Introduction
Hepatitis C virus (HCV), a member of family
Flaviviridae (1), has infected an
estimated 170 million people worldwide and patients have high risk
of chronic liver diseases and liver cancer (2–4). The
delayed responses of lymphocytes are linked to chronic HCV
infection (5). Efforts to improve
the understanding of the pathogenesis of the disease and to
identify novel therapeutic targets are emergent.
Indoleamine 2,3-dioxygenase (IDO) is a
heme-containing, immunosuppressive enzyme which degrades tryptophan
into kynurenine (6).
Lipopolysaccharide (LPS), interleukin-1 (IL-1) and tumor necrosis
factor (TNF) act synergistically with interferon-γ (IFN-γ) to
enhance IDO expression in vitro (7). IFN-γ is upregulated in the livers of
patients with chronic hepatitis C (CHC) infection (8). The overexpression of IDO induces
tolerance and immunosuppression, and studies of different models
have confirmed that IDO is a potent regulator of adaptive immune
responses that has the ability to suppress T-cell proliferation
through tryptophan depletion (9–11).
IDO activity is inhibited by 1-methyl tryptophan (1-MT) (11). A role of IDO in immune evasion by
cancer has been proposed and the inhibition of IDO in vivo
could be a promising antitumor adjuvant therapy (12).
A high expression of IDO in the liver has been
described in humans with chronic infection and HCV clearance has
been found to be associated with the normalization of the levels of
IDO (13). IDO may suppress T-cell
reactivity to viral antigens in CHC infection. A study revealed
that in HCV-infected chimpanzees that cleared the infection, the
hepatic IDO expression level was normal; however, it was high in
those who developed liver cirrhosis (13). In humans, T-regulatory cells are
expended for the period of acute HCV infection (14–16),
maintained during the chronic stage (14,17–20)
and decrease in individuals who recover from HCV (14,18).
Fallarino et al (21)
observed that tryptophan-derived catabolites and tryptophan
starvation can transform naïve CD4+ CD25– T
cells into CD4+ CD25+ and FoxP3+
regulatory T cells. A previous study has shown that mature
dendritic cells expand CD4+CD25 high regulatory T cells
in an IDO-dependent manner (22).
IDO-expressing dendritic cells (DCs) enhance the function of Tregs
(23). IDO inhibits T-cell
responses through tryptophan metabolites that are produced by the
kynurenine pathway (24). The
blocking of IDO by 1-MT might result in augmented T-cell
propagation in DC-T cell co-culture in vitro (25), which may be promising new adjuvant
therapeutic target for HCV. The authors of the present study
hypothesized that IDO may be involved in HCV-induced liver
cirrhosis. Thus, i) the functional enzymatic activity of IDO in the
serum samples of patients with HCV and controls and; ii) the
expression of IDO in control and HCV-infected liver tissues was
investigated in the current study.
Materials and methods
Patients
A total of 240 individuals were involved in the
current study. The IDO enzymatic activity was analyzed in the serum
samples of 200 individuals (100 HCV cases and 100 controls). After
blood was obtained, the serum samples were immediately isolated and
were stored at −20°C and paraffin-embedded blocks were stored at
room temperature for one week. Immunohistochemistry was performed
on liver sections from HCV-infected patients (n=35) and healthy
controls (n=5). This study was conducted with the approval of the
institutional review board (IRB) of Atta-ur-Rahman School of
Applied Biosciences (ASAB), National University of Sciences &
Technology (NUST; Islamabad, Pakistan). Samples were taken for this
study with the consent of patients.
