Introduction
Neutropenia is a common clinical complication of
chemotherapy in cancer patients. It is also an important factor
that delays the course of standard treatments in patients.
Recombinant human granulocyte colony-stimulating factor (rhG-CSF)
is an effective drug for the treatment of chemotherapy-induced
neutropenia. However, for patients with grade IV neutropenia,
multiple rhG-CSF treatments are usually required. This is likely to
extend the antitumor treatment period and increase physical and
mental stress in patients. Pegylated recombinant human granulocyte
colony-stimulating factor (PEG-rhG-CSF) is rhG-CSF chemically
modified by a single methoxy polyethylene glycol group; it is able
to alleviate neutropenia with a single dose (1,2)
However, due to the short time that it has been used in China,
oncologists have many questions about the use, dosage and safety of
this therapy in the treatment of patients with grade IV
neutropenia. The questions concern whether the same single dose of
PEG-rhG-CSF should be used in all patients; whether PEG-rhG-CSF
should be added if the neutropenia is not improved in the short
term; and whether the side-effects of PEG-rhG-CSF are significantly
increased compared with those of rhG-CSF due its greater molecular
weight. The present study analyzed the efficacy and safety of
PEG-rhG-CSF in 114 patients with concurrent
chemoradiotherapy-induced grade IV neutropenia. The results may
provide a basis for the clinical application of PEG-rhG-CSF.
Materials and methods
General information
From April 2012 to July 2013, a total of 114
patients with concurrent chemoradiotherapy-induced grade IV
neutropenia were enrolled at the Radiotherapy Department of the
Fourth Hospital of Hebei Medical University (Shijiazhuang, China).
Of these, 69 cases were male and 45 cases were female. The patients
were aged 35 to 70 years with a median age of 52 years. All
patients were in general good condition with a Karnofsky
Performance Status score ≥60 points. Blood diseases, bone
metastasis, previous history of chemotherapy and radiotherapy were
not found, and the liver and kidney function was normal. There were
62 cases of esophageal cancer; the treatment program was
three-dimensional conformal radiotherapy/intensity-modulated
radiation therapy (IMRT), the total dose was PTV60-66Gy/30
fractions (f), 1 f/day for 5 days; 43 cases had the LFP regimen
(leucovorin, fluorouracil and cisplatin) and 19 cases had the DC
regimen (docetaxel and cisplatin) for chemotherapy. In addition,
there were 52 cases of lung cancer; the treatment program was
three-dimensional conformal radiotherapy/IMRT at the same dosage
used for esophageal cancer; 28 cases had the EP regimen (etoposide
and cisplatin) and 23 cases had the DC regimen for chemotherapy.
Single or combined symptoms of fever, muscle pain, fatigue and
digestive disorders were present in 83 patients. The study was
approved by the Medical Ethics committee of the fourth Medical
College of Hebei University (Hebei, China).
Experimental and treatment groups
According to the World Health Organization grading
standards for common adverse reactions of anticancer drugs
(3), patients were diagnosed with
grade IV neutropenia when the absolute neutrophil count (ANC) was
<0.5×109/l. A randomized approach was used to divide
the patients into an experimental group and a control group. The
experimental group included three subgroups, namely the P-50, P-100
and P + R groups. The P-50 group contained 42 cases, which were
given a single 50 μg/kg subcutaneous injection of PEG-rhG-CSF
(Jinyouli™; Shijiazhuang Pharmaceutical Group Co., Ltd),
Shijiazhuang, China). The P-100 group contained 30 cases, which
received a single 100-μg/kg subcutaneous injection of PEG-rhG-CSF.
The P + R group contained 22 cases, which were given a single
50-μg/kg subcutaneous injection of PEG-rhG-CSF and 5 μg/kg/day
rhG-CSF (Lishengsu™; Beijing SL Pharmaceutical Co., Ltd. Beijing,
China); when the ANC was ≥2.0×109/l, the administration
of rhG-CSF was stopped. The control group (RC group) comprised 20
patients who received rhG-CSF 5 μg/kg/day by subcutaneous injection
until the ANC was ≥2.0×109/l. All patients were given
prophylactic anti-inflammatory treatment, based on clinical
symptoms, and also received symptomatic and supportive treatment.
