Introduction
Cardiac transplantation is a viable therapy for
patients with end-stage heart disease. The development of novel
immunosuppressive agents has improved early survival following
cardiac transplantation; however, long-term survival has linearly
decreased (1). A second
transplantation surgery is inevitable if previous grafts are
completely rejected (2).
Furthermore, a number of factors, including the initial transplant
graft recipient, blood transfusion, pregnancy and continued
exposure to symbiotic microbial pathogens, may stimulate memory T
(Tm) cell proliferation; 40–50% of T cells express a memory-like
phenotype in peripheral blood (3,4). Tm
cells have become an inevitable barrier in transplant tolerance in
retransplantation due to their ability to respond more rapidly and
effectively to the previously encountered pathogens and their
longevity (5).
Heart retransplantation studies face difficulties in
establishing a retransplantation murine model, particularly a model
of solid organ retransplantation with blood vessel anastomosis,
since few mice can withstand a second surgical trauma. In the
present study, an advanced microsurgical technique was used to
adapt a consecutive skin-heart retransplantation murine model. As a
potent immunogen, alloskin has the ability to vigorously induce
alloantigen-specific Tm cells, resulting in a reliable
retransplantation murine model.
Cardiac allograft vasculopathy (CAV) is the major
cause of late morbidity and mortality among heart retransplantation
recipients. Progressive neointimal proliferation leads to ischemic
injury and failure of the allograft (6). Although the pathogenesis of CAV is
not fully understood, a number of studies have found that the
infiltration of T cells (including naive T cells and Tm cells) and
their expression products is a distinctive characteristic of CAV
(7,8). Researchers have attempted to
investigate immunosuppressive regimens for Tm cells from multiple
perspectives; however, previous studies have indicated that
conventional immunosuppressive regimens, such as antirejection
drugs (including cyclosporin A, FK506 and rapamycin) and classic
costimulatory pathway blockade are ineffective in retransplantation
(9,10).
Chemokines are a family of ~50 cytokines that
mediate cell chemotaxis and activation. Chemokine levels are known
to significantly increase during acute rejection episodes following
first organ transplantations (11,12).
A previous study indicated that specific chemokines may play a
critical role in Tm-cell recruitment during transplantation
allograft rejection (13). We
previously demonstrated the presence of higher levels of RANTES
expression and secretion in retransplantation or Tm-cell-transfer
models (14). In the present
study, the effects of mouse C-X-C motif chemokine 9 (CXCL9)
affinity purified polyclonal antibody (Ab), mouse CXCL10 monoclonal
Ab and FTY720 on Tm-cell infiltration and allograft survival time
were investigated in a murine retransplantation model.
Materials and methods
Animals
BALB/c (H2d) and C57BL/6 (H2b)
mice were used as transplant donors and recipients, respectively.
The mice (female; age, 8–12 weeks; weight, 20–25 g) were purchased
from the Shanghai Laboratory Animal Center, CAS (Shanghai, China)
and housed in the animal facilities at Xiamen University (Xiamen,
China) under pathogen-free conditions. The animals received human
care in compliance with the Guide for the Care and Use of
Laboratory Animals published by the United States Department of
Health and Human Services (8th edition, National Academies Press,
Washington, USA, D.C., 2011). The current study was approved by the
Ethics Committee of the First Affiliated Hospital of Xiamen
University (Xiamen, China).
Skin transplantation
Orthotopic full-thickness skin grafts were obtained
from the BALB/c donors, cut into circular pieces (area, 1.5
cm2) and sutured bilaterally into the flanks of the
C57BL/6 recipients. The BALB/c donor skin grafts on the lumbar
region of the C57BL/6 recipients were examined daily.
Heterotopic heart transplantation and Ab
treatment
Mouse hearts were transplanted to a heterotopic neck
location by anastomosis of the neck vessels using a microsurgery
nonsuture cuff technique (15).
The BALB/c (H2d) mice were used as heart transplant
donors, while C57BL/6 (H2b) mice received the heart
transplants four weeks after the skin transplantation. Graft
viability was assessed by palpation twice a day. The
post-transplantation treatment regimens are shown in Table I.
 | Table IPost-transplantation treatment
regimens. |
Table I
Post-transplantation treatment
regimens.
| Group | Treatment
(intraperitoneal) |
|---|
| Control | NS |
| CXCL9 Ab and CXCL10
Ab | CXCL9 Ab + CXCL10 Ab
+ NS |
| FTY720 | FTY720 + NS |
| Combined | CXCL9 Ab + CXCL10 Ab
+ FTY720 + NS |
The mice were divided into four groups: i) Control
(n=6), ii) CXCL9 Ab and CXCL10 Ab (n=6), iii) FTY720 (n=6) and iv)
combined (n=6). Mouse polyclonal CXCL9 Ab and mouse monoclonal
CXCL10 Ab (cat. nos. AF-492-NA and MAB66, respectively; R&D
Systems, Inc., Minneapolis, MN, USA) were administered [150 μg;
intraperitoneal (ip)] to the transplant recipients on days −1, +1,
+3 and every two days thereafter until rejection (the day of
transplant counted as day 0). Similarly, FTY720 (R&D Systems,
Inc.) was administered (0.2 mg; ip) to the transplant recipients on
days −1, +1, +3 and every two days until rejection. Mice in the
control group were treated with equivalent doses of normal saline.
In the combined group, 150 μg CXCL9 Ab ip, 150 μg CXCL10 Ab ip and
0.2 mg FTY720 ip were administered to the transplant recipients on
days 1, +1, +3 and every two days until rejection.
