal-wall veins (Fig. 3).
HV-inferior phrenic vein
collaterals
In eight cases (10.0%), three HVs of this type were
obstructed to different degrees without dilated accessory HVs.
Blood in the obstructed HVs flowed through the inferior phrenic,
intercostal and retroperitoneal veins, and then into the systemic
venous system (Fig. 4).
Extrahepatic collateral circulations
General manifestations
Extrahepatic collaterals resulted from an
obstruction in the IVC. The extrahepatic collateral circulations
were further classified as one of the following five types.
IVC-lumbar vein-ascending lumbar
vein-azygos/hemiazygos vein collaterals
Collateral circulations were shown with CTA in all
patients (100.0%). Venous flow within the IVC reversed to the
lumbar vein and then continued through the ascending lumbar veins,
anastomosing with the azygos/hemiazygos vein system (Fig. 5).
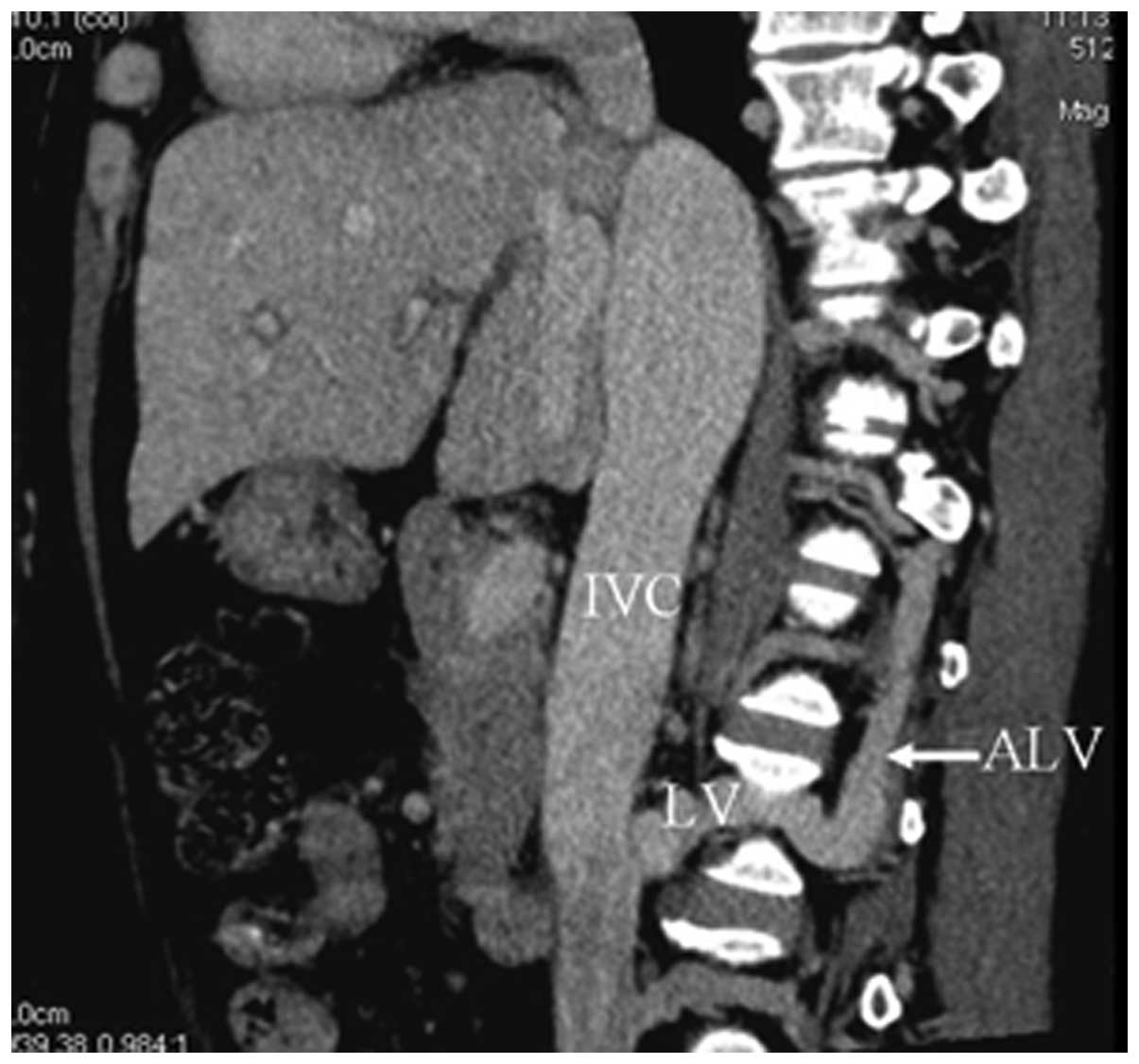
IVC-left renal vein-ascending lumbar
vein-hemiazygos vein collaterals
In 75 cases (93.8%), CTA showed dilated left renal
veins connecting with apparently dilated and tortuous hemiazygos
veins by means of the lumbar vein and ascending lumbar vein
(Fig. 6).