All patients enrolled voluntarily and provided signed informed
consent.
Detection indices
All patients received a blood routine test to detect
the blood neutrophil count (ANC values) at 12, 24, 36, 48, 72, 96,
120 and 144 h after the first application of PEG-rhG-CSF or
rhG-CSF. The changes in the neutrophil proliferation rate and ANC
values occurring over time after treatment initiation were
documented for statistical analysis. The neutrophil proliferation
rate was calculated as follows: Neutrophil proliferation rate (%) =
(current ANC value/previous ANC value) × 100. For patients with
neutropenia-induced adverse symptoms in each group, the symptom
remission time after treatment was recorded for statistical
analysis. For patients without neutropenia-induced adverse symptoms
prior to treatment, the incidence of drug reactions after treatment
was recorded for statistical analysis.
Statistical methods
SPSS statistical software, version 17.0 (SPSS, Inc.,
Chicago, IL, USA), was used for statistical analysis of
experimental data. The neutrophil proliferation rate and ANC values
at each time point were compared using analysis of variance of
repeated measured data. For patients with neutropenia-induced
adverse symptoms, the symptom remission times after treatment were
compared with single factor analysis of variance. For patients
without neutropenia-induced adverse symptoms prior to treatment,
the incidence of drug reactions after treatment were compared by
χ2 test.
