Introduction
Osteoarthritis (OA), a common chronic disease
increasingly prevalent with age, is manifested by joint pain,
stiffness, disability and/or swelling, and reduces the overall
quality of life of affected individuals (1,2).
Cartilage degradation, an important feature of OA, is caused by the
interplay of metabolic, genetic, biochemical and biomechanical
factors. This degradation results in the imbalance of catabolism
and anabolism, which degrades the structural and functional
integrity of the extracellular matrix (ECM). The integrity of the
ECM is regulated by chondrocytes, the only type of cell in
cartilage (3). Due to the poor
intrinsic repair capacity of chondrocytes, articular cartilage
damage is often progressive and is generally permanent; therefore,
improving and maintaining the proliferation potential and phenotype
of chondrocytes may potentially be an effective method to delay the
development and progression of OA.
Efficient cell cycle regulation is vital for cell
growth and differentiation. The family of cyclin-dependent kinases
(CDKs) is one of the key regulators in the cell cycle process
(4,5). CDKs pair with cell cycle-specific
regulatory subunits known as cyclins to govern the cell cycle. The
activity of the CDKs is controlled at multiple levels:
Transcriptionally, post-translationally and by CDK inhibitors
(CDKIs) (6). Multiple cell cycle
genes have been implicated in chondrocyte proliferation, including
cyclin D1, CDK4, CDK6 and p21, affecting G1 phase progression as
well as S phase entry (7).
Since there are numerous adverse side effects of
conventional western medication in the treatment of OA (8), the use of complementary and
alternative medicine to improve the signs and symptoms of diseased
joints has been widely accepted by patients with OA (9). Traditional Chinese Medicine (TCM) has
been used for the treatment of OA in China for thousands of years.
According to the theory of TCM, OA is believed to originate in the
Jin-Gu (tendons and bones) but presents as Ben-Xu (visceral
insufficiency) and Biao-Shi (asthenia in superficiality). The
invasion of pathogens due to ‘wind-cold-dampness’ (three pathogenic
factors of TCM) is linked to blood stasis, which then leads to Bi
Zheng (arythromyodonia) and Wei Zheng (wilting syndrome) (10,11).
Bi Zheng is treated by blood activation and wind-cold-dampness
purging, and Wei Zheng is treated by visceral reinforcement.
Bushen Zhuangjin decoction (BZD), a TCM formulation,
consists of 10 component herbs: 12 g Shu Di Huang (steamed Chinese
foxglove, Radix Rehmanniae Glutinosae Conquitae), 12 g Dang
Gui (Angelica sinensis), 10 g Niu Xi (Achyranthes
bidentatae), 12 g Shan Zhu Yu (Cornus officinalis), 12 g
Fu Ling (Poria cocos), 12 g Xu Duan (Dipsacus), 10 g
Du Zhong (Eucommia bark) 10 g Bai Shao (white peony root,
Radix Paeoniae Alba), 5 g Qing Pi (immature tangerine peel,
Pericarpium Citri Reticulatae Viride) and 10 g Wu Jia Pi
(Acanthopanax root bark). These natural products together
confer BZD properties of nourishing Gan (liver) and Shen (kidney)
and strengthening tendons and bones, according to the theories of
TCM (12). BZD has a long history
of use in the treatment of OA, and has been shown to improve
osteoarthritic symptoms in clinical trials (12–14);
however, the molecular mechanism of BZD in the treatment of OA is
complicated and multifaceted and is thus not fully understood. In
the present study, the effect of BZD on chondrocyte proliferation
was investigated to elucidate the mechanisms underlying the
therapeutic effects of BZD in OA.
Materials and methods
Animals
Four-week-old male Sprague Dawley rats of Specific
Pathogen Free status were purchased from Shanghai SLAC Laboratory
Animal Co. (Shanghai, China). The present study was approved by the
Institutional Animal Care and Use Committee of Fujian University of
TCM (Fuzhou, China).
Preparation of BZD
The herbs of BZD, obtained from the Third People’s
Hospital of Fujian University of TCM (Fuzhou, China), were dried in
an air-circulating oven (model SFG-02.600; Hengfeng Medical
Instrument Co., Ltd., Huangshi, China) at 50°C for 24 h, and then
crushed to an appropriate particle size in a high-speed rotary
cutting mill (model ZN-400A; Zhongnan Pharmaceutical Machinery
Factory, Changsha, China). According to the proportion of BZD, 105
g herbal powder was extracted with 1,050 ml 85% ethanol using a
refluxing method and filtered. The filtrate was evaporated on a
rotary evaporator (model RE-2000; Shanghai Yarong Biochemistry
Instrument Factory, Shanghai, China) and then dried to a constant
weight in a vacuum drying oven (model DZF-300; Shanghai Yiheng
Scientific Instrument Co., Ltd., Shanghai, China). The BZD was
dissolved in dimethylsulfoxide (DMSO; Hengxing Chemical Preparation
Co., Ltd., Tianjin, China) to a stock concentration of 100 mg/ml
and stored at −20°C. The working concentrations of BZD were made by
diluting the stock solution in Dulbecco’s modified Eagle’s medium
(DMEM) containing 10% fetal bovine serum (FBS) (both HyClone
Laboratories, Inc., Logan, UT, USA), and then filtering through a
0.22-μm filter and storing at 4°C. The final concentration of DMSO
in the DMEM was <0.5%.