Allograft survival time
The cardiac allografts were examined by palpation
daily, at 8:00 a.m. and 5:00 p.m. Transplant rejection was defined
as the absence of heartbeat, which was later confirmed by
histological examination. Four groups were studied in total,
containing 6 animals/group: positive control (n=6), CXCL9 Ab and
CXCL10 Ab (n=6), FTY720 (n=6) and combined (n=6). All reagents were
administered to the transplant recipients on days 1, +1, +3 and
every 2 days thereafter until rejection. Mice in the control group
were treated with equivalent doses of normal saline. The allograft
survival time was recorded in each group.
Histological examination
The cardiac allografts were harvested four days
after transplantation and incised with a scalpel along the septal
plane. Half of each sample was placed in 10% neutral buffered
formalin (Shanghai HuaYi Bio-tech Co. Ltd., Shanghai, China) and
embedded in paraffin (Shanghai HuaYi Bio-tech Co. Ltd.).
Subsequently, 5-μm sections were cut, stained with hematoxylin and
eosin (Shanghai HuaYi Bio-tech Co. Ltd.) and observed under an
optical microscope (BM2000; Ronbio Scientific Co., Ltd., Shanghai,
China). Allograft rejection level determination was performed
according to the International Society for Heart and Lung
Transplantation (ISHLT) standards (16,17).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the remainder of the
cardiac allograft samples using TRIzol® reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA), according to the
manufacturer’s instructions. A total of 2 μg RNA was
reverse-transcribed into cDNA using an ABI StepOnePlus™ system
(Applied Biosystems Life Technologies, Foster City, CA, USA).
RT-qPCR was performed using an MJ Research DNA Engine with a Chrome
4 Detector (PTC-200; MJ Research, Inc., St. Bruno, QC, Canada),
with endogenous β-actin used as a reference. The gene expression
levels of interleukin (IL)-2, interferon (IFN)-γ, IL-10 and
transforming growth factor (TGF)-β were determined in each group.
The primers used in this study (designed using Primer Premier 5.0
software; Premier Biosoft, Palo Alto, Canada) were as follows:
β-actin, 5′-CATCCGTAAAGACCTCTATGCCAAC-3′ (forward) and
5′-ATGGAGCCACCGATCCACA-3′ (reverse); IFN-γ,
5′-CGGCACAGTCATTGAAAGCCTA-3′ (forward) and
5′-GTTGCTGATGGCCTGATTGTC-3′ (reverse); IL-2,
5′-GGAGCAGCTGTTGATGGACCTAC-3′ (forward) 5′-AATCCAGAACATGCCGCAGAG-3′
(reverse); IL-10, 5′-GACCAGCTGGACAACATACTGCTAA-3′ (forward)
5′-GATAAGGCTTGGCAACCCAAGTAA-3′ (reverse); and TGF-β,
5′-TGACGTCACTGGAGTTGTACGG-3′ (forward) and
5′-GGTTCATGTCATGGATGGTGC-3′ (reverse). The cycle conditions were as
follows: 10 min denaturation at 95°C followed by a total of 40
cycles (95°C for 30 sec and 55°C for 1 min) and 1 min elongation at
72°C.
Enzyme-linked immunosorbent assay
(ELISA)
Blood was collected from the eyeballs of the
recipient mice in the first four days after transplantation. The
serum was separated by centrifugation (1,000 × g at 25°C for
10min), and the expression levels of IL-2, IFN-γ, IL-10 and TGF-β
were determined using an ELISA kit (Shanghai Yikesai Bioproduct
Co., Ltd., Shanghai, China). The serum optical density values were
subsequently determined using an ELISA analyzer (iMark™;
Bio-Rad Laboratories, Inc., Hercules, CA, USA), and the
concentration of the cytokines was calculated using a standard
curve.
Mixed lymphocyte reaction
Spleen removal was performed in the C57BL/6 mice
four weeks after skin transplantation. Lymphocytes were isolated
from the spleen samples using an EZ-Sep™ lymphocyte separation kit
(Dakewe Biotech Co., Ltd., Shenzhen, China) and the T cells were
passed through nylon wool (Wako Pure Chemical Industries, Ltd.,
Osaka, Japan). The spleen cells of BALB/c mice were collected (4
weeks after transplant donation) and treated with 40 g/ml mitomycin
(Amresco, Solon, OH, USA). The BALB/c and C57BL/6 cells were mixed
at a ratio of 1:10 and cultured at 37°C for 72 h in triplicate. The
degree of proliferation was measured in each group using a cell
proliferation ELISA BrdU kit (Roche Applied Science, Mannheim,
Germany).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. SPSS 13.0 (SPSS Inc., Chicago, IL, USA) and GraphPad
Prism 5 (GraphPad Software, San Diego, CA, USA) software were used
to perfom the statistical analysis. Survival times were analyzed
using the Kaplan-Meier method. All the animal group comparisons
were evaluated using the Student’s t-test. P<0.05 and P<0.01
were considered to indicate a statistically significant
difference.