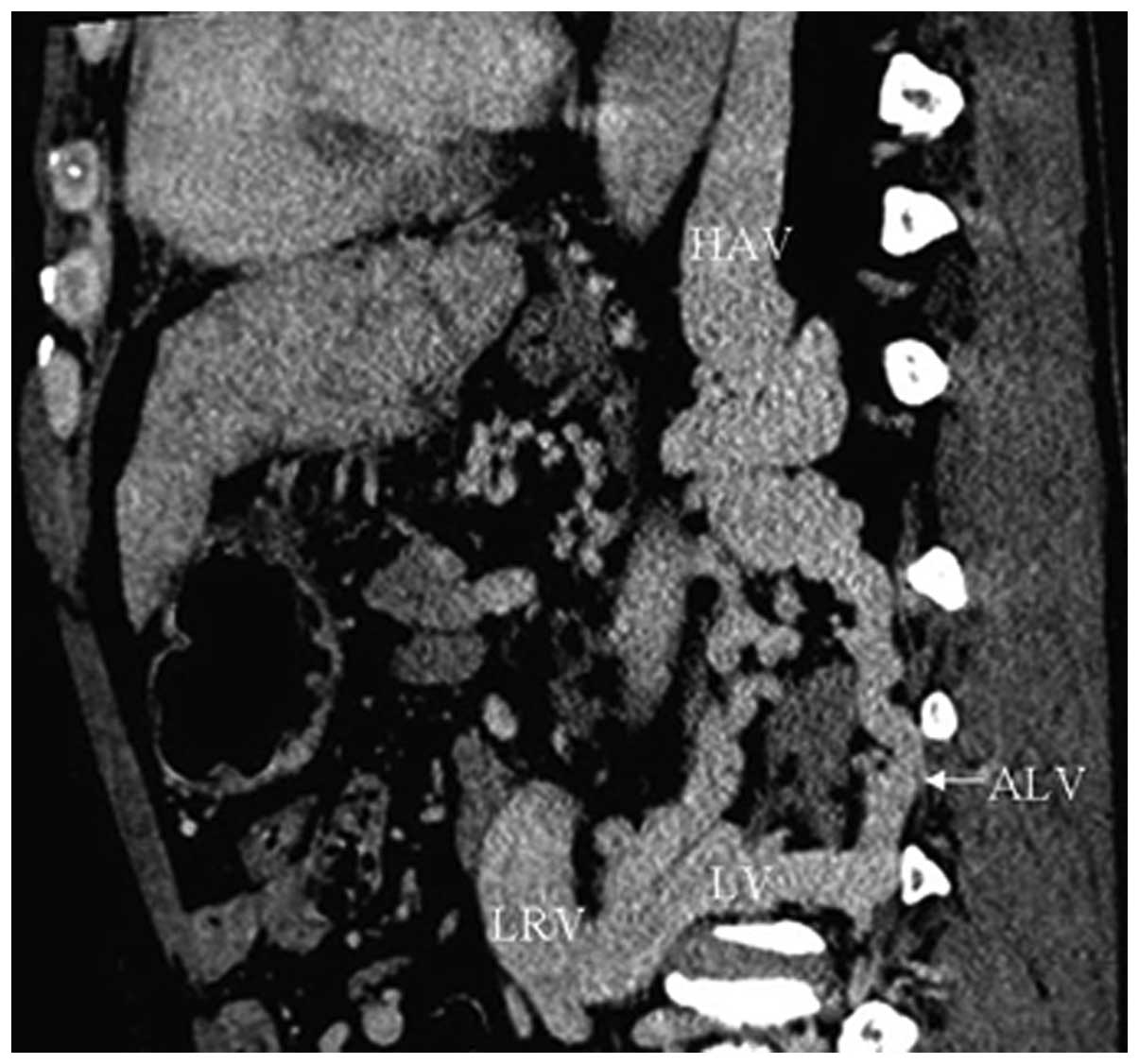
IVC-left renal vein-left inferior
phrenic vein collaterals
In 49 cases (61.3%), CTA showed blood flow from the
IVC reversed to the dilated left renal vein and then to the left
inferior phrenic vein. Tortuous blood vessels in the cardiophrenic
angle were also found in five patients simultaneously (Fig. 7).