Immunohistochemistry
Following de-paraffinization, the liver sections
were subjected to antigen retrieval by 1 min in a pressure cooker
in Tris/EDTA buffer pH 8.0 (T9285; Sigma-Aldrich, St. Louis, MO,
USA) followed by rapid cooling in running tap water. The sections
were then washed with phosphate-buffered saline (PBS) containing
0.1% Tween 20. Sections were soaked in 3%
H2O2 methanol solution for 5 min, followed by
15 min in biotin blocking solution (X0590; Dako Agilent
Technologies, Glostrup, Denmark) and washing with Tris-buffered
saline (TBS) 3X for 5 min. To prevent non-specific binding, the
sections were incubated at room temperature (RT) for 1 h in 20%
normal swine serum (S-4000; Vector Laboratories, Burlingame, CA,
USA) in TBS. Mouse monoclonal anti-IDO antibody (ab55305; Abcam,
Cambridge, UK) was applied at a dilution of (1:100) in normal swine
serum at 4°C in a humidified chamber overnight. Following three
washes with TBS for 5 min, the secondary antibody peroxidase horse
anti-mouse IgG antibody (PI-2000; Vector Laboratories) diluted
1:100 in normal swine serum was applied for 1 h at RT in a
humidified chamber. Following three washes with PBS, the slides
were developed with a diaminobenzidine (DAB) peroxidase substrate
kit (SK-4100 Vector Laboratories), and counterstained with Mayer’s
hematoxylin (Dako, Agilent Technologies) and the staining was
visualized with a microscope (B-350; Optika Italy, Ponteranica,
Italy).
Colorimetric assay
Kynurenine was measured spectrophotometrically, as
previously described (26,27). In brief, following the addition of
50 μl 30% trichloroacetic acid to 100 μl serum sample, the serum
samples were vortexed and centrifuged at 10,000 × g for 5 min.
Then, 75 μl of the supernatant was added to an equal volume of
Ehrlich’s reagent (100 mg p-dimethylaminobenzaldehyde and 5
ml glacial acetic acid) in a microtiter plate well (96-well
format). Optical density was measured at 492-nm using an ELx800
Absorbance Microplate reader (Dynex Technologies, Chantilly, VA,
USA). A standard curve of defined kynurenine concentration (0–100
μM) permitted the analysis of unknown concentrations.