Results
Neutrophil proliferation rate changes
with time after treatment
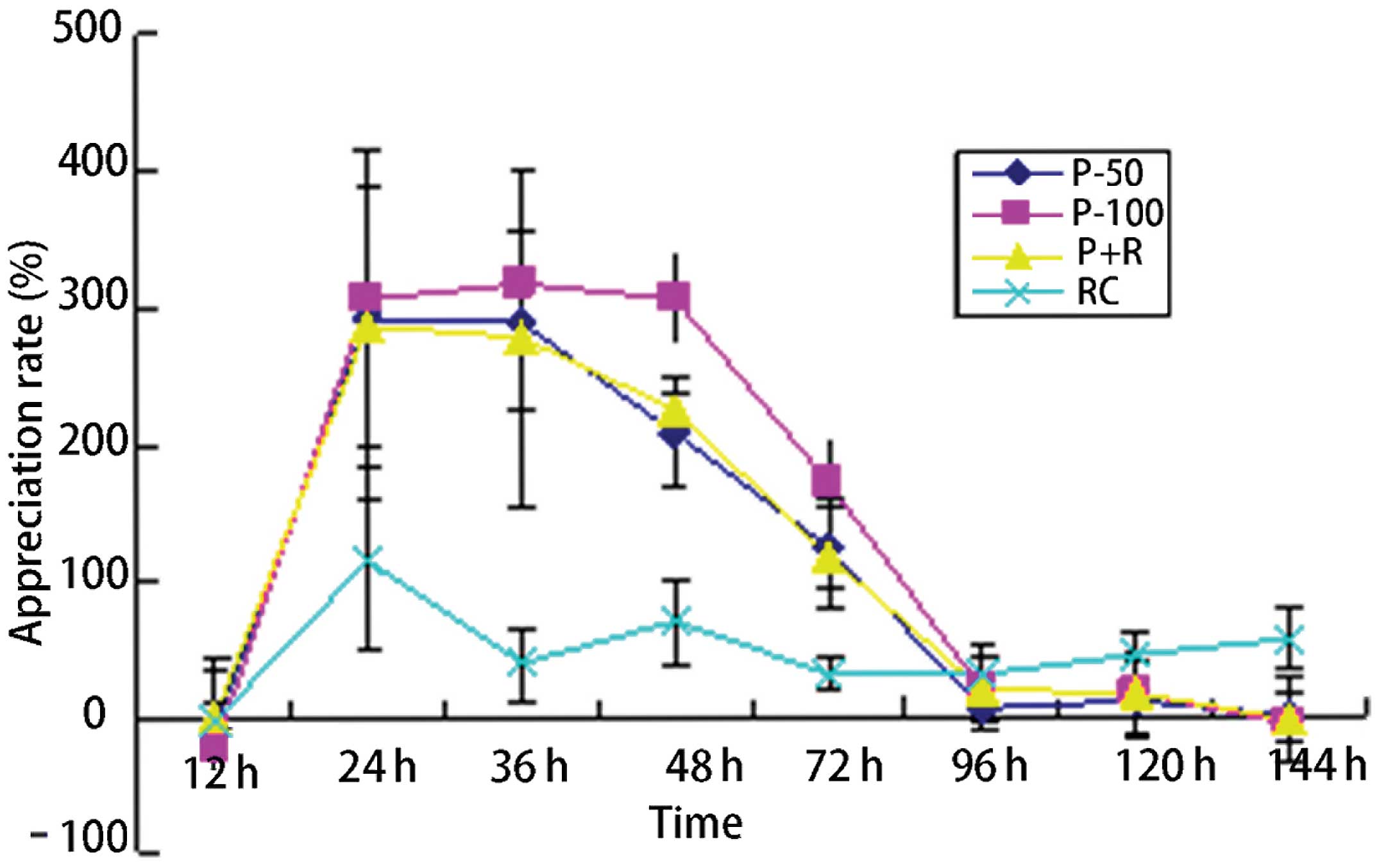
In the experimental group, the neutrophil
proliferation rate was significantly higher than that in the
control group, and the difference was statistically significant
(P<0.05). In each experimental subgroup, the neutrophil
proliferation rate showed no significant difference between each
pair of subgroups (P>0.05). Between the experimental subgroups
and the control group, the pairwise comparisons showed
statistically significant differences (P<0.05). At 12 h after
administration in each group, the mean neutrophil proliferation
rates were negative values; in the experimental group, the
proliferation rate peaks in the P-50 and P + R groups occurred
24–36 h after treatment initiation. The P-100 peak proliferative
rate occurred 24–48 h after treatment. At 144 h after treatment
initiation, the mean neutrophil proliferation rates were negative
values in each experimental group (Table I, Fig.
1).
 | Table IChanges in the neutrophil
proliferation rate in patients with grade IV neutropenia over time
after treatment (%). |
Table I
Changes in the neutrophil
proliferation rate in patients with grade IV neutropenia over time
after treatment (%).
| | Time after
treatment | | |
|---|
| |
| | |
|---|
| Group | n | 12 h | 24 h | 36 h | 48 h | 72 h | 96 h | 120 h | 144 h | F-statistic | P-value |
|---|
| P-50 | 42 | −9.82±38.37 | 291.00±42.47 | 289.54±43.6 | 207.64±34.47 | 122.43±30.18 | 2.97±25.16 | 9.06±23.92 | −2.30±8.41 | | |