Results
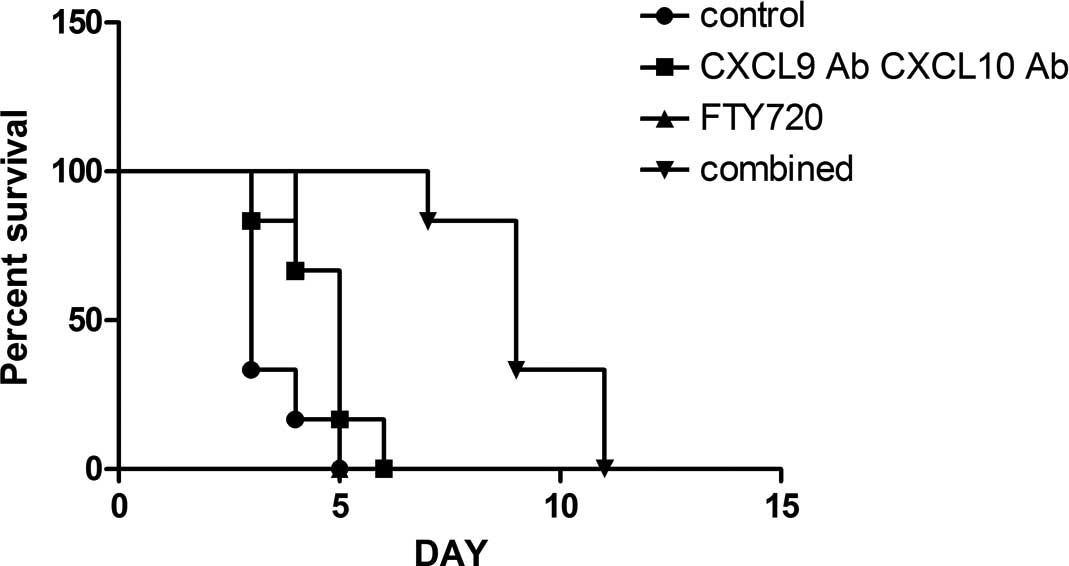
Allograft survival time
All the recipient mice survived the study, even
following transplant rejection. In the murine heterotopic cardiac
transplantation model, the mean graft survival time was found to be
3.5 days in the control group; the mean allograft survival time was
prolonged in the CXCL9 Ab and CXCL10 Ab (4.7 days) and the FTY720
(4.7 days) groups (n=6; P<0.05 vs. the control group).
Furthermore, the mean allograft survival time was significantly
prolonged in the combined group (9.3 days) (n=6; P<0.01 vs. the
control group) (Fig. 1). The group
survival time comparisons were evaluated using the Kaplan-Meier
method.
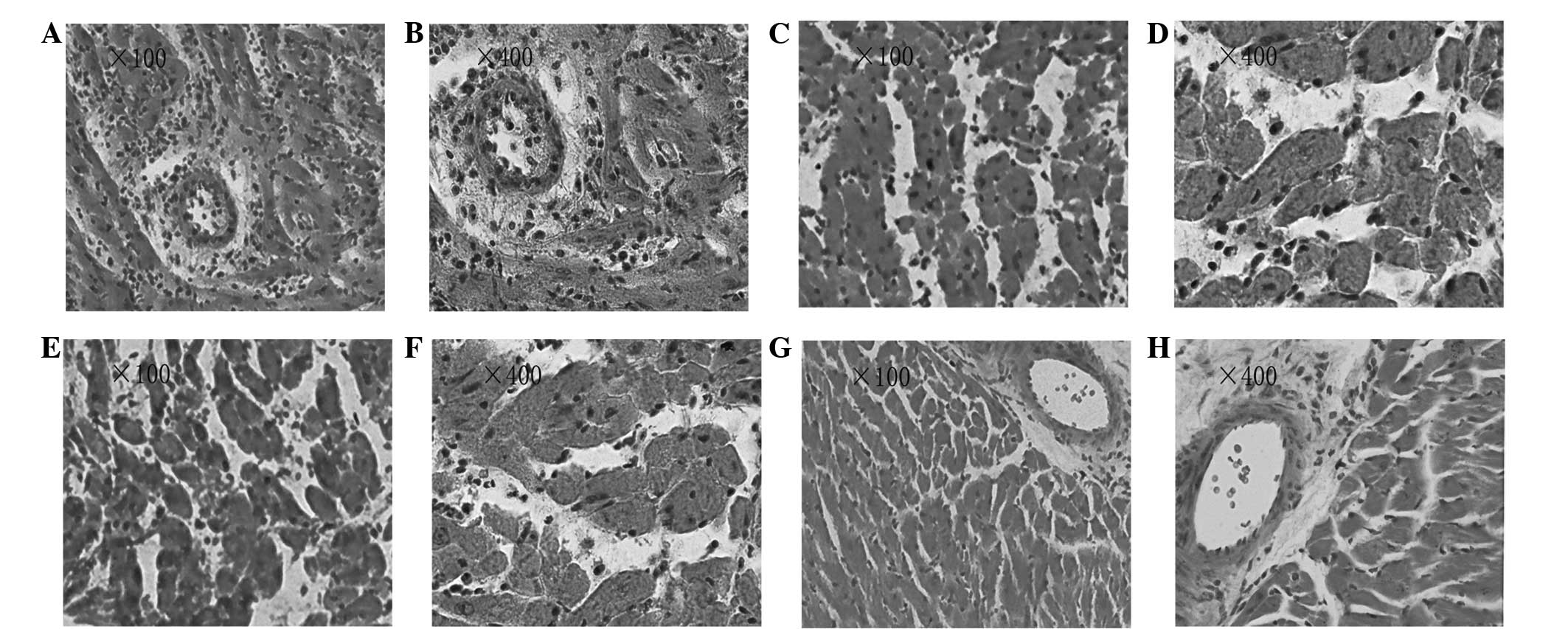
Histological examination
Heterotopic cardiac graft tissues were obtained on
day 4 after transplantation. The heterotopic cardiac grafts in the
control group showed severe acute rejection. Diffuse infiltration
of various cell types was observed, along with edema, hemorrhage
and extensive myocardial necrosis (Fig. 2A and B; ISHLT grade 4). By
contrast, the CXCL9 Ab and CXCL10 Ab group grafts showed multifocal
moderate acute rejection with multifocal inflammatory cell
infiltration and myocardial necrosis (Fig. 2C and D; ISHLT grade 3A). The FTY720
group grafts showed diffuse severe acute rejection, with diffuse
inflammatory cell infiltration and myocardial necrosis (Fig. 2E and F; ISHLT grade 3B). In the
combined group, the grafts showed focal mild acute rejection. Focal
myocardial interstitial and perivascular inflammatory cell
infiltration was observed, with no evidence of myocardial necrosis
(Fig. 2G and H; ISHLT grade 1A).