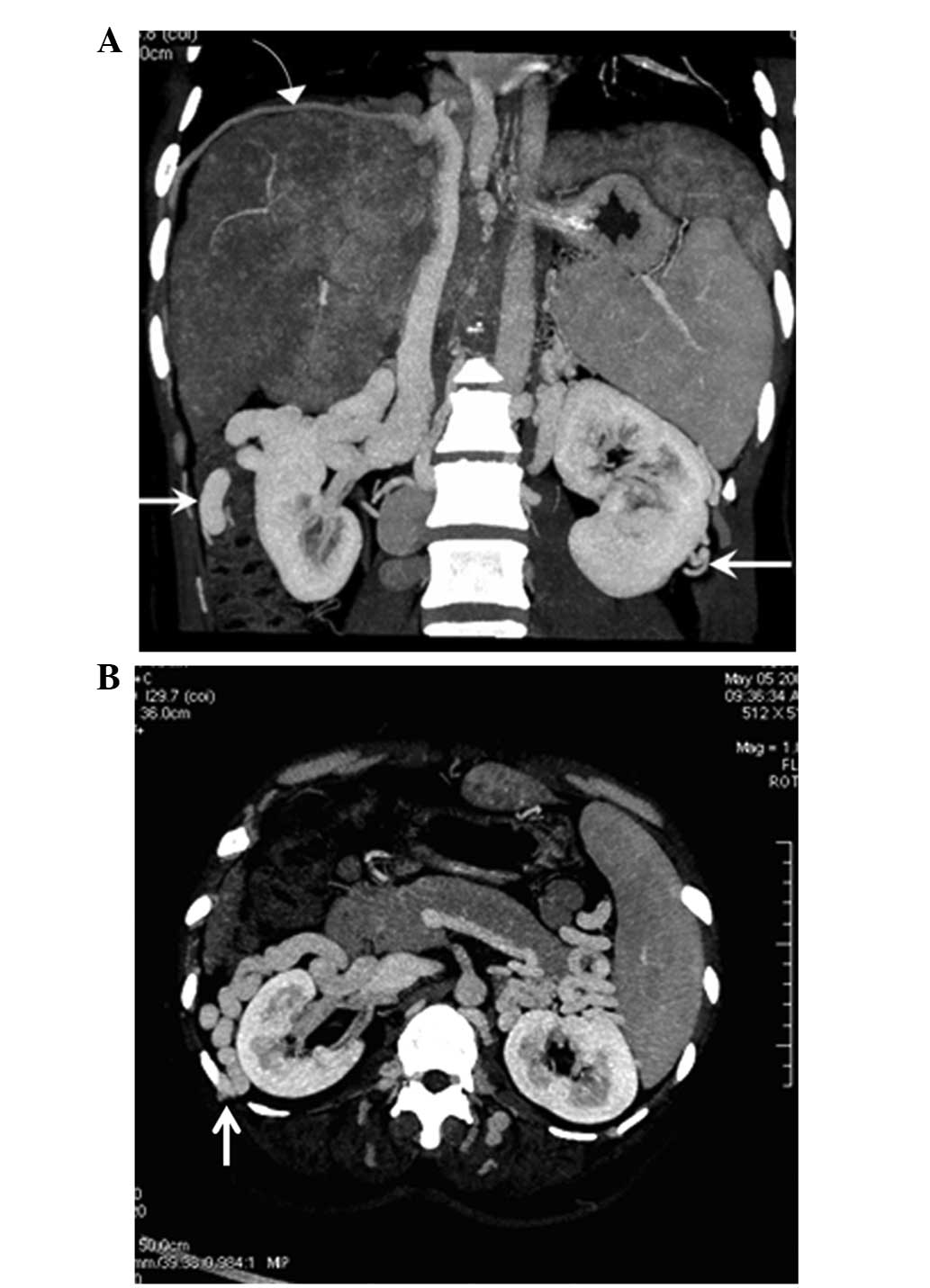
IVC-renal vein-peri-renal
vein-superficial epigastric vein collaterals
In 26 cases (32.5%), CTA showed communication
between bilateral renal veins and peri-renal veins that were
evidently distended and anastomosed with the superficial epigastric
vein (Fig. 8).