| P-100 | 30 | −22.83±43.49 | 304.85±96.34 | 317.54±62.33 | 305.99±39.03 | 171.60±30.90 | 18.40±8.32 | 38.23±28.41 | −7.67±17.64 | | |
| P + R | 22 | −1.04±10.89 | 287.12±127.19 | 277.60±123.78 | 223.66±10.69 | 117.32±40.62 | 19.36±33.39 | 16.34±31.15 | −4.90±31.19 | | |
| RC | 20 | −4.26±47.43 | 114.84±66.48 | 37.52±26.40 | 67.98±32.40 | 30.14±13.17 | 30.08±13.81 | 43.92±16.63 | 54.92±22.53 | 12.94 | 0.002 |
ANC values changes over time after
treatment
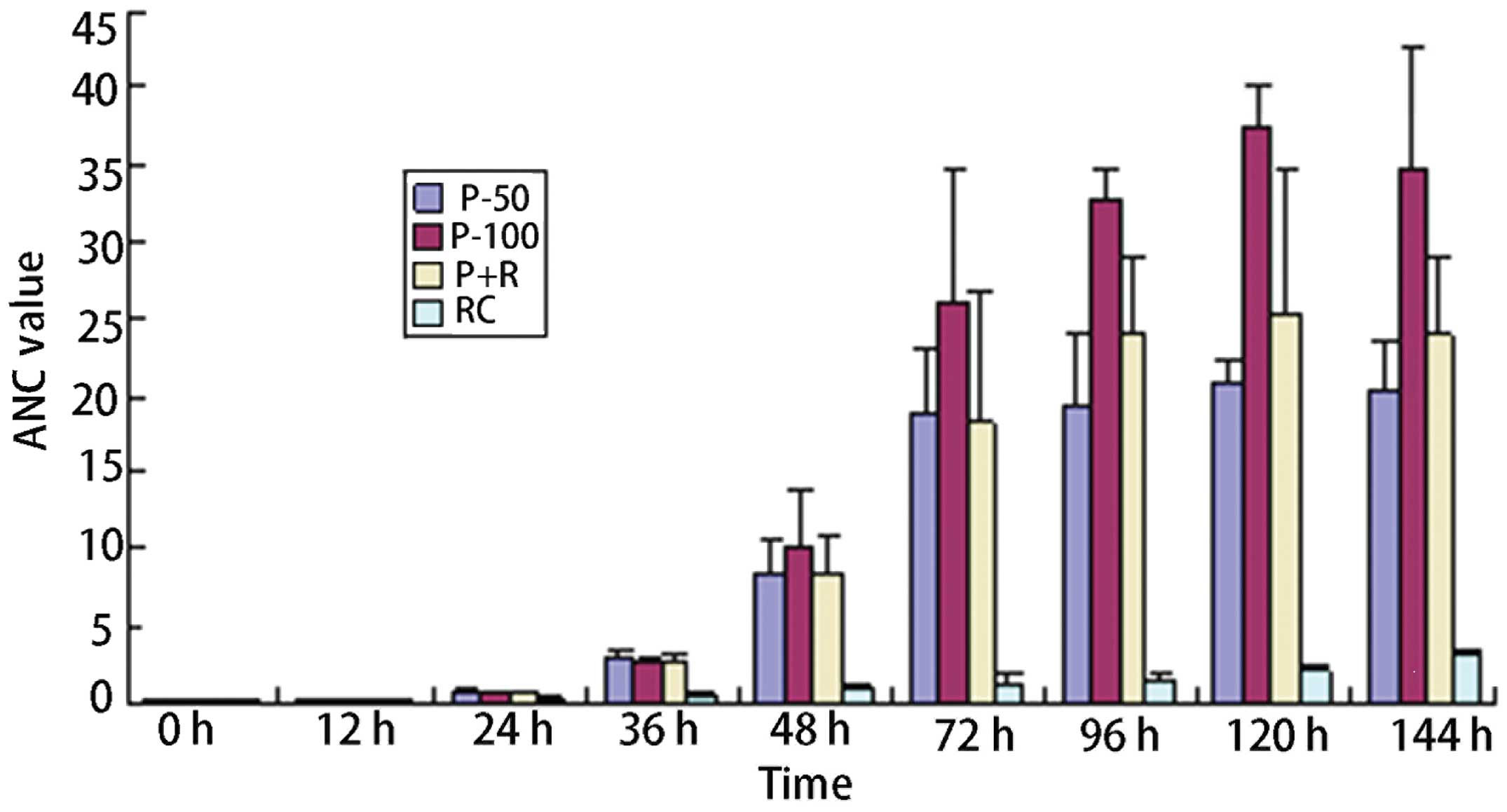
In the experimental group, the ANC values were
significantly higher than those in the control group; the
differences were statistically significant (P<0.05). At 12 h
after the initial drug administration, the blood routine test
showed that the mean ANC values had decreased in the patients in
each group; the proliferative effect began 12–24 h after the
administration of medication. The ANC values in the P-50 and P + R
groups showed no significant differences, whereas the P-100 group
exhibited statistically significant differences from the P-50 and P
+ R groups. At 36 h after the initiation of treatment, all three
subgroups of the experimental group basically achieved the clinical
purpose of completely improving the neutropenia. However, 120 h
after the initial administration of medication, ANC values reached
2.1×109/l in the control group; the average amount of
rhG-CSF required was 1,650 μg/kg. (Table II, Fig. 2).