Compared with the control group, graft rejection was alleviated to
varying degrees in the treatment groups. The graft rejection
pathology scores were 3.62±0.23 in the control group and 0.50±0.21
in the combined group; a statistically significant difference was
observed between the two groups (P<0.01). By contrast, the graft
rejection pathological scores were 2.50±0.20 in the CXCL9 Ab and
CXCL10 Ab group and 2.62±0.23 in the FTY720 group; these scores
were significantly different from the score in the control group
(P<0.05).
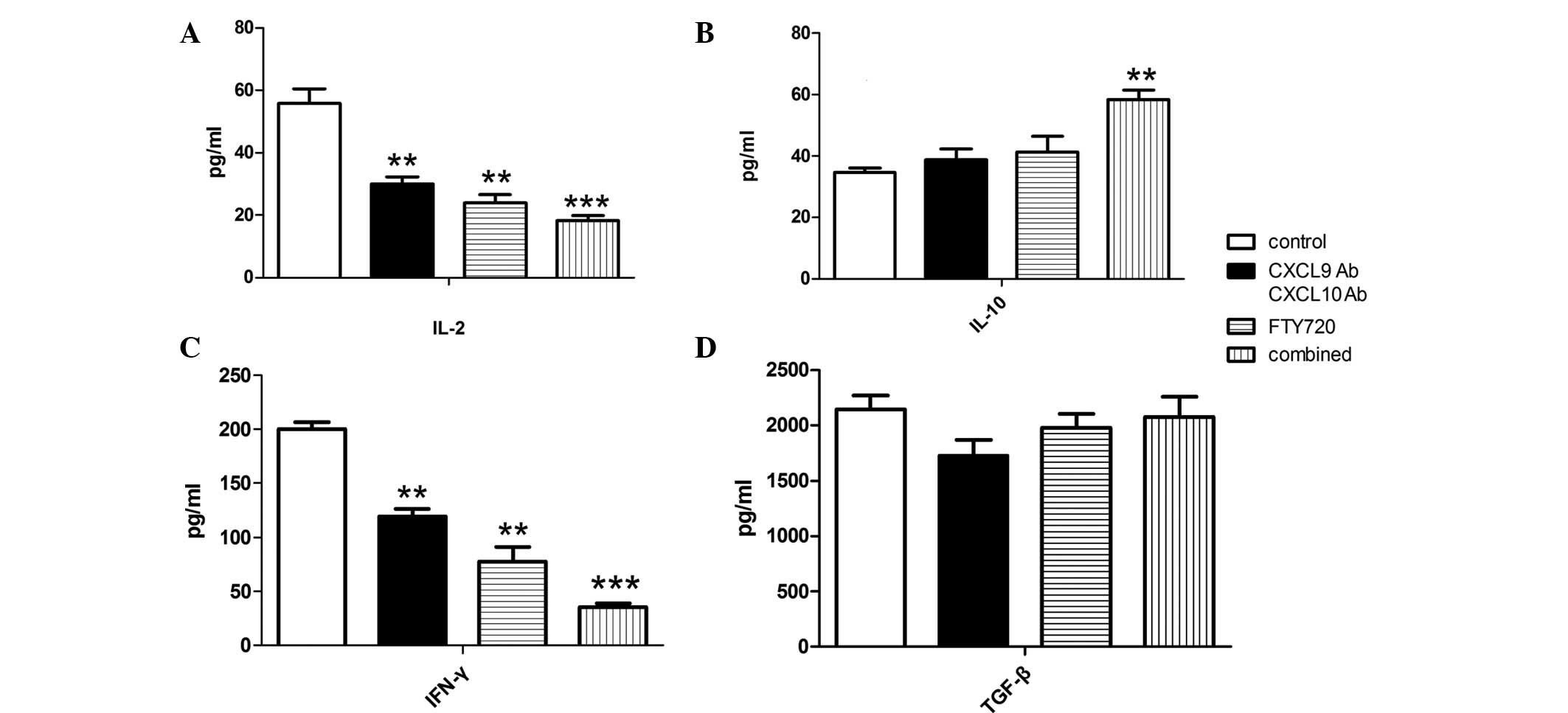
Allograft cytokine gene expression
levels
Allograft mRNA was harvested from the heart samples
obtained at day 4 post-transplantation. As shown in Fig. 3, the gene expression of IL-2 was
downregulated, whereas the expression of IL-10 and TGF-β was
upregulated. Statistical analysis revealed that IL-2 expression was
significantly inhibited in the CXCL9 Ab and CXCL10 Ab and FTY720
groups compared with the control group (P<0.01), while the
inhibition was even more marked in the combined group (P<0.001).
