Introduction
Alzheimer’s disease (AD) is a progressive,
neurodegenerative disease, which is pathologically characterized by
senile plaques, neurofibrillary tangles and neural cell death. The
etiology and pathogenesis of the disease remain unknown (1). It is generally believed that cerebral
ischemia and hypoxia, caused by head trauma, brain vascular
inflammation, cerebral vascular stenosis or partial embolism,
contribute to the disease onset and development (2); therefore, the study of brain vascular
lesions has always been an important topic in research into AD.
Vascular endothelial growth factor (VEGF) is one of
the most important regulatory factors in vascular growth and
development. VEGF can promote angiogenesis and increase the blood
supply to support metabolic processes (3). It has been found in a previous study
that the expression of VEGF is altered in the occurrence and
development of AD, which may be associated with the disease process
(4). Recently, the role of
microRNA (miRNA) in post-transcriptional gene regulation has been
uncovered (5). VEGF has been
observed to be one of the targets of miRNA-210 (6), and a previous study has shown that
the upregulation of miRNA-210 can increase the expression of VEGF
in kidney tissue (7). Due to its
stability and specificity, miRNA-210 has been used as a genetic
marker for the early diagnosis and treatment of VEGF-associated
diseases; however, the quantitative detection of miRNA-210 in the
cerebrospinal fluid (CSF) and serum in AD, particularly regarding
the regulation of VEGF, has, to the best of our knowledge, not been
fully elucidated.
In the present study, the expression levels of VEGF
and miRNA-210 in the CSF and serum of patients with AD were
detected, and their association with the disease severity was
investigated.
Materials and methods
Patients and sample collection
A total of 56 patients with dementia, who had been
admitted to Zaozhuang Municipal Hospital between January 2012 and
August 2013, were enrolled in the present study. Based on the
National Institute of Neurological and Communicative Disorders and
Stroke and the Alzheimer’s Disease and Related Disorders
Association (NINCDS-ADRDA) diagnostic criteria amendment, published
by the National Institute on Aging and the Alzheimer’s Association
(NIA-AA) in April 2011 (8), these
patients were diagnosed with AD and categorized into the mild
cognitive impairment (MCI) (n=30) and AD (n=26) groups. A total of
42 healthy individuals were used as controls. The MCI group
comprised 18 male and 12 female patients, with ages ranging from 61
to 82 years (mean age, 71.6 years); the AD group comprised 12 male
and 14 female patients, with ages ranging from 60 to 84 years (mean
age, 72.3 years); and the control group comprised 23 male and 19
female subjects, with ages ranging from 62 to 85 years (mean age,
71.9 years). Prior written and informed consent was obtained from
every patient prior to their participation in the current study,
and the study was approved by the Ethics Review Board of Zaozhuang
Municipal Hospital (Zaozhuang, China). Furthermore, eight patients
with AD, with sufficient finances, underwent intracranial pressure
monitoring, brain oxygen tension monitoring and computed tomography
(CT).
For sample collection, 2 ml CSF was extracted from
each patient in the morning, centrifuged at 1,200 × g for 5 min at
4°C and then stored at −20°C until required. For the serum samples,
peripheral blood was collected from each patient in the morning,
after fasting for 12 h. The serum was separated by centrifugation
at 12,000 × g for 5 min at 4°C and the samples were stored at −80°C
until required.
Intracranial pressure monitoring
Intracranial pressure was detected by inserting an
intraventricular catheter with a sensor (ICP Express; Codman &
Shurtleff, Inc., Raynham, MA, USA), into the lateral ventricle of
patients with AD. This area of the brain contains liquid that
protects the brain and spinal cord. The intracranial pressure was
monitored whilst the fluid was drained out through the catheter,
which was connected to an Intracranial Pressure Monitoring system
(Spiegelberg GmbH & Co. KG, Hamburg, Germany).