Superficial epigastric vein
collaterals
In 12 cases (15.0%). CTA showed that the dilated
inferior epigastric vein, arising from the external iliac vein,
anastomosed with the superior epigastric vein above the umbilicus
and with the internal mammary vein to reach the subclavian vein
(Fig. 9).
Eighty patients with intrahepatic collateral
circulations were combined with one or more extrahepatic collateral
circulations in this group.
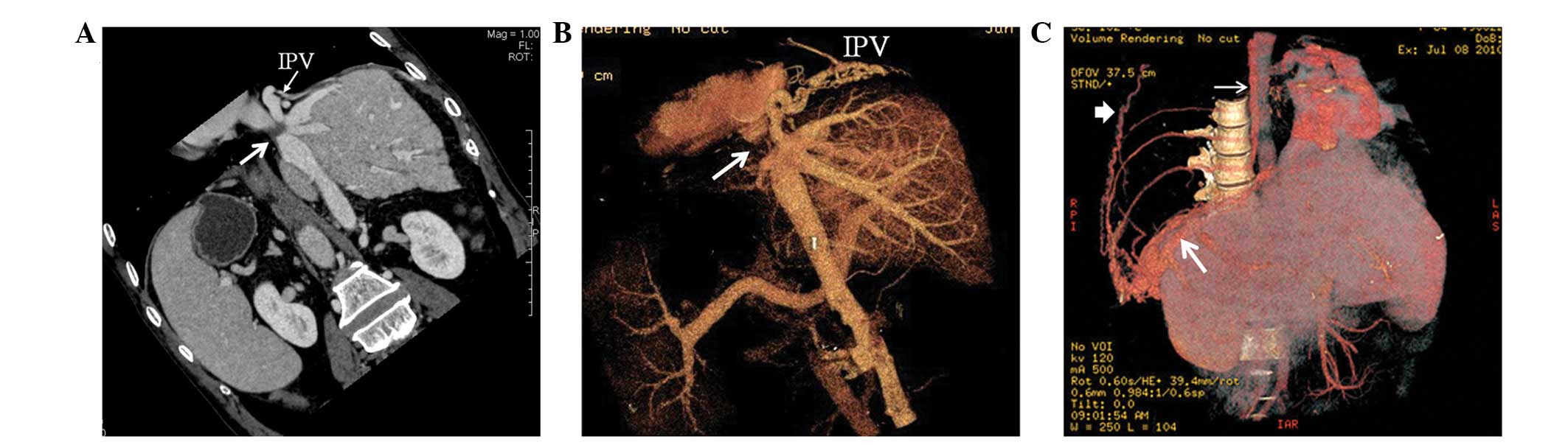
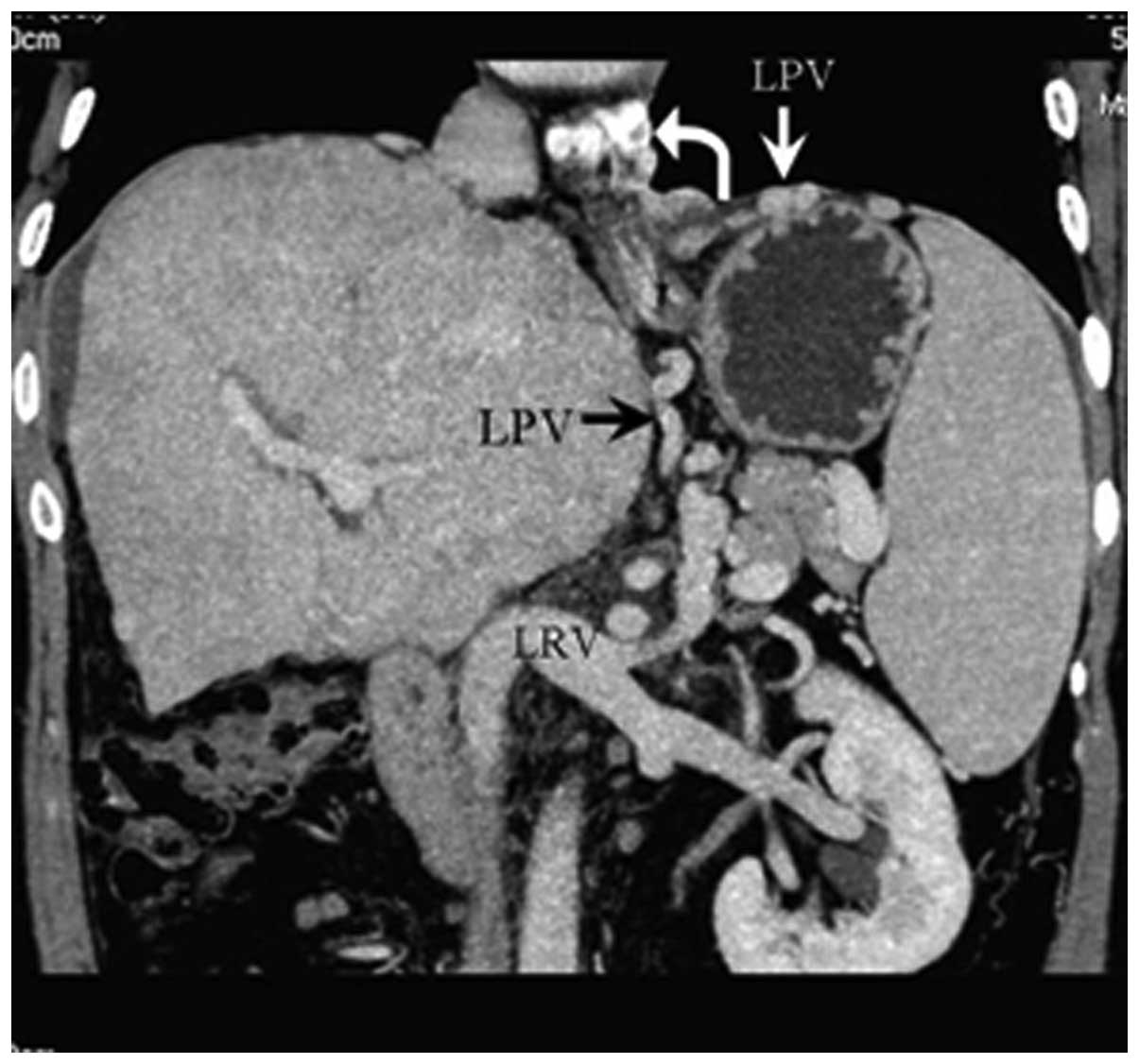
Portosystemic shunts
Spontaneous portosystemic shunts were found in 16
cases. CTA showed portal veins connected with patent hepatic,
accessory hepatic or left renal veins. Among the 16 patients,
portal veins communicated with inferior HVs, caudate lobe veins and
left HVs in eight, four and two cases, respectively, and
communication with left renal veins through splenic veins occurred
in two cases (Fig. 10). Blood
within the portal veins flowed into the systemic venous system via
the corresponding patent vessels. Hepatic cirrhosis was
simultaneously shown on CT scan. In sixteen patients, spontaneous
portosystemic shunts were combined with one or more type of intra-
or extrahepatic collateral circulations.
Discussion
Imaging of the HVs on enhancement CT examination
occurs as of result of the back-streaming of contrast medium from
the hepatic sinusoid into different-grade branches of the HVs. The
enhancement of HVs is affected not only by the dose, concentration
and injected flow rate of the contrast medium, but also by the
delayed scan time. Normally, image formation of the HVs is more
difficult than that of the hepatic arteries and portal veins. In
patients with BCS, the time interval to the peak enhancement of the
HV is prolonged due to the disordered hepatic blood flow that
occurs as a result of obstructions in the HV and/or IVC; therefore,
in the present study, a time interval of 75–80 sec after injection
was selected. As a result, the afflictions of the HVs and IVCs, as
well as the collateral circulations, were satisfactorily
displayed.
Diverse compensatory intra- and extrahepatic
circulation collaterals are demonstrated with CTA due to the
different obstruction positions and lengths of the HVs and IVCs.