Statistical analysis
Statistical analysis was carried out using GraphPad
Prism 3.0 software (GraphPad Software, San Diego, CA, USA). Unless
otherwise stated, graphical data represents the mean value of an
experiment performed in triplicate using the Student’s t-test if
the level of significance reached P<0.05.
Results
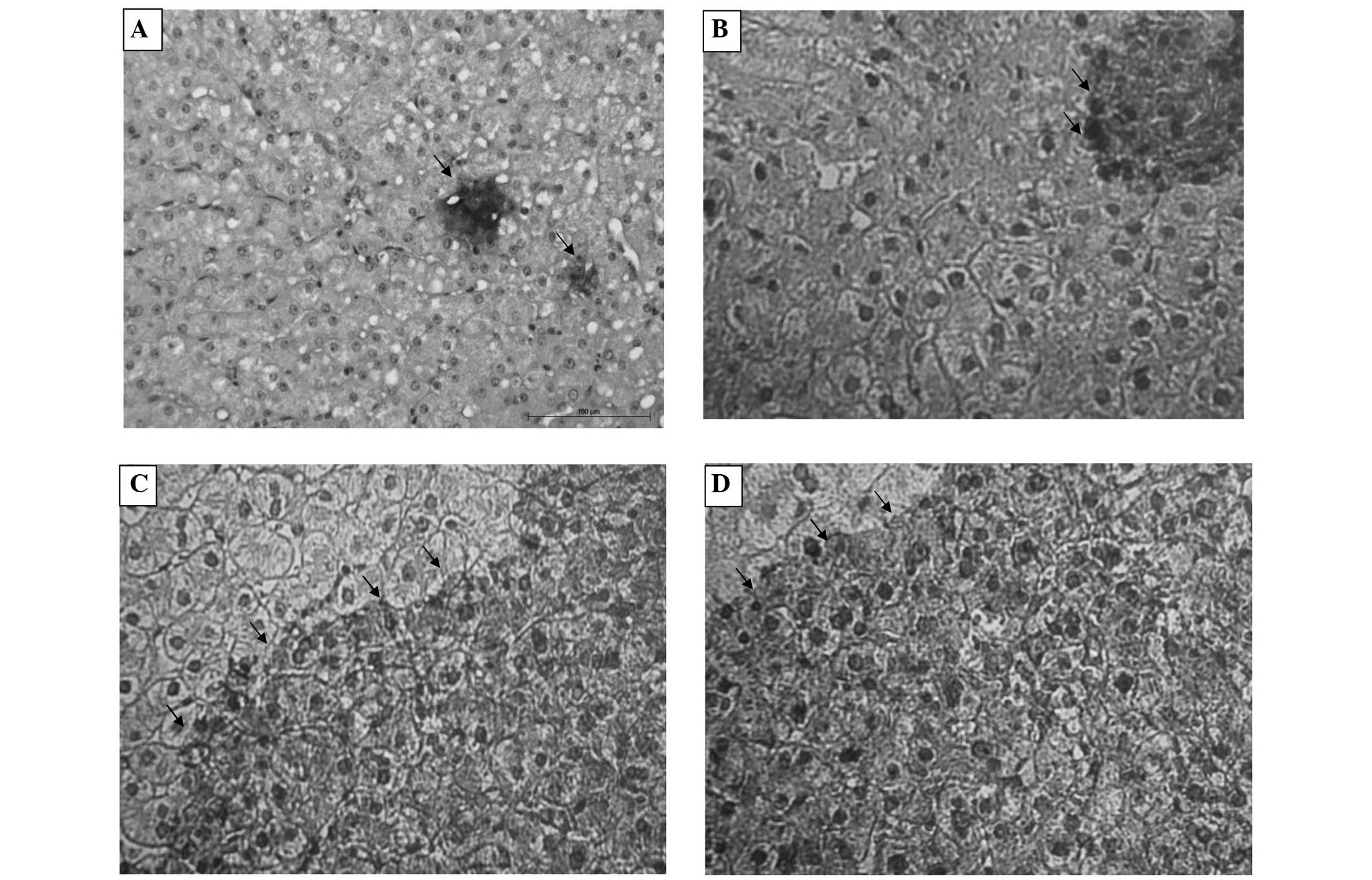
Immunohistochemical staining of IDO in
liver samples with HCV-induced cirrhosis
In order to observe the exact status of IDO
expression in vivo, IDO protein expression was investigated
by immunohistochemistry. No IDO-positive staining was detected in
the sections of normal liver tissues, with the exception of minimal
expression in hepatocytes Fig. 1A,
whereas in the hepatocytes of the 35 liver sections from
HCV-infected patients, mild IDO expression was exhibited in two
(5.7%) cases (Fig. 1B), moderate
IDO expression was present in five (14.2%) cases (Fig. 1C) and high expression of IDO was
observed in 28 (80%) cases. The high expression of IDO may
represent a significant role in the pathogenesis of disease.
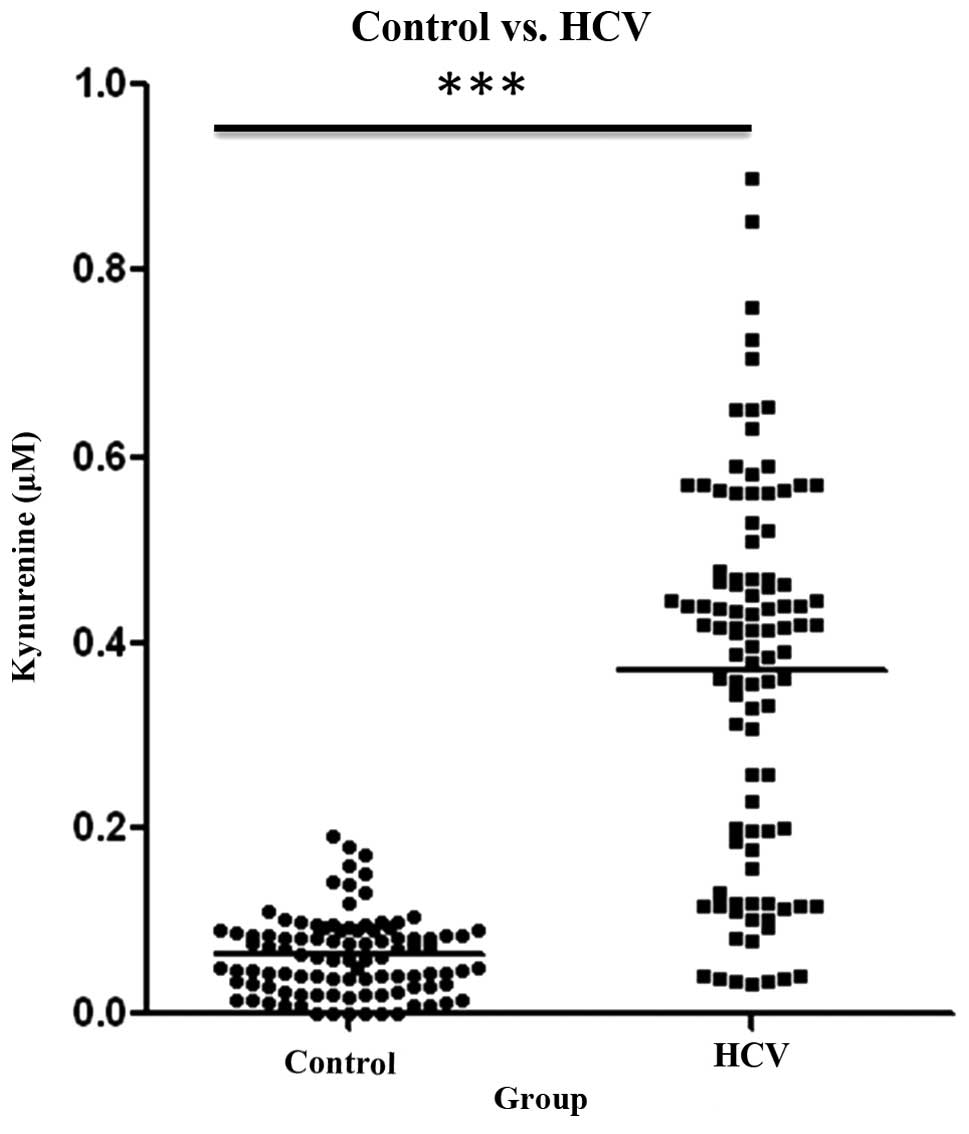
IDO activity in patient sera
It was imperative to evaluate IDO enzymatic
activity, as there is a documented incongruity between IDO activity
and expression, indicating probable post-translational regulation
of the enzyme (28,29). The functional enzymatic activity of
IDO may be determined by quantifying kynurenine (the first
catabolite in the kynurenine pathway). To confirm this approach, a
standard curve for quantifying kynurenine was established.
Significantly high levels of kynurenine were observed in the
HCV-infected patients as compared with those in the control group
(Fig. 2).
Discussion
In the current study, IDO expression was analyzed in
liver tissue by immunohistochemistry and the correlation of IDO
expression level and functional enzymatic activity was evaluated.
To the best of our knowledge, this is the first time that this has
been evaluated in Pakistan. The findings demonstrate that IDO
expression was detectable in cirrhotic cells. High IDO expression