Brain oxygen tension monitoring
Following calibration to anoxia and ambient oxygen,
an oval polymethylmethacrylate conformer, containing a conjunctival
sensor (LICOX CMP system; Integra Neurosciences, Inc., San Diego,
CA, USA), was placed on the superolateral side of the head in order
to allow the sensor to engage the superolateral intracranial
conjunctiva, while preventing contact between the conformer and the
cornea. The sensor was then connected to the conjunctival oxygen
monitor (TO2M 2000 Tissue Oxygen Monitoring system; Biomedical
Sensors, High Wycombe, UK).
Computed tomography (CT)
All CT scans were obtained on a single-detector
helical HiSpeed CT/i scanner (GE Medical Systems, Milwaukee, WI,
USA). All subjects were subjected to a nonenhanced head CT (axial
5-mm collimation), following placement of a brain-tissue oxygen
probe. The axial level corresponding to the most inferior aspect of
the probe was used. CT perfusion images were acquired by Advantage
Workstation (GE Medical Systems).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA in the CSF and serum samples was extracted
using TRIzol® (Invitrogen Life Technologies, Carlsbad,
CA, USA) with miRcute miRNA isolation kits (Tiangen Biotech,
Beijing, China). The purity of the samples was measured at
A260/A280 with an ultraviolet spectrophotometer (DR 6000™ UV VIS;
HACH, Loveland, CO, USA), and cDNA was obtained by reverse
transcription with an miRcute miRNA First-Strand cDNA Synthesis kit
(Tiangen Biotech). The RT-qPCR was performed with an iQ5 RT-qPCR
detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The reaction conditions for VEGF were as follows: Pre-denaturation
at 94°C for 2 min; denaturation at 94°C for 30 sec, annealing at
55°C for 30 sec and extension at 71°C for 1 min, (35 cycles); and a
final extension step at 71°C for 2 min. The RT-qPCR reaction
conditions for miRNA-210 detection were as follows:
Pre-denaturation at 95°C for 10 min; denaturation at 95°C for 15
sec, annealing at 60°C for 1 min and extension at 72°C for 2 min
(40 cycles); and a final extension step at 72°C for 7 min. Results
were calculated using the 2−ΔΔCt method, and β-actin and
U6 were used as the controls for VEGF and miRNA-210, respectively.
The RT-qPCR primers (Tiangen Biotech) for the measurement of VEGF
mRNA and miRNA-210 expression are listed in Table I.
 | Table IPrimer sets used for the reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primer sets used for the reverse
transcription-quantitative polymerase chain reaction.
| Primer sets | Sequences |
|---|
| VEGF |
| Forward |
5′-TTGCCTTGCTGCTCTACCTC-3′ |
| Reverse |
5′-AAATGCTTTCTCCGCTCTGA-3′ |
| β-actin |
| Forward |
5′-TGACGTGGACATCCGCAAAG-3′ |
| Reverse |
5′-CTGGAAGGTGGACAGCGAGG-3′ |
| miRNA-210 |
| Forward |
5′-CTGTGCGTGTGACAGCGGCTGA-3′ |
| Reverse |
5′-GCGAGCACAGAATTAATACGAC-3′ |
| U6 |
| Forward |
5′-CGCTTCGGCAGCACATATACTA-3′ |
| Reverse |
5′-CGCTTCACGAATTTGCGTGTCA-3′ |
Western blot analysis
The total proteins in the CSF and serum samples were
extracted with protein lysis solution (Tiangen Biotech). Protein
concentration was measured with a bicinchoninic acid kit (Tiangen
Biotech). SDS-PAGE sample buffer (Tiangen Biotech) was added to the
protein samples, and the samples were boiled for 5 min. A total of
20 μg protein was used for SDS-PAGE with 10% acrylamide gels, and
the protein was then transferred onto a membrane at a constant
voltage of 100 V for 2 h under ice-cold conditions. The membrane
was blocked with 5% skimmed milk for 1 h at room temperature.