By contrast, the gene expression of IL-10 was not affected by the
CXCL9 Ab and CXCL10 Ab and FTY720 treatments, although it was
upregulated in the combined treatment group. No statistically
significant differences were observed in the gene expression of
IFN-γ among the experimental groups. Compared with the control
group, allografts in the CXCL9 Ab and CXCL10 Ab, FTY720 and
combined groups showed varying degrees of TGF-β gene expression
upregulation (P<0.05).
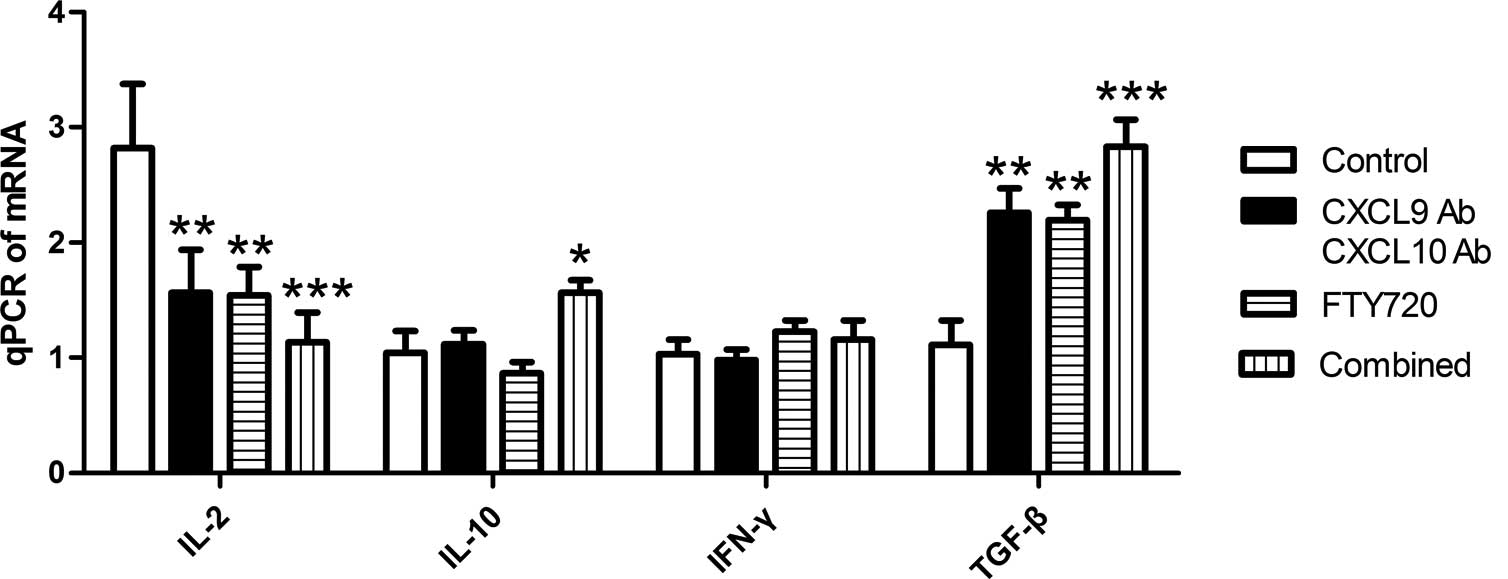 | Figure 3Relative gene expression levels of
IL-2, IL-10, IFN-γ and TGF-β in the cardiac allografts of each
group. Allograft mRNA was analyzed using qPCR. The gene expression
level of IL-2 was significantly downregulated, while the gene
expression level of TGF-β was significantly upregulated in the
experimental groups compared with the control group.
*P<0.05, **P<0.01 and
***P<0.001 vs. the control group. Combined group,
CXCL9 Ab + CXCL10 Ab + FTY720; CXCL, C-X-C motif chemokine; Ab,
antibody; IL, interleukin; IFN, interferon; TGF, transforming
growth factor; qPCR, quantitative polymerase chain reaction. |
Cytokine expression in peripheral
blood
To determine the cytokine levels in peripheral
blood, serum was collected on day 4 after cardiac transplantation.
Serum levels of IL-2, IL-10, IFN-γ and TGF-β were measured using
ELISA, and the IL-2 and IFN-γ levels were found to be lower in the
experimental groups than those in the control group. Statistical
analysis revealed that the serum levels of IL-2 and IFN-γ in the
CXCL9 Ab and CXCL10 Ab and FTY720 groups showed a decreasing trend
(P<0.05) compared with those in the control group. Furthermore,
the serum concentration of IL-2 and IFN-γ decreased more
significantly in the combined group (P<0.001). As shown in
Fig 4, administration of CXCL9 Ab
and CXCL10 Ab or FTY720 alone did not affect the concentration of
IL-10. However, IL-10 serum concentration was increased in the
combined group (P<0.05). No statistically significant
differences were observed in the TGF-β concentration among the
groups (Fig. 4D).
