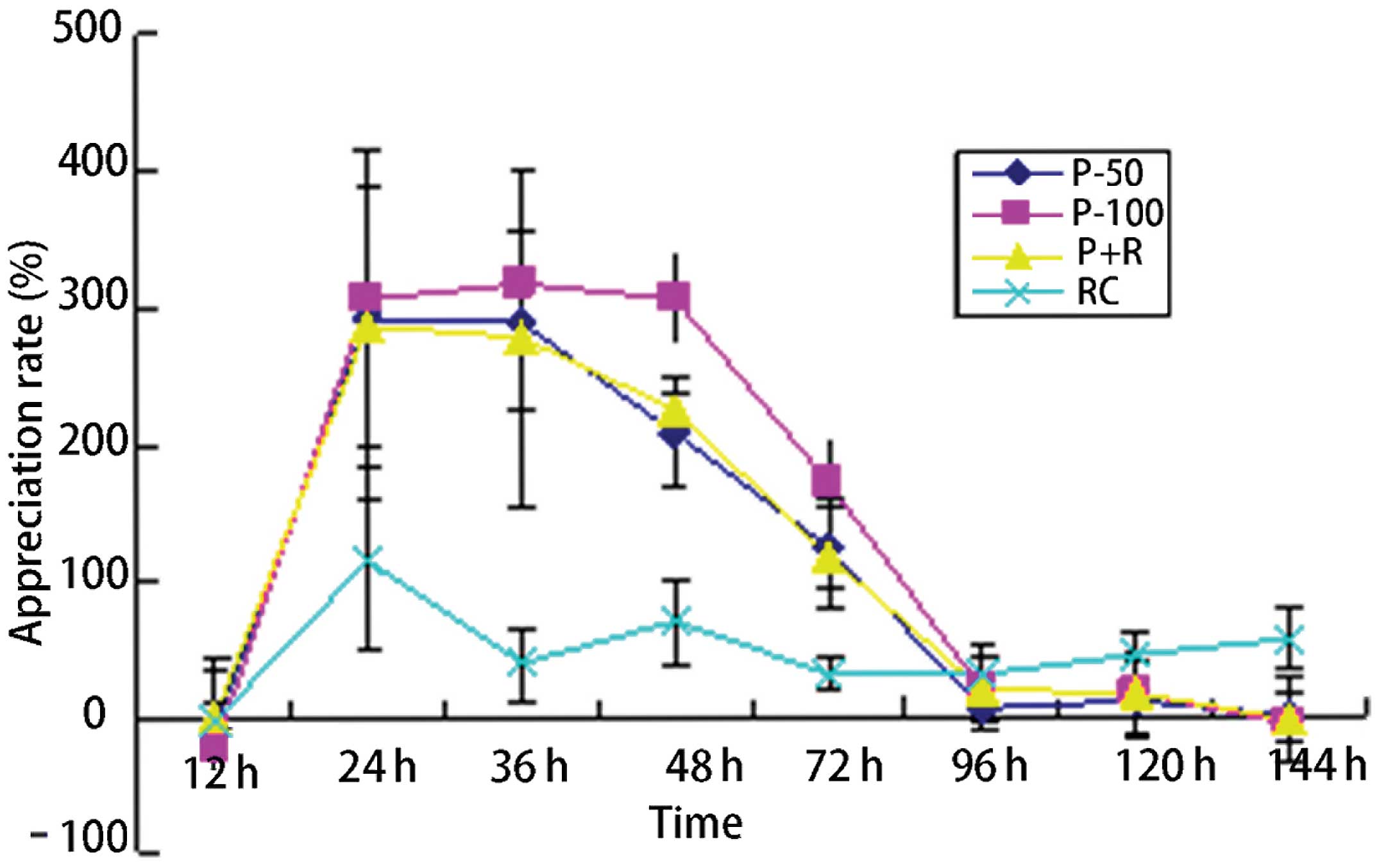
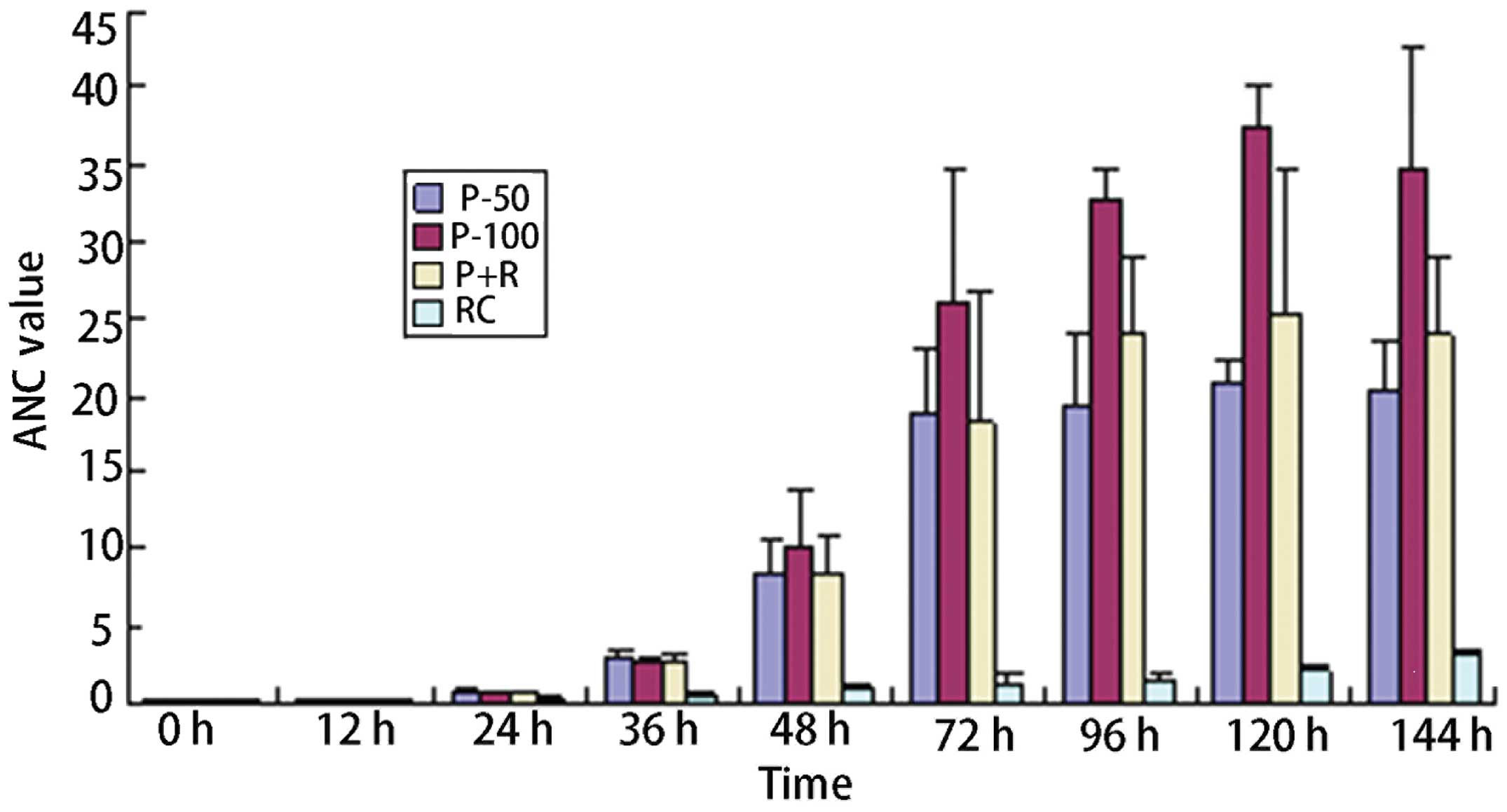
Spandidos Publications style
Wu FP, Wang J, Wang H, Li N, Guo Y, Cheng YJ, Liu Q and Yang XR: Clinical observation of the therapeutic effects of pegylated recombinant human granulocyte colony-stimulating factor in patients with concurrent chemoradiotherapy-induced grade IV neutropenia. Exp Ther Med 9: 761-765, 2015.
APA
Wu, F., Wang, J., Wang, H., Li, N., Guo, Y., Cheng, Y. ... Yang, X. (2015). Clinical observation of the therapeutic effects of pegylated recombinant human granulocyte colony-stimulating factor in patients with concurrent chemoradiotherapy-induced grade IV neutropenia. Experimental and Therapeutic Medicine, 9, 761-765. https://doi.org/10.3892/etm.2014.2160
MLA
Wu, F., Wang, J., Wang, H., Li, N., Guo, Y., Cheng, Y., Liu, Q., Yang, X."Clinical observation of the therapeutic effects of pegylated recombinant human granulocyte colony-stimulating factor in patients with concurrent chemoradiotherapy-induced grade IV neutropenia". Experimental and Therapeutic Medicine 9.3 (2015): 761-765.
Chicago
Wu, F., Wang, J., Wang, H., Li, N., Guo, Y., Cheng, Y., Liu, Q., Yang, X."Clinical observation of the therapeutic effects of pegylated recombinant human granulocyte colony-stimulating factor in patients with concurrent chemoradiotherapy-induced grade IV neutropenia". Experimental and Therapeutic Medicine 9, no. 3 (2015): 761-765. https://doi.org/10.3892/etm.2014.2160