Rabbit anti-human monoclonal anti-VEGF primary antibody (1:1,000;
cat. no. ab52917, Abcam, Cambridge, MA, USA) and the internal
reference rabbit anti-human monoclonal anti-β-actin primary
antibody (1:5,000; cat. no. ab115777, Abcam) were added for
incubation with the membrane at 4°C overnight. A goat anti-rabbit
polyclonal immunoglobulin G secondary antibody (1:3,000; cat. no.
ab97047, Abcam) was subsequently added for incubation at room
temperature for 1 h. The membrane was placed in an enhanced
chemiluminescence solution (Tiangen Biotech) and then exposed to
the Gel Doc™ EZ Imager (Bio-Rad Laboratories, Inc.). Image Lab
software version 3.0 (Bio-Rad Laboratories, Inc.) was used for
protein band analysis. The relative content of the target protein
was determined as the ratio to the internal reference β-actin.
Statistical analysis
Data are expressed as the mean ± standard deviation.
All data were processed using SPSS 18.0 software (SPSS, Inc.,
Chicago, IL, USA). One-way analysis of variance was performed for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
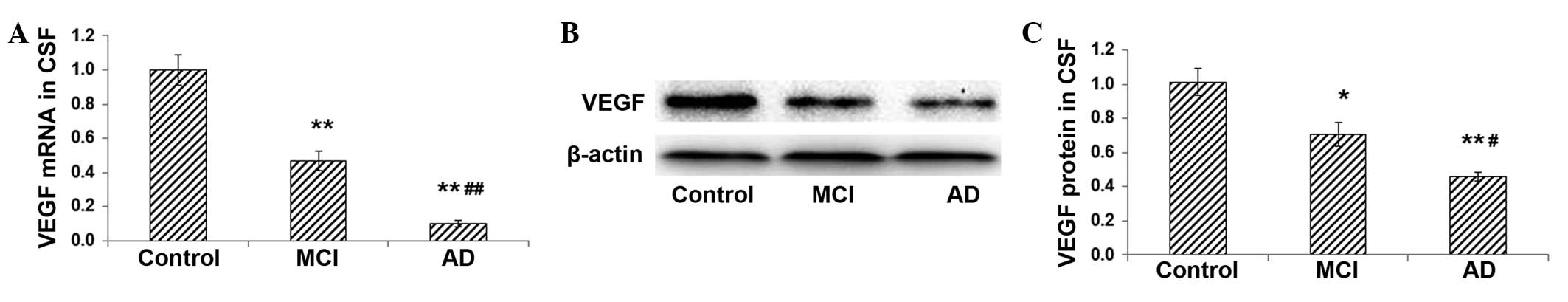
Expression levels of VEGF mRNA and
protein are decreased in the CSF of patients with MCI and AD
To investigate the role of VEGF in the pathogenesis
of AD, the mRNA and protein expression levels of VEGF in the CSF
were detected with RT-qPCR and western blot analysis, respectively.
The enrolled patients were divided into the MCI (n=30) and AD
(n=26) groups, and normal individuals (n=42) were used as controls.
Results from the RT-qPCR demonstrated that, compared with the
expression in the control group, VEGF mRNA expression in the CSF in
the MCI group was significantly reduced (P<0.01), and was
further reduced in the AD group (AD versus control, P<0.01; AD
versus MCI, P<0.01) (Fig. 1A).
Similar results were obtained with the western blot analysis. The
protein expression of VEGF was significantly lower in the MCI and
AD groups compared with that in the control group (control versus
MCI, P<0.05; control versus AD, P<0.01). Furthermore, VEGF
protein expression was lower in the AD group than that in the MCI
group (P<0.01) (Fig. 1B and C).
These results indicate that the mRNA and protein expression levels
of VEGF in the CSF are decreased in patients with MCI and AD.