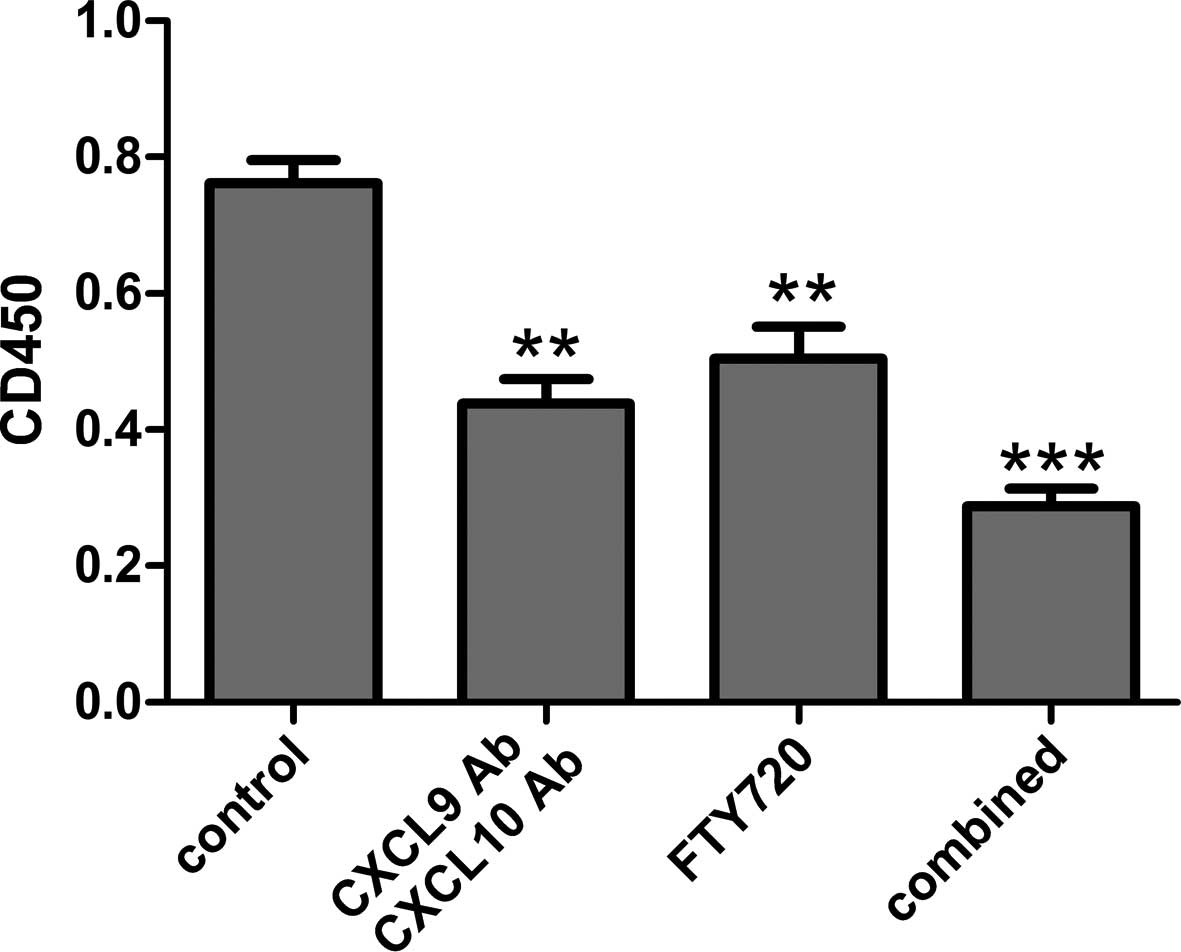
Comparison of mixed lymphocyte
reaction
To assess the proliferative response differences
among the groups, a mixed lymphocyte reaction was performed.
Compared with the control group, the cell proliferative response
was suppressed in the CXCL9 Ab and CXCL10 Ab and FTY720 groups
(P<0.01). In addition, a significant difference in the
proliferative response was observed in the combined group compared
with the control group (P<0.001).
Discussion
Acute cardiac allograft rejection is characterized
by perivascular lymphocyte and monocyte infiltration; however, the
exact mechanism leading to leukocyte recruitment in cardiac
allografts has not been fully elucidated. A number of studies have
indicated that Tm cells play an important role in this process
(18,19). Allograft rejection mediated by Tm
cells is resistant to conventional immunosuppressive treatments,
such as administration of cyclosporin A, FK506 and rapamycin.
Furthermore, conventional strategies and costimulatory blockades do
not affect Tm cell-mediated rejection (20).
The role of chemokines in inflammatory processes has
been studied extensively. The C-C chemokine receptor type 5
(CCR5)-binding chemokines CXCL9 and CXCL10 belong to the CXC
family. It has previously been demonstrated that the expression
levels of CXCL9, CXCL10 and CCR5 are widely upregulated in early
retransplantation models (21).
CXCL9 and CXCL10 bind the G-protein-coupled receptor C-X-C
chemokine receptor type 5 (expressed on multiple cell types, but
predominantly on memory-phenotype cells). Chemotaxis of
inflammatory cells to inflammation sites activates Tm cells and
accelerates the process of rejection. These observations indicate
that CXCL9 and CXCL10 are closely associated with Tm cell-mediated
retransplantation graft rejection (13); however, immunosuppressive treatment
of secondary organ transplantation has been rarely reported.
In the present study, administration of CXCL9 Ab and
CXCL10 Ab was shown to decrease the gene expression levels of the
rejection cytokines IL-2 and IFN-γ in the graft and peripheral
blood of transplant recipient mice. By contrast, the gene
expression of the tolerance cytokines IL-10 and TGF-β was found to
be upregulated (Figs. 3 and
4). The results indicated that
CXCL9 Ab and CXCL10 Ab play an important role in the regulation of
various cytokines secreted by Tm cells. The Tm-cell proliferation
in the mixed lymphocyte reaction was also investigated. CXCL9 Ab
and CXCL10 Ab administration was found to have an inhibitory effect
on T-cell proliferation (Fig. 5).