possibly arose due to active inflammation; this is consistent with
results obtained by Pan et al (30). The IDO expression levels were
significantly higher in the HCV-infected patients as compared with
those in the control group. This requires investigation with a
broader cohort to establish a definitive correlation.
Numerous studies have confirmed that the clearance
of HCV infection is linked to HCV-specific CD4+ T-cell responses
(31,32,33).
The exact mechanism underlying the failure of certain individuals
to resolve HCV infection is poorly understood. The overexpression
of IDO induces immunosuppression. IDO has the ability to suppress
T-cell proliferation through tryptophan depletion (9–11).
It has previously been shown that IDO creates a transitional
pathway in dendritic cell maturation leading to the expansion of
CD4+CD25 high regulatory T cells (22). IDO-expressing DCs enhance the
function of Tregs (23) and an
augmented number of Tregs at the commencement of infection has been
defined as a chronic infection (34). Ino et al (35) and Brandacher et al (36) have confirmed that high IDO
expression levels contribute to the metastasis of endometrial and
colorectal cancer. Moreover, the high immunoreactivity of IDO is
significantly associated with the frequency of liver metastases
(37). The data in the present
study indicate that IDO is a crucial player that may contribute to
the poor outcome of patients in a manner that remains unknown.
The role of IDO in HCV is only just beginning to be
studied in detail. The current study proposes that IDO mediates the
immune escape employed by HCV in chronic patients and, therefore,
more studies into the exact mechanism by which HCV signaling leads
to the upregulation of IDO are warranted. These data support the
hypothesis that an immunosuppressive environment created by IDO may
lead patients with chronic HCV infection progressively toward liver
cirrhosis. IDO has the potential to become a useful marker for
HCV-induced liver cirrhosis. Thus, the inhibition of IDO activity
may contribute to the application of adjuvant therapy intervention
for HCV.