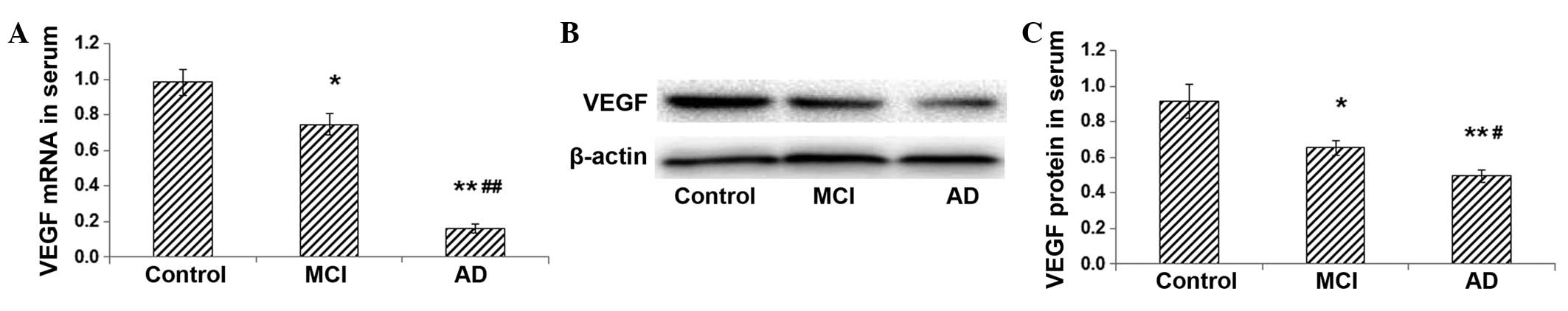
Expression levels of VEGF mRNA and
protein in the serum are decreased in patients with MCI and AD
To further confirm the association between VEGF
expression and the pathogenesis of AD, the serum expression of VEGF
was determined. The RT-qPCR results showed that the serum
expression level of VEGF mRNA was significantly lower in the MCI
group than that in the control group (P<0.05) (Fig. 2A). In the AD group, the serum VEGF
mRNA expression was further decreased and was significantly
different from that in the MCI group (P<0.01) (Fig. 2A). Western blot analysis
demonstrated that the protein expression level of VEGF in the serum
was markedly lower in the MCI group than that in the control group
(P<0.05), and it was further decreased in the AD group
(P<0.01) (Fig. 2B and C). These
results indicate that the VEGF mRNA and protein expression levels
in the serum decrease with the increase in disease severity.
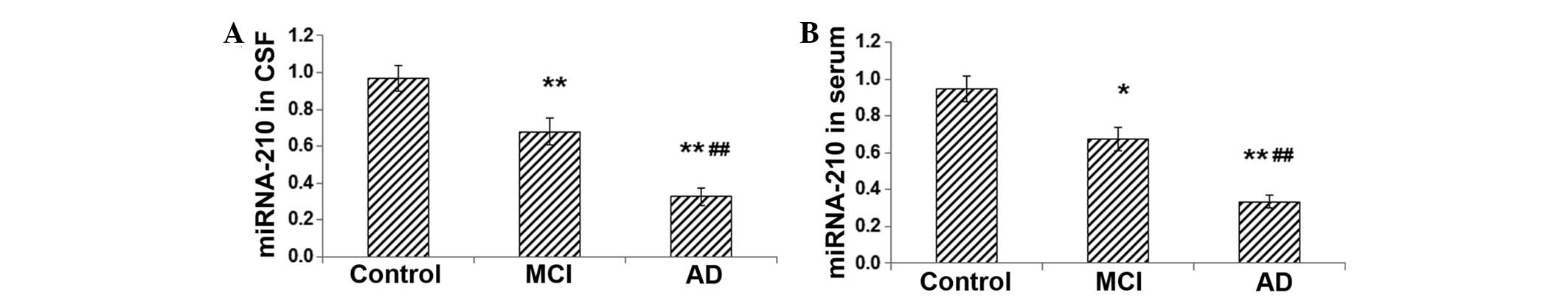
Expression levels of miRNA-210 are
reduced in the CSF and serum of patients with MCI and AD
Since VEGF is one of the targets of miRNA-210, the
current study investigated whether the level of miRNA-210 was
altered in patients with MCI and AD. The expression levels of
miRNA-210 in the CSF and serum were determined using RT-qPCR. The
results indicated that the levels of miRNA-210 in the CSF and serum
were significantly decreased in the MCI group compared with those
in the control group (for CSF, P<0.01; for serum, P<0.05)
(Fig. 3A and B). Furthermore, in
the AD group, the expression levels of miRNA-210 in the CSF and
serum were markedly lower than those in the MCI group (both
P<0.01) (Fig. 3A and B). These
results indicate that, similar to VEGF, the expression levels of
miRNA-210 in the CSF and serum decrease as the severity of AD
increases.