 | Table IIChanges in ANC values in patients with
grade IV neutropenia over time after (×109). |
Table II
Changes in ANC values in patients with
grade IV neutropenia over time after (×109).
| | Time after treatment
initiation | | |
|---|
| |
| | |
|---|
| Group | n | 0 h | 12 h | 24 h | 36 h | 48 h | 72 h | 96 h | 120 h | 144 h | F-statistic | P-value |
|---|
| P-50 | 42 | 0.22±0.11 | 0.17±0.05 | 0.73±0.18 | 2.73±0.83 | 8.41±2.02 | 18.67±4.32 | 19.32±4.49 | 20.96±1.20 | 20.47±3.03 | | |
| P-100 | 30 | 0.26±0.08 | 0.19±0.04 | 0.60±0.17 | 2.51±0.64 | 10.19±3.64 | 26.05±8.54 | 32.71±2.04 | 37.61±2.44 | 34.72±8.04 | | |
| P+R | 22 | 0.21±0.14 | 0.19±0.10 | 0.69±0.13 | 2.60±0.65 | 8.42±2.46 | 18.27±8.51 | 24.07±5.10 | 25.22±9.48 | 23.98±5.01 | | |
| RC | 20 | 0.19±0.12 | 0.18±0.09 | 0.34±0.08 | 0.51±0.16 | 0.86±0.41 | 1.12±0.87 | 1.46±0.52 | 2.10±0.13 | 3.06±0.28 | 65.23 | <0.01 |
Comparison of the remission time of
neutropenia-induced symptoms in the experimental and control
groups
In the experimental group, the remission time of
neutropenia-induced fever and muscle pain after administration was
significantly shorter than the corresponding time in the control
group; there was a statistically significant difference between
these groups (P<0.05). However, no significant difference was
observed in the remission time of neutropenia-induced fatigue and
gastrointestinal symptoms between these two groups (Table III).
 | Table IIIRemission time comparison of
neutropenia-induced symptoms after therapy in the patients with
grade IV neutropenia in the experimental and control groups. |
Table III
Remission time comparison of
neutropenia-induced symptoms after therapy in the patients with
grade IV neutropenia in the experimental and control groups.
| Symptoms | Group | n | Remission time
(h) | F-statistic | P-value |
|---|
| Fever | Experimental | 63 | 30.00±7.48 | 85.79 | <0.01 |
| Control | 12 | 72.00±17.89 | | |
| Weakness | Experimental | 64 | 66.00±11.14 | 2.12 | 0.10 |
| Control | 17 | 78.00±11.56 | | |
| Skeletal muscle
pain | Experimental | 51 | 30.00±5.10 | 81.11 | <0.01 |
| Control | 10 | 59.00±11.46 | | |
| Digestive tract
symptoms | Experimental | 33 | 66.00±11.14 | 3.11 | 0.10 |
| Control | 8 | 78.00±11.56 | | |
Incidence of adverse drug reactions in
patients with no neutropenia-induced adverse clinical symptoms
pretherapy
The simultaneous or separate incidence of fever,
muscle pain, skin rashes, gastrointestinal reaction and other
symptoms was 25.3% in the experimental group and 24% in the control
group following treatment; there was no statistically significant
difference between the two groups (P>0.05).
Discussion
Numerous studies have confirmed that the
modification of protein drugs with polyethylene glycol can create a
modified drug with better biological activity and a longer
half-life. When compared with rhG-CSF, the polyethylene
glycol-modified derivative PEG-rhG-CSF has similar efficacy and
safety. It also has a long half-life and a self-regulation effect
on blood concentration (4–7). In developed countries such as those
in Europe and the USA, PEG-rhG-CSF is mainly used in the preventive
treatment of chemotherapy-induced non-marrow-derived neutropenia.
In China, the Cancer Hospital of Chinese Academy of Medical
Sciences led the research program for domestic PEG-rhG-CSF
(Jinyouli), and completed a phase III clinical trial in the
prophylactic treatment of chemotherapy-induced neutropenia. The
results demonstrated that the efficacy and adverse reactions of
single PEG-rhG-CSF administration were similar to those of repeated
administration of rhG-CSF (8–9).