According to Gray’s Anatomy (8),
in addition to the main blood draining vessels, including the left,
middle and right HVs of the liver, there is another inferior group
of small-diameter HVs, which drain blood from the inferior portion
of the right and caudal lobe of the liver. The HVs in this inferior
group are also known as accessory HVs. Intrahepatic collaterals can
be found between HVs and accessory HVs, HVs and HVs or HVs and
portal veins in normal subjects (9,10).
With the development of obstructions in the HVs, blood from the
obstructed HVs can be compensatorily drained through the dilated
collateral circulations (11,12).
The intrahepatic blood drainage route is the shortest pathway with
the lowest resistance. Furthermore, the IVC-HV/accessory
HV-HV-right atrium collateral can efficiently compensate the
outflow of the IVC to the heart, as shown in the literature
(13,14) and in our previous study (15). The pathology underlying this type
of collateral is that the blood pressure below the obstruction of
the IVC exceeds that of the HVs, and blood from the IVC reverses
through the intrahepatic collaterals to the right atrium.
The diagnosis of the HV-umbilical vein and
HV-inferior phrenic vein drainage types predominantly depends on
DSA, and reports of CTA descriptions are rare. The results of the
present study show that CTA can clearly demonstrate not only the
afflictions of the HVs but also the intra- or extrahepatic
collateral circulations.
In the event of chronic IVC occlusion, collateral
pathways must develop to maintain venous drainage to the right
atrium. As a result, blood from the IVC may flow retrogradely
through the lumbar vein-ascending lumbar vein-azygos vein and then
through the hemiazygos vein into the right atrium. This is the most
common extrahepatic approach and was demonstrated in all 80
patients in this group.
Anatomically, the left renal vein receives blood
from multiple vessels, including the inferior phrenic, renal
capsule, adrenal gland and gonadal veins; however, there is still
an anatomical shunt, in which the left renal vein and the ascending
lumbar, hemiazygos and vertebral veins are directly or indirectly
connected through pre-existing lumbar veins (16). Ordinarily, the lumen of this shunt
is minimal and without any physiological significance. In cases of
IVC obstruction, as the IVC hypertension passes on to the left
renal vein, the shunt may dilate and become another main
blood-draining route for the IVC. The presence of such a pathway
may result in para-aortic dilated and tortuous vessels connecting
with the left renal vein on CTA. These collaterals were found in
most of the patients (93.8%) in the present study.
As noted previously (16), the left inferior phrenic vein has
two branches, one ending in the left renal vein and the other
ending in the IVC. When the left renal vein is patent, a direct
connection with the inferior phrenic vein may occur. As a result of
the pericardiacophrenic vein, a tributary of the left
brachiocephalic vein, which has diaphragmatic branches that
anastomose with the inferior phrenic vein, hypertension due to IVC
obstruction may result in pericardiacophrenic varices, which can
appear as a vascular mass in the left cardiophrenic angle and be
traced upward along the left border of the left ventricle with
CTA.
Unlike the left inferior phrenic vein, the right
inferior phrenic vein usually drains directly into the IVC
(16); therefore, blood in the
right renal vein cannot be drained by the right inferior phrenic
vein. By contrast, blood flow from the right renal vein can be
drained into the right atrium via renal -peri-renal-superficial
epigastric venous collateral pathways, which can be clearly
observed with CTA. As mentioned above, venous pressure of both
renal veins can be effectively relieved by shunting blood via the
collaterals, thus preserving renal function.
The superficial epigastric collateral found in the
patients in the present study is another blood-draining pattern
arising from an obstructed IVC. Although the collaterals of the
superficial epigastric veins may be manifested on physical
examination, they can be observed more clearly with CTA.
It has been reported that portosystemic connections
exist both in healthy individuals and in normal individuals
following mortality (17,18). In normal circumstances, there is
likely to be no blood flowing through these connections due to the
balanced blood pressure in the portal vein and venous system;
however, when portal hypertension exists in BCS, the connections
expand and shunt blood from the portal vein to the venous system,
which may effectively relieve the portal vein pressure. This may
therefore decrease the hepatic sinusoidal pressure and ameliorate
liver function (19). The
identification of spontaneous shunts using CTA may be useful in
estimating the shunting capacity of blood prior to surgery.
Therapeutic strategies for BCS are based on imaging,
which can demonstrate the lesions and collateral circulations of
obstructed IVCs and HVs prior to surgery. The results of the
present study show that the application of CTA on patients with BCS
can accurately demonstrate lesions of the IVCs and HVs, as well as
intra- and extrahepatic collateral circulations, which may be
beneficial for therapeutic planning.