Discussion
AD is one of the most common types of dementia and
is caused by chronic pathological changes in the central nervous
system. The clinical features of AD include amnesia, spatial
learning and memory disability, language barriers, apraxia,
cognitive impairment, executive dysfunction, and changes in
personality and behavior. The risk of the disease increases with
age, with a higher incidence in individuals >70 years old. The
onset and pathogenesis of AD remains incompletely understood
(9). Under current medical
standards, clinical diagnostic tools for AD include
neuropsychological tests, hematological examination, neuroimaging,
electroencephalography and CSF detection (10). Hematological examination is
primarily used to detect concomitant diseases, complications and
potential risk factors, and to help exclude dementia caused by
other medical conditions. Although it has good sensitivity and
specificity, the application of CSF detection is currently limited
due to the lack of uniform testing and sample processing methods,
which could lead to clinical diagnostic errors and missed
diagnoses. For these reasons, it is necessary to determine other
more reliable and stable genetic markers in the CSF and blood
detection.
Cerebral vascular changes, which contribute to the
pathological changes in nerve cells, may be one of the important
risk factors for a number of neurological diseases. These
pathological changes result in cerebral insufficiency, low brain
oxygen tension, low oxygen exchange rate and CO2
poisoning (11). It has been found
that the temporal parietal and prefrontal lobes in the brains of
patients with AD exhibit significant atrophy and that there are
numerous neurofibrillary tangles in the nerve cells; these
observations are associated with microvascular pathological changes
(12). Brain vascular lesions may
also cause ischemia and hypoxia, which induce β-amyloid deposition,
abnormal phosphorylation of tau protein and neuronal degeneration
and death (13). These findings
indicate that there are vascular pathological changes in the brains
of individuals with AD, which may contribute to the occurrence and
development of the disease. In the present study, certain patients
with AD were subjected to intracranial pressure monitoring, brain
oxygen tension monitoring and computed tomography, and the results
showed increased intracranial pressure, decreased oxygen partial
pressure and intracranial vascular blockage and/or stenosis, which
indirectly demonstrated the presence of vascular lesions in the
brains of the patients with AD (data not shown).
VEGF is one of the most effective angiogenic growth
factors in the human body and is able to promote angiogenesis and
increase the blood supply (3).
Solerte et al (14) found
that the level of immune cell-released VEGF in patients with AD was
lower than the normal level. A decrease in the immune cell-released
VEGF could reduce the protection of neuronal cells and inhibit
microvascular nutritional factors, resulting in an increase in
brain injuries under hypoxia (14). Detection of the level of VEGF in
the brain tissue could indicate the neurodegenerative pathological
processes in cases of AD; however, considerable difficulty exists
in the detection of VEGF expression in biopsy brain tissue samples.
Furthermore, it has been demonstrated that, when brain lesions are
severe, VEGF can enter the blood circulation from the brain due to
the rupture of nerve cells (15).
The detection of VEGF levels in the CSF can therefore be used as an
indicator for VEGF expression in the brain. In the present study,
the mRNA and protein expression levels of VEGF in the CSF and serum
were significantly downregulated in the MCI group when compared
with those in the control group. Furthermore, the expression of
VEGF was further deceased in the AD group relative to that in the
MCI group. These results indicate a downward trend in VEGF
expression with increasing severity of dementia. The expression
level of VEGF was decreased in both the CSF and serum; however, the
expression levels differed slightly between the two, indicating the
differential sensitivities of the same indicator in the CSF and
serum in reflecting the pathological conditions of AD.