Given the social and economic factors in China, certain
difficulties remain in PEG-rhG-CSF application for preventive
treatment in relatively underdeveloped areas. As the drug has been
used in China only for a short time, oncologists have questions
concerning its use in the salvage treatment of chemotherapy-induced
non-marrow-derived neutropenia.
The present study included 114 patients with grade
IV chemotherapy-induced neutropenia. The results showed that
PEG-rhG-CSF and rhG-CSF both had a beneficial effect on
neutrophils. These and other study results are consistent in
indicating that PEG-rhG-CSF can be applied as a preventive therapy
for chemotherapy-induced neutropenia (9–11).
The positive results may also be associated with the fact that the
patients in the present study were all chest cancer patients, and
the radiation treatment used three-dimensional conformal
intensity-modulated technology to better reduce the radiation dose
of neighboring flat bones and irregular bones, and therefore, the
effects of radiation on the proliferation of bone marrow. The 114
patients received different radiotherapy doses and chemotherapy
plans. However, all patients enrolled in the pre-experiment had
been confirmed to have no signs of bone metastases. Therefore, the
grade IV neutropenia was considered as concurrent
chemoradiotherapy-induced non-myeloid-derived neutropenia. In the
three subgroups of the experimental group, the neutrophil
proliferation rate and ANC values were significantly higher than
those in the control group. ANC values in the experimental group
increased to the normal range 48 h after treatment, whereas those
in the control group only reached 2.0×109/l 120 h after
treatment initiation, indicating that a single dose of PEG-rhG-CSF
had greater effects on the proliferation of neutrophils than
multiple doses of rhG-CSF. PEG-rhG-CSF also significantly shortened
the time taken for chemoradiotherapy-induced myeloid-derived
non-neutropenia to be improved, and so can reduce the clinical risk
of neutropenia.
In the three subgroups of the experimental group,
the neutrophil proliferation rates did not show significantly
statistical difference. In terms of ANC values, the P-50 group
showed no significant difference from the P + R group, and these
two groups were statistically different from the P-100 group. In
the P-100, P-50 and P + R groups, symptoms of neutropenia were
improved 48 h after treatment, so the purpose of clinical treatment
was achieved. The difference between the P-100 group and the latter
two was that the ANC values of the P-100 group increased more
significantly 72 h after treatment. Accordingly, it is proposed
that oncologists should use a single subcutaneous dose of 50 μg/kg
PEG-rhG-CSF in the treatment of grade IV non-marrow-derived
neutropenia to achieve the therapeutic purpose. It is noteworthy
that in the experimental and control groups, 12 h after dosing, the
neutrophil proliferation rates were negative and ANC mean values
showed a downward trend. The reason may be as follows: when grade
IV neutropenia was found in certain patients, the ANC values had
not dropped to the lowest point induced by the toxicity of
chemotherapy; it took some time for rhG-CSF to stimulate the
differentiation and maturation of granulocytes. Therefore, the
blood ANC values dropped shortly following the injection of
PEG-rhG-CSF or rhG-CSF instead of rising. At 24 h after medication,
the three experimental subgroups exhibited peak neutrophil
proliferation rates, and at 36 h, the ANC mean values all exceeded
2.0×109/l. It is proposed that the blood routine test
should be performed 12 h after the application of PEG-rhG-CSF; even
if the ANC shows no significant increase, no supplementary
administration of rhG-CSF is necessary and the focus should be on
changes in ANC values 24–48 h after dosing.
The mean time required to alleviate the neutropenic
fever and muscle pain in experimental group was 30 h, compared with
72 and 59 h, respectively, in the control group. This is consistent
with the time at which ANC mean values reached 2.0×109/l
in the two groups of patients. The times in the two groups were
significantly different, indicating that PEG-rhG-CSF had advantages
over rhG-CSF in relieving the symptoms of fever and muscle pain
caused by neutropenia. In alleviating fatigue and digestive
symptoms, PEG-rhG-CSF showed significant advantages over rhG-CSF.