Isolation, culture and identification of
chondrocytes
Chondrocytes from the knee articular cartilage of
rats were isolated, cultured and identified as previously described
(4). Briefly, the chondrocytes
from the knee articular cartilage of four-week-old rats were
isolated using 0.2% type II collagenase (Sigma-Aldrich, St. Louis,
MO, USA) in magnesium- and calcium-free phosphate buffered saline
(pH 7.4) (HyClone Laboratories, Inc.) for 1 h at 37°C. The cells
were then resuspended in DMEM supplemented with 10% FBS, 100 μg/ml
streptomycin and 100 U/ml penicillin (HyClone Laboratories, Inc.),
and seeded in a monolayer at a density of 5×105
cells/cm2. The second passage cells were identified by
type II collagen immunohistochemistry. Briefly, sterilized
coverslips were placed into the wells of a 24-well plate, the cells
were seeded onto the coverslips (2×105 cells/well) and
were cultured for 48 h at 37°C in an incubator containing 5%
CO2. The cells were then fixed with 4% paraformaldehyde
(Sigma-Aldrich) at 4°C for 30 min, and blocked with 10% bovine
serum albumin (Sigma-Aldrich) for 30 min. The slides were then
incubated with a polyclonal rabbit antibody targeting type II
collagen (1:100; BS1071, Bioworld Technology, Inc., St. Louis Park,
MN, USA) overnight at 4°C, followed by an incubation with
biotinylated goat anti-rabbit secondary antibody immunoglobulin
(Ig)G (1:2,000; ZB-2301, Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China) for 30 min. The slides
were then incubated with streptavidin-horseradish peroxidase (HRP)
conjugate (Zhongshan Golden Bridge Biotechnology Co., Ltd.) for 30
min, before the antibody-HRP complex was visualized by incubation
with diaminobenzidine (ZLI-9018 DAB kit; Zhongshan Golden Bridge
Biotechnology Co., Ltd.) for 5 min. The slides were briefly
counter-stained with hematoxylin (Sigma-Aldrich) and dehydrated,
and cover slips were added. Images were captured under a light
microscope (BH2; Olympus, Tokyo, Japan). Chondrocytes were treated
with 0, 100, 200 and 400 μg/ml BZD for 48 h.
Cell viability analysis
The chondrocytes were treated with BZD at different
concentrations (0, 50, 100, 200, 300, 400, 600, 800 and 1,200
μg/ml) and for different lengths of time (24, 48 or 72 h). A total
of 100 μl 0.5% MTT (Sigma-Aldrich) was added, and the cells were
incubated at 37°C for 4 h. The purple-blue MTT formazan precipitate
was dissolved in 150 μl DMSO and agitated for 10 min. The
absorbance was measured at 490 nm using an ELISA reader (model
EXL800, BioTek, Winooski, VT, USA).
Cell cycle analysis
The cell cycle of the chondrocytes was determined
with a cell cycle assay kit (KeyGEN Biotech, Nanjing, China) using
a fluorescence-activated cell sorting (FACS) machine (FACSCaliber™;
Becton-Dickinson, San Diego, CA, USA). Staining was performed
according to the manufacturer’s instructions. The percentage of
cells in the different phases was calculated using ModFit software
(Verity Software House, Topsham, ME, USA) including the G0/G1, S,
G2 and M phases.
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR) analysis
The total RNA was extracted with TRIzol™ reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) and reverse
transcribed into complementary DNA with SuperScript® II
reverse transcriptase (Promega Corp, Madison, WI, USA). The
analysis of the mRNA expression of cyclin D1, CDK4, CDK6 and p21
was performed using PCR. The primers (Sangon Biotech Co., Ltd.,
Shanghai, China) used for the PCR were as follows: Cyclin D1
forward, 5′-AAT GCC AGA GGC GGA TGA GA-3′ and reverse, 5′-GCT TGT
GCG GTA GCA GGA GA-3′ (189 bp); CDK4 forward, 5′-GAA GAC GAC TGG
CCT CGA GA-3′ and reverse, 5′-ACT GCG CTC CAG ATT CCT CC-3′ (109
bp); CDK6 forward, 5′-TTG TGA CAG ACA TCG ACG AG-3′, and reverse,
5′-GAC AGG TGA GAA TGC AGG TT-3′ (151 bp); p21 forward, 5′-CGG GCA
GTC CCT TCT AGT TCC-3′ and reverse, 5′-AAT GCT TGA GCA CAC ACG
AG-3′ (204 bp); β-actin forward, 5′-CGT TGA CAT CCG TAA AGA CC-3′
and reverse, 5′-GGA GCC AGG GCA GTA ATC T-3′ (108 bp). The DNA
bands were examined using a Gel Documentation system (Model Gel Doc
2000; Bio-Rad, Hercules, CA, USA) and normalized to β-actin.