There are a number of factors affecting the
regulation of mRNA expression. Recent studies have revealed that
miRNAs are a class of endogenous, small, non-coding RNA molecules
in cells that are important modulators for normal development and
physiological and pathological conditions (16,17).
miRNAs regulate target mRNAs in a negative feedback loop, cutting
the mRNAs and inhibiting their translation. In the processes of
tumor formation and development (18), angiogenesis and nerve repair
(19), capillary formation
(20) and ligament repair
(21), miRNA-210 has been observed
to upregulate VEGF to promote the growth of vascular cells. The
present results demonstrated that miRNA-210 was downregulated with
decreasing VEGF expression in the CSF and serum in patients with
AD, which was in line with the results of other studies (18–21).
The results of the current study demonstrated that
disease severity, VEGF expression and miRNA-210 level are
associated with disease development. The expression of miRNA-210 in
the CSF and serum may indirectly reflect the severity of dementia
due to AD, and the regulation of miRNA-210 expression may affect
the disease processes. The patients enrolled in the present study
were categorized with the NINCDS-ADRDA diagnostic criteria
amendment published by the NIA-AA, and the basic information about
the patients, including their gender, age and medical history, was
matched as far as possible. Despite this, the results may be
limited due to the limited sample size and the regional differences
among the patients, as well as the fact that there are numerous
other factors associated with AD pathogenesis besides VEGF and
miRNA-210 (20–24). Furthermore, the actions of
miRNA-210 may differ from case to case, and so further studies are
required to detail and validate the specific underlying mechanisms
of miRNA-210 in cellular experiments, animal model studies and
clinical trials.
In conclusion, the present results showed that the
expression levels of VEGF mRNA and protein were significantly
decreased in the CSF and serum of patients with MCI and AD, with an
evident decreasing trend with increased disease severity. Notably,
as a modulator of VEGF expression, the level of miRNA-210 also
declined in the CSF and serum in patients with MCI and AD. These
results demonstrate that miRNA-210 is not only indicative of AD
pathogenesis, but may also provide novel insights into the
prevention and treatment of the disease.
Acknowledgements
The authors would like to thank Professor Guangrun
Xu (Department of Neurology, Qilu Hospital of Shandong University)
and Dr Jianwei Li (Chief Physician at the Department of Anesthesia,
Zaozhuang Municipal Hospital) for their conscientious efforts and
helpful advice in the study design, sample collection, statistical
analysis and manuscript preparation.
References
|
1
|
Duran-Aniotz C, Martínez G and Hetz C:
Memory loss in Alzheimer’s disease: are the alterations in the UPR
network involved in the cognitive impairment? Front Aging Neurosci.
6:82014. View Article : Google Scholar
|
|
2
|
de la Monte SM and Tong M: Brain metabolic
dysfunction at the core of Alzheimer’s disease. Biochem Pharmacol.
88:548–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roberts E, Cossigny DA and Quan GM: The
role of vascular endothelial growth factor in metastatic prostate
cancer to the skeleton. Prostate Cancer. 2013:4183402013.
View Article : Google Scholar
|
|
4
|
Mao X, Wang T, Liu Y, et al:
N-acetylcysteine and allopurinol confer synergy in attenuating
myocardial ischemia injury via restoring VEGF/HO-1 signaling in
diabetic rats. PLoS One. 8:e689492013. View Article : Google Scholar
|
|
5
|
In Lee S, Ji MR, Jang YJ, et al:
Characterization and miRNA-mediated posttranscriptional regulation
of vitelline membrane outer layer protein I in the adult chicken
oviduct. In Vitro Cell Dev Biol Anim. Nov 8–2014.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu SC, Chuang SM, Hsu CJ, et al: CTGF
increases vascular endothelial growth factor-dependent angiogenesis
in human synovial fibroblasts by increasing miR-210 expression.
Cell Death Dis. 5:e14852014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li H, Wang Y, Liu F, et al: Dynamic
expressions of hypoxia-inducible factor-1α and its target gene
miRNA-210 and vascular endothelial growth factor after renal
ischemia-reperfusion injury. Zhong Hua Shi Yan Wai Ke Za Zhi.