Histological examination revealed that CXCL9 Ab and CXCL10 Ab
reduced the infiltration of lymphocytes in second organ
retransplantation grafts (Fig. 2B and
F). In addition, CXCL9 Ab and CXCL10 Ab treatment was shown to
prolong the average survival time (Fig. 1). It was therefore demonstrated
that CXCL9 Ab and CXCL10 Ab played an important role in Tm
cell-mediated retransplantation graft rejection.
FTY720 is a novel potential immunosuppressant drug
and is a form of Cordyceps, the active ingredient in
Traditional Chinese Medicine. According to previous studies on
FTY720, the drug induces lymphocyte homing to promote the apoptosis
of lymphocytes and has an immunosuppressive role (22,23).
FTY720 prompts the peripheral circulation to reduce the number of
lymphocytes, reducing the lymphocyte infiltration of the graft or
damaged tissue, thus delaying the rejection of the development
process. Numerous studies have reported that the use of FTY720
prolongs graft survival in the skin, heart, liver and small bowel
of animal transplantation models (24–27).
The results of the present study demonstrated that
the use of FTY720 delayed the rejection of cardiac allografts
(Fig. 1). The expression of TGF-β
was upregulated in cardiac allografts, while the expression levels
of IL-2 and IFN-γ were downregulated in the recipients’ peripheral
blood (Figs. 3 and 4). Cytokine analysis revealed that FTY720
could regulate the cytokine levels in the grafts and peripheral
blood. The mixed lymphocyte reaction results confirmed that FTY720
inhibited T-cell proliferation in vitro (Fig. 5), which is consistent with the
results of Kim et al (28).
In addition, histological examination of the FTY720 group revealed
that FTY720 reduced the graft infiltration of lymphocytes.
In the present study, CXCL9 Ab, CXCL10 Ab and FTY720
were found to prolong cardiac allograft survival through various
mechanisms. CXCL9 Ab and CXCL10 Ab prolong allograft survival by
inhibiting the proliferation of activated Tm cells, whereas FTY720
prolongs allograft survival by accelerating lymphatic homing. The
finding of the present study indicated that the application of
CXCL9 Ab and CXCL10 Ab or FTY720 alone on Tm-cell-mediated second
cardiac transplantation resulted in a certain inhibitory effect;
however, the antirejection effect was not satisfactory. A combined
blocking immunosuppressive regimen was therefore developed. It was
hypothesized that combining the inhibitory effect of CXCL9 Ab and
CXCL10 Ab on Tm-cell proliferation and the inducing effect of
FTY720 on homing would efficiently prevent Tm and other
inflammatory cells from migrating to the graft, and thus reduce or
avoid the occurrence of acute rejection. In the combined group, the
gene expression level of IL-2 in the graft was found to be
significantly lower compared with that in the control group, while
the gene expression level of TGF-β was found to be increased
(Fig. 3). Furthermore, the
expression levels of IL-2 and IFN-γ in the peripheral blood of the
combined group were significantly lower than those in the control
group, whereas the expression level of IL-10 was increased
(Fig. 4). The combined group
additionally showed the stronger inhibition of T-cell proliferation
in vitro (Fig. 5).
Pathological observations revealed that the combined application
also significantly decreased the infiltration of lymphocytes in the
grafts.
In conclusion, the results of the present study
indicated that administration of CXCL9 Ab and CXCL10 Ab or FTY720
reduced the graft infiltration of inflammatory cells, inhibited
T-cell proliferation and prolonged graft survival. Combined therapy
with CXCL9 Ab, CXCL10 Ab and FTY720 was found to prolong the
allograft survival by a considerably greater extent than
single-drug therapy. The combined treatment may therefore be a
novel therapeutic approach for the prevention of cardiac
retransplantation graft failure.
Acknowledgements
This study was supported by a grant from the Key
Project of Program of Science and Technology of Fujian Province of
China (no. MKJ 2008-59).
References
|
1
|
Taylor DO, Edwards LB, et al: Registry of
the International Society for Heart and Lung Transplantation:
twenty-third official adult heart transplantation report - 2006. J
Heart Lung Transplant. 25:869–879. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pour-Reza-Gholi F, Nafar M, Saeedinia A,
Farrokhi F, Firouzan A, Simforoosh N, Basiri A and Einollahi B:
Kidney retransplantation in comparison with first kidney
transplantation. Transplant Proc. 37:2962–2964. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amir AL, D’Orsogna LJ, Roelen DL, van
Loenen MM, Hagedoorn RS, de Boer R, van der Hoorn MA, Kester MG,
Doxiadis II, Falkenburg JH, et al: Allo-HLA reactivity of
virus-specific memory T cells is common. Blood. 115:3146–3157.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bingaman AW and Farber DL: Memory T cells
in transplantation: generation, function, and potential role in
rejection. Am J Transplant. 4:846–852. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Valujskikh A and Li XC: Frontiers in
nephrology: T cell memory as a barrier to transplant tolerance. J
Am Soc Nephrol. 18:2252–2261. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yun JJ, Whiting D, Fischbein MP, Banerji
A, Irie Y, Stein D, Fishbein MC, Proudfoot AE, Laks H, Berliner JA
and Ardehali A: Combined blockade of the chemokine receptors CCR1
and CCR5 attenuates chronic rejection. Circulation. 109:932–937.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iida S, Suzuki T, Tanabe K, Valujskikh A,
Fairchild RL and Abe R: Transient lymphopenia breaks costimulatory
blockade-based peripheral tolerance and initiates cardiac allograft
rejection. Am J Transplant. 13:2268–2279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang H, Zhang Z, Tian W, Liu T, Han H,
Garcia B, Li XC and Du C: Memory T cells mediate cardiac allograft
vasculopathy and are inactivated by anti-OX40L monoclonal antibody.