Acknowledgements
This study was supported by the Shandong Provincial
Science and Technology Development Project Foundation of China
(nos. 2012GSF11820 and 2013GSF11827).
References
|
1
|
Janssen HL, Garcia-Pagan JC, Elias E, et
al: European Group for the Study of Vascular Disorders of the
Liver: Budd-Chiari syndrome: a review by an expert panel. J
Hepatol. 38:364–371. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bogin V, Marcos A and Shaw-Stiffel T:
Budd-Chiari syndrome: in evolution. Eur J Gastroenterol Hepatol.
17:33–35. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gai YH, Cai SF, Guo WB, Zhang CQ, Liang B,
Jia T and Zhang GQ: Sonographic classification of draining pathways
of obstructed hepatic veins in Budd-Chiari syndrome. J Clin
Ultrasound. 42:134–142. 2014. View Article : Google Scholar
|
|
4
|
Bargalló X, Gilabert R, Nicolau C, et al:
Sonography of Budd-Chiari Syndrome. AJR Am J Roentgenol.
187:W33–W41. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kanamura T, Murakami G, Hirai I, et al:
High dorsal drainage routes of Spiegel’s lobe. J Hepatobiliary
Pancreat Surg. 8:549–556. 2001. View Article : Google Scholar
|
|
6
|
Bargalló X, Gilabert R, Nicolau C, et al:
Sonography of the caudate vein: value in diagnosing Budd-Chiari
syndrome. AJR Am J Roentgenol. 181:1641–1645. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xing X, Li H and Liu WG: Clinical studies
on inferior right hepatic veins. Hepatobiliary Pancreat Dis Int.
6:579–584. 2007.PubMed/NCBI
|
|
8
|
Gabella G: Hepatic vein. Gray’s Anatomy.
Williams PL and Bannister LH: 38th edition. Churchill Livingstone;
London: pp. 16021995
|
|
9
|
Kapur S, Paik E, Rezaei A and Vu DN: Where
there is blood, there is a way: unusual collateral vessels in
superior and inferior vena cava obstruction. Radiographics.
30:67–78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakamura S and Tsuzuki T: Surgical anatomy
of the hepatic veins and the inferior vena cava. Surg Gynecol
Obstet. 152:43–50. 1981.PubMed/NCBI
|
|
11
|
Ueda K, Matsui O, Kadoya M, et al: CTAP in
Budd-Chiari syndrome: evaluation of intrahepatic portal flow. Abdom
Imaging. 23:304–308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karaosmanoglu D, Karcaaltincaba M, Akata
D, Ozmen M and Akhan O: CT, MRI, and US findings of incidental
segmental distal hepatic vein occlusion: a new form of Budd-Chiari
syndrome? J Comput Assist Tomogr. 32:518–522. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Akaki S, Kanazawa S, Gochi A, et al:
Asymptomatic membranous obstruction of the inferior vena cava due
to large intrahepatic collaterals. Cardiovasc Intervent Ridio.
18:403–405. 1995. View Article : Google Scholar
|
|
14
|
Kamba M, Ochi S, Ochi H, et al:
Asymptomatic membranous obstruction of the inferior vena cava
forming intrahepatic collateral pathways. J Gastroenterol.
30:783–785. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gai YH, Cai SF, Fan HL and Liu QW:
Diagnosis of the cavo-hepato-atrial pathway in Budd-Chiari syndrome
by ultrasonography. Exp Ther Med. 8:793–796. 2014.PubMed/NCBI
|
|
16
|
Cho OK, Koo JH, Kim YS, et al: Collateral
pathways in Budd-Chiari syndrome: CT and venographic correlation.
AJR Am J Roentgenol. 167:1163–1167. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Edwards EA: Functional anatomy of the
porta-systemic communications. AMA Arch Intern Med. 88:137–154.
1951. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fleming RJ and Seaman WB: Roentgenographic
demonstration of unusual extra-esophageal varices. Am J Roentgenol
Radium Ther Nucl Med. 103:281–290. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Terasaki M, Kitai T, Morimoto T, et al:
Hemodynamics and hepatic energy metabolism in canine model of acute
hepatic venous occlusion with mesocaval shunt. Eur Surg Res.
26:19–27. 1994. View Article : Google Scholar : PubMed/NCBI
|