28:2074–2076. 2011.(In Chinese).
|
|
8
|
Azheimer’s Association. 2011 Alzheimer’s
disease facts and figures. Alzheimers Dement. 7:208–244. 2011.
View Article : Google Scholar
|
|
9
|
Petrella JR: Neuroimaging and the search
for a cure for Alzheimer disease. Radiology. 269:671–691. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li S, Okonkwo O, Albert M and Wang MC:
Variation in variables that predict progression from MCI to AD
dementia over duration of follow-up. Am J Alzheimers Dis
(Columbia). 2:12–28. 2013.
|
|
11
|
Hill CB, Grandgeorge SH and Bavis RW:
Developmental hyperoxia alters CNS mechanisms underlying hypoxic
ventilatory depression in neonatal rats. Respir Physiol Neurobiol.
189:489–505. 2013. View Article : Google Scholar
|
|
12
|
Sopova K, Gatsiou K, Stellos K and Laske
C: Dysregulation of neurotrophic and haematopoietic growth factors
in Alzheimer’s disease: from pathophysiology to novel treatment
strategies. Curr Alzheimer Res. 11:27–39. 2014. View Article : Google Scholar
|
|
13
|
Damodaran T, Hassan Z, Navaratnam V, et
al: Time course of motor and cognitive functions after chronic
cerebral ischemia in rats. Behav Brain Res. 275:252–258. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Solerte SB, Ferrari E, Cuzzoni G, et al:
Decreased release of the angiogenic peptide vascular endothelial
growth factor in Alzheimer’s disease: recovering effect with
insulin and DHEA sulfate. Dement Geriatr Cogn Disord. 19:1–10.
2005. View Article : Google Scholar
|
|
15
|
Pikula A, Beiser AS, Chen TC, et al: Serum
brain-derived neurotrophic factor and vascular endothelial growth
factor levels are associated with risk of stroke and vascular brain
injury: Framingham Study. Stroke. 44:2768–2775. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng CJ, Bahal R, Babar IA, et al:
MicroRNA silencing for cancer therapy targeted to the tumor
microenvironment. Nature. Nov 17–2014.(Epub ahead of print).
View Article : Google Scholar
|
|
17
|
Jayanthy A and Selaturi V: Light regulated
microRNAs. Photochem Photobiol. Nov 10–2014.(epub ahead of
print).
|
|
18
|
Alaiti MA, Ishikawa M, Masuda H, et al:
Up-regulation of miR-210 by vascular endothelial growth factor in
ex vivo expanded CD34+ cells enhances cell-mediated
angiogenesis. J Cell Mol Med. 16:2413–2421. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zeng L, He X, Wang Y, et al: MicroRNA-210
overexpression induces angiogenesis and neurogenesis in the normal
adult mouse brain. Gene Ther. 21:37–43. 2014. View Article : Google Scholar
|
|
20
|
Lou Y, Gao F, Xie A, et al: MicroRNA-210
modified human umbilical vein endothelial cells induce capillary
formation. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 26:587–591.
2012.(In Chinese). PubMed/NCBI
|
|
21
|
Shoji T, Nakasa T, Yamasaki K, et al: The
effect of intra-articular injection of microRNA-210 on ligament
healing in a rat model. Am J Sports Med. 40:2470–2478. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nagpure BV and Bian JS: Hydrogen sulfide
inhibits A2A adenosine receptor agonist induced β-amyloid
production in SH-SY5Y neuroblastoma cells via a cAMP dependent
pathway. PLoS One. 9:e885082014. View Article : Google Scholar
|
|
23
|
Zhang Y, Pan C, Wu X, et al: Different
effects of anesthetic isoflurane on caspase-3 activation and
cytosol cytochrome c levels between mice neural progenitor cells
and neurons. Front Cell Neurosci. 8:142014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Y, Wang B, Liu D, et al: Hsp90
chaperone inhibitor 17-AAG attenuates A β-induced synaptic toxicity
and memory impairment. J Neurosci. 34:2464–2470. 2014. View Article : Google Scholar : PubMed/NCBI
|