Acknowledgements
The authors would like to express their extreme
gratitude to Dr M. Idrees Awan and Dr Nasir Iqbal Sheikh (Hashim
Welfare Hospital, Kharian, Pakistan) for providing liver biopsy
sections.
References
|
1
|
Tan SL, Pause A, Shi Y and Sonenberg N:
Hepatitis C therapeutics: current status and emerging strategies.
Nat Rev Drug Discov. 1:867–881. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alter HJ and Seef LB: Recovery,
persistence, and sequelae in hepatitis C virus infection: a
perspective on long-term outcome. Semin Liver Dis. 20:17–35. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lauer GM and Walker BD: Hepatitis C virus
infection. N Engl J Med. 345:41–52. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El-Serag HB: Hepatocellular carcinoma and
hepatitis C in the United States. Hepatology. 36(5 Suppl 1):
S74–S83. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bowen DG and Walker CM: Adaptive immune
responses in acute and chronic hepatitis C virus infection. Nature.
436:946–952. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stone TW and Darlington LG: Endogenous
kynurenines as targets for drug discovery and development. Nat Rev
Drug Discov. 1:609–620. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Robinson CM, Shirey KA and Carlin JM:
Synergistic transcriptional activation of indoleamine dioxygenase
by IFG-gamma and tumor necrosis factor-alpha. J Interferon Cytokine
Res. 23:413–421. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abbate I, Romano M, Longo R, et al:
Endogenous levels of mRNA for IFNs and IFN-related genes in hepatic
biopsies of chronic HCV-infected and non-alcoholic steatohepatitis
patients. J Med Virol. 70:581–587. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grohmann U, Fallarino F and Puccetti P:
Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol.
24:242–248. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Munn DH, Sharma MD and Mellor AL: Ligation
of B7-1/B7-2 by human CD4+ T cells trigger indoleamine
2,3-dioxgenase activity in dendritic cells. J Immunol.
172:4100–4110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mellor AL and Munn DH: IDO expression by
DCs: tolerance and tryptophan catabolism. Nat Rev Immunol.
4:762–774. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Uyttenhove C, Pilotte L, Théate I, et al:
Evidence for a tumoral immune resistance mechanism based on
tryptophan degradation by indoleamine 2,3-dioxgenase. Nat Med.
9:1269–1274. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Larrea E, Riezu-Boj JI, Gil-Guerrero L, et
al: Upregulation of indoleamine 2,3-dioxgenase in hepatitis C virus
infection. J Virol. 81:3662–3666. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sugimoto K, Ikeda F, Stadanlick J, et al:
Suppression of HCV-specific T-cells without differential hierarchy
demonstrated ex vivo in persistent HCV infection. Hepatology.
38:1437–1448. 2003.PubMed/NCBI
|
|
15
|
Perrella A, Vitiello L, Atripaldi L, et
al: Elevated CD4+/CD25+ T cell frequency and
function during acute hepatitis C presage chronic evolution. Gut.
55:1370–1371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ulsenheimer A, Gerlach JT, Gruener NH, et
al: Detection of functionally altered hepatitis C virus-specific
CD4 T cells in acute and chronic hepatitis C. Hepatology.
37:1189–1198. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cabrera R, Tu Z, Xu Y, et al: An
immunomodulatory role for CD4+, CD25+,
regulatory T lymphocytes in hepatitis C virus infection.
Hepatology. 40:1062–1071. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boettler T, Spangenberg HC,
Neumann-Haefelin C, et al: T cells with a
CD4+CD25+ regulatory phenotype suppress in
vitro proliferation of virus-specific CD+ T cells during
chronic hepatitis C virus infection. J Virol. 79:7860–7867. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bolacchi F, Sinistro A, Ciaprini C, et al:
Increased hepatitis C virus (HCV)-specific CD4+
CD25+ regulatory T lymphocytes and reduced HCV-specific
CD4+ T cell response in HCV-infected patients with
normal versus abnormal alanine aminotransferase levels. Clin Exp
Immunol. 144:188–196. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ebinuma H, Nakamoto N, Li Y, et al:
Identification and in vitro expansion of functional
antigen-specific CD25+ FoxP3+ regulatory
T-cells in hepatitis C virus infection. J Virol. 82:5043–5053.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fallarino F, Grohmann U, You S, et al: The
combined effects of tryptophan starvation and tryptophan
catabolites down-regulate T-cell receptor zeta-chain and induce a
regulatory phenotype in naïve T cells. J Immunol. 176:6752–6761.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hill M, Tanguy-Royer S, Royer P, et al:
IDO expands human CD4+CD25 high regulatory T cells by
promoting maturation of LPS-treated dendritic cells. Eur J Immunol.