Cardiovasc Drugs Ther. 28:115–122. 2014. View Article : Google Scholar
|
|
9
|
Liang H, Liao C, Qi Z, Sha C, Xie B, Chen
J, Xia J, Wang Y, Yao Q and Zhao Y: Rapamycin or tacrolimus alone
fails to resist cardiac allograft accelerated rejection mediated by
alloreactive CD4(+) memory T cells in mice. Transpl Immunol.
22:128–136. 2010. View Article : Google Scholar
|
|
10
|
Larsen CP, Knechtle SJ, Adams A, Pearson T
and Kirk AD: A new look at blockade of T-cell costimulation: a
therapeutic strategy for long-term maintenance immunosuppression.
Am J Transplant. 6:876–883. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sallusto F, Schaerli P, Loetscher P,
Schaniel C, Lenig D, Mackay CR, Qin S and Lanzavecchia A: Rapid and
coordinated switch in chemokine receptor expression during
dendritic cell maturation. Eur J Immunol. 28:2760–2769. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhai Y, Wang Y, Wu Z and Kupiec-Weglinski
JW: Defective alloreactive CD8 T cell function and memory response
in allograft recipients in the absence of CD4 help. J Immunol.
179:4529–4534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rosenblum JM, Shimoda N, Schenk AD, Zhang
H, Kish DD, Keslar K, Farber JM and Fairchild RL: CXC chemokine
ligand (CXCL) 9 and CXCL10 are antagonistic costimulation molecules
during the priming of alloreactive T cell effectors. J Immunol.
184:3450–3460. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou X, Shan Z, Liang H, et al: Role of
regulated upon activation normal T-cell expressed and secreted in a
model of retransplantation acute rejection mediated by alloreactive
memory CD4+T cells. Transplant Proc. 45:546–551. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen ZH: A technique of cervical
heterotopic heart transplantation in mice. Transplantation.
52:1099–1101. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Billingham ME, Cary NR, Hammond ME, et al:
A working formulation for the standardization of nomenclature in
the diagnosis of heart and lung rejection: Heart Rejection Study
Group. The International Society for Heart Transplantation. J Heart
Transplant. 9:587–593. 1990.PubMed/NCBI
|
|
17
|
Winters GL, Marboe CC and Billingham ME:
The International Society for Heart and Lung Transplantation
grading system for heart transplant biopsy specimens: clarification
and commentary. J Heart Lung Transplant. 17:754–760.
1998.PubMed/NCBI
|
|
18
|
Salom RN, Maguire JA and Hancock WW:
Endothelial activation and cytokine expression in human acute
cardiac allograft rejection. Pathology. 30:24–29. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Setoguchi K, Hattori Y, Iida S, Baldwin WM
III and Fairchild RL: Endogenous memory CD8 T cells are activated
within cardiac allografts without mediating rejection. Am J
Transplant. 13:2293–2307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Farivar AS, Mackinnon-Patterson BC,
McCourtie AS, Ward PA and Mulligan MS: The role of CC and CXC
chemokines in cardiac allograft rejection in rats. Exp Mol Pathol.
78:171–176. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou X, Shan Z, Liang H, Lin Z, Qiu S,
Kuang F, Zhuang J and Qi Z: Role of regulated upon activation
normal T-cell expressed and secreted in a model of
retransplantation acute rejection mediated by alloreactive memory
CD4+ T cells. Transplant Proc. 45:546–551. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Jiang J, Xiao H, Wang X, et al:
Topical application of FTY720 and cyclosporin A prolong corneal
graft survival in mice. Mol Vis. 18:624–633. 2012.PubMed/NCBI
|
|
23
|
Heng Y, Ma Y, Yin H, Duan L, et al:
Adoptive transfer of FTY720-treated immature BMDCs significantly
prolonged cardiac allograft survival. Transpl Int. 23:1259–1270.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pearl JP, Parris J, Hale DA, Hoffmann SC,
Bernstein WB, McCoy KL, Swanson SJ, Mannon RB, Roederer M and Kirk
AD: Immunocompetent T-cells with a memory-like phenotype are the
dominant cell type following antibody-mediated T-cell depletion. Am
J Transplant. 5:465–474. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang L, Zhu T, Sun EW, Shen SQ, Min ZL
and Chen ZK: Pretreatment with FTY720 alone induced long-term
survival of mouse heart allograft. Transplant Proc. 35:567–568.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang ME, Tejpal N, Qu X, Yu J, Okamoto M,
Stepkowski SM and Kahan BD: Immunosuppressive effects of FTY720
alone or in combination with cyclosporine and/or sirolimus.
Transplantation. 65:899–905. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yanagawa Y, Hoshino Y and Chiba K: The
significance of timing of FTY720 administration on the
immunosuppressive effect to prolong rat skin allograft survival.
Int J Immunopharmacol. 22:597–602. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim MG, Lee SY, Ko YS, Lee HY, Jo SK, Cho
WY and Kim HK: CD4+ CD25+ regulatory T cells partially mediate the
beneficial effects of FTY720, a sphingosine-1-phosphate analogue,
during ischaemia/reperfusion-induced acute kidney injury. Nephrol
Dial Transplant. 26:111–24. 2011. View Article : Google Scholar
|