This may be due to physical weakness and intestinal dysfunction in
certain patients following concurrent chemoradiotherapy.
In terms of safety, the incidence of fever, skeletal
muscle pain, skin rashes, gastrointestinal reactions and other
symptoms was 25.3% in the experimental group and 24% in the control
group; the difference was not statistically significant. The
results were comparable with those in the study by Lan et al
(12). Accordingly the authors of
the present study consider that PEG-rhG-CSF is clinically a safe
and reliable drug used in patients with non-myeloid-derived
neutropenia.
In conclusion, this study showed that PEG-rhG-CSF
can be used in concurrent radiotherapy-induced grade IV neutropenia
preventive therapy. It demonstrated similar clinical safety to
rhG-CSF and significant advantageous effects. A single dose of
PEG-rhG-CSF can improve neutropenia and some secondary symptoms,
ensure the course of antitumor therapy in these patients, and
reduce the pain of repeated rhG-CSF injections, indicating that is
has good prospects for the future.
References
|
1
|
Molineux G: Pegylation: engineering
improved pharmaceuticals for enhanced therapy. Cancer Treat Rev.
28(Suppl 1): 13–16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Klastersky J and Awada A: Prevention of
febrile neutropenia in chemotherapy-treated cancer patients:
Pegylated versus standard myeloid colony stimulating factors. Do we
have a choice? Crit Rev Oncol Hematol. 78:17–23. 2011. View Article : Google Scholar
|
|
3
|
National Cancer Institute. Cancer Therapy
Evaluation Program. Common toxicity criteria (CTC) version 2.0.
1999
|
|
4
|
Bailon P and Berthold W: Polyethylene
glycol-conjugated pharmaceutical proteins. Pharm Sci Technol Today.
1:352–356. 1998. View Article : Google Scholar
|
|
5
|
Crawford J: Clinical benefits of pegylated
proteins in oncology. Cancer Treat Rev. 28(Suppl 1): 1–2. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reddy KR: Controlled-release, pegylation,
liposomal formulations: new mechanisms in the delivery of
injectable drugs. Ann Pharmacother. 34:915–923. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Molineux G: Pegfilgrastim: using
pegylation technology to improve neutropenia support in cancer
patients. Anticancer Drugs. 14:259–264. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi YK, Liu P, Yang S, Han XH, He XH, Ai
B, Qin Y, Li B, Huang DZ, Zhang CG and Sun Y: Phase I clinical
trial of intravenous pegylated recombinant human granulocyte
colony-stimulating factor. Ai Zheng. 25:495–500. 2006.(In Chinese).
PubMed/NCBI
|
|
9
|
Shi YK, He XH, Yang S, et al: Treatment of
chemotherapy-induced neutropenia pegylated recombinant human
granulocyte colony-stimulating factor: a multi-center randomized
controlled phase II clinical study. Zhonghua Yi Xue Za Zhi.
86:3414–3419. 2006.(In Chinese).
|
|
10
|
Holmes FA, Jones SE, O’Shaughnessy J, et
al: Comparable efficacy and safety profiles of once-per-cycle
pegfilgrastim and daily injection filgrastim in
chemotherapy-induced neutropenia: a multicenter dose-finding study
in women with breast cancer. Ann Oncol. 13:903–909. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Holmes FA, O’Shaughnessy JA, Vukelja S,
Jones SE, Shogan J, Savin M, Glaspy J, Moore M, Meza L, Wiznitzer
I, Neumann TA, Hill LR and Liang BC: Blinded, randomized,
multicenter study to evaluate single administration pegfilgrastim
once per cycle versus daily filgrastim as an adjunct to
chemotherapy in patients with high-risk stage II or stage III/IV
breast cancer. J Clin Oncol. 20:727–731. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lan HT, Luo SC, Hu HL and Wu Z: Clinical
observation of pegylated recombinant human granulocyte
colony-stimulating factor in prevention chemotherapy-induced
neutropenia. Sichuan Medical Journal. 32:1879–1882. 2011.(In
Chinese).
|