37:3054–3062. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sharma MD, Baban B, Chandler P, et al:
Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes
directly activate mature Tregs via indoleamine 2,3-dioxgenase. J
Clin Invest. 117:2570–2582. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Terness P, Bauer TM, Röse L, et al:
Inhibition of allogenic T cell proliferation by indoleamine
2,3-dioxgenase-expressing dendritic cells: mediation of suppression
by tryptophan metabolites. J Exp Med. 447–457. 2002. View Article : Google Scholar
|
|
25
|
Munn DH and Mellor AL: IDO and tolerance
to tumors. Trends Mol Med. 10:15–18. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takikawa O, Kuroiwa T, Yamazaki F and Kido
R: Mechanism of interferon-gamma action. characterization of
indoleamine 2,3-dioxygenase in cultured human cells induced by
interferon-gamma and evaluation of the enzyme-mediated tryptophan
degradation in its anticellular activity. J Biol Chem.
263:2041–2048. 1988.PubMed/NCBI
|
|
27
|
Grant RS, Naif H, Thuruthyil SJ, et al:
Induction of indolamine 2,3-dioxygenase in primary human
macrophages by human immunodeficiency virus type 1 is strain
dependent. J Virol. 74:4110–4115. 2000. View Article : Google Scholar
|
|
28
|
Fallarino F, Vacca C, Orabona C, et al:
Functional expression of indoleamine 2,3-dioxygenase by murine CD8
alpha+ dendritic cells. Int Immunol. 14:65–68. 2002.
View Article : Google Scholar
|
|
29
|
Grohmann U, Bianchi R, Orabona C, et al:
Functional plasticity of dendritic cell subsets as mediated by CD40
versus B7 activation. J Immunol. 171:2581–2587. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pan K, Wang H, Chen MS, et al: Expression
and prognosis role of indoleamine 2,3-dioxygenase in hepatocellular
carcinoma. J Cancer Res Clin. 134:1247–1253. 2008. View Article : Google Scholar
|
|
31
|
Day CL, Lauer GM, Robbins GK, et al: Broad
specificity of virus-specific CD4+ T-helper-cell responses in
resolved hepatitis C virus infection. J Virol. 76:12584–12595.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Semmo N and Klenerman P: CD4+ T cell
responses in hepatitis C virus infection. World J Gastroenterol.
13:4831–4838. 2007.PubMed/NCBI
|
|
33
|
Chang KM, Thimme R, Melpolder JJ, Oldach
D, et al: Differential CD4 (+) and CD8(+) T-cell responsiveness in
hepatitis C virus infection. Hepatology. 33:267–276. 2001.
View Article : Google Scholar
|
|
34
|
Perrella A, Vitiello L, Atripaldi L, et
al: Elevated CD4+/CD25+ T cell frequency and
function during acute hepatitis C presage chronic evolution. Gut.
55:1370–1371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ino K, Yoshida N, Kajiyama H, et al:
Indoleamine 2,3-dioxygenase is a novel prognostic indicator for
dendometrial cancer. Br J Cancer. 95:1555–1561. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brandacher G, Perathoner A, Ladurner R, et
al: Prognostic value of indoleamine 2,3-dioxygenase expression in
colorectal cancer: effect on tumor-infiltrating T cells. Clin
Cancer Res. 12:1144–1151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ishio T, Goto S, Tahara K, et al:
Immunoactivative role of indoleamine 2,3-dioxygenase in human
hepatocellular carcinoma. J Gastroenterol Hepatol. 19:319–326.
2004. View Article : Google Scholar : PubMed/NCBI
|