Western blot analysis
Total proteins were collected immediately in lysis
buffer (Beyotime Institute of Biotechnology, Haimen, China), stored
for 30 min on ice and quantified using the bicinchoninic acid
assay. A total of 20 μg protein was separated on a 12% SDS-PAGE gel
and transferred onto polyvinylidene fluoride membranes. The
membranes were blocked, and incubated with rabbit polyclonal
antibodies targeting cyclin D1 (1:1,000; sc-718), CDK4 (1:1,000;
sc-260), CDK6 (1:1,000; sc-177), p21 (1:1,000; sc-397) and β-actin
(1:1,000; sc-7210) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) overnight at 4°C. Goat anti-rabbit horseradish
peroxidase-conjugated secondary antibody IgG (1:10,000; ZB-2301,
Zhongshan Golden Bridge Biotechnology Co., Ltd.) was added to the
membranes at room temperature. The immunocomplexes were visualized
by the enhanced chemiluminescence method. The bands were quantified
by scanning densitometry (Molecular Imager ChemiDoc X-Ray
Spectroscopy System, cat. no. 170-8070; Bio-Rad). Protein
concentrations were determined using the Tocan 190 protein assay
system (Bio-Rad) and normalized to β-actin.
Statistical analysis
All data are presented as the mean ± standard
deviation from at least three independent experiments. Statistical
analysis was performed using SPSS software (version 13.0; SPSS
Inc., Chicago, IL, USA) with one-way analysis of variance, and
multiple comparisons were performed with the Student-Newman-Keuls q
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
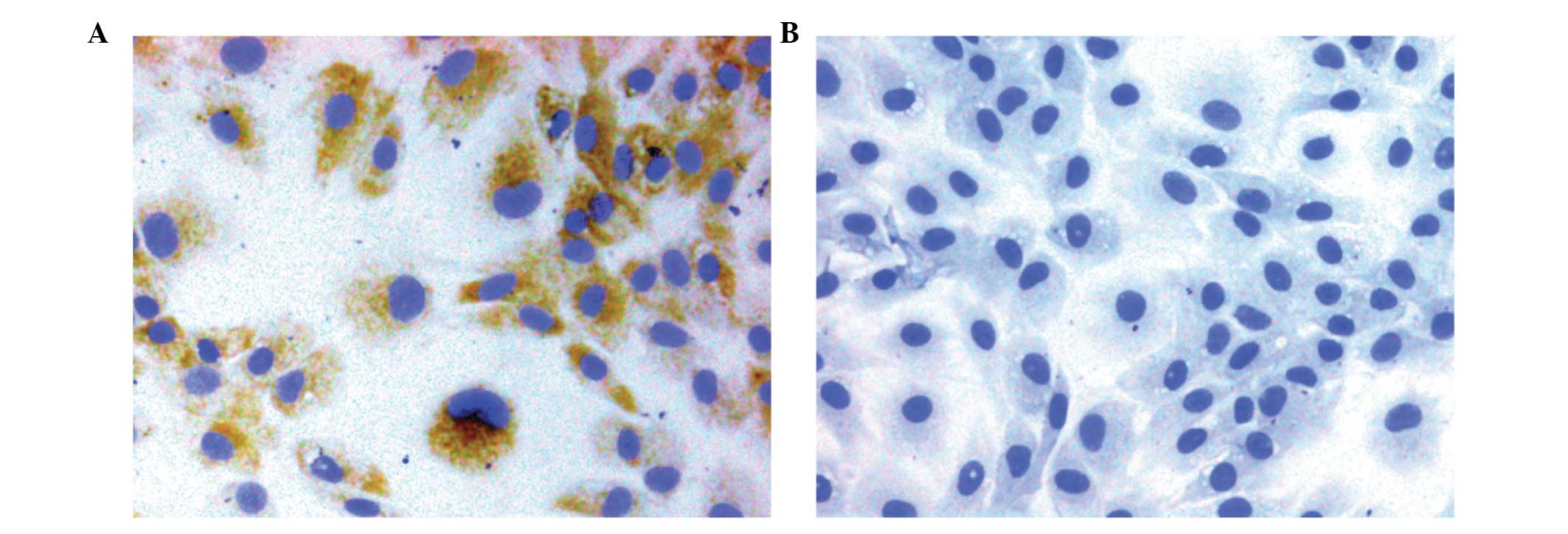
Morphology and identification of the
chondrocytes
The chondrocytes exhibited a typical morphology with
a polygonal or spherical shape, as described in previous studies
(4,15). The type II collagen in the
cytoplasm of the passage 2 chondrocytes was stained brown, which
represented positive expression; no staining was observed in the
negative control cells (Fig. 1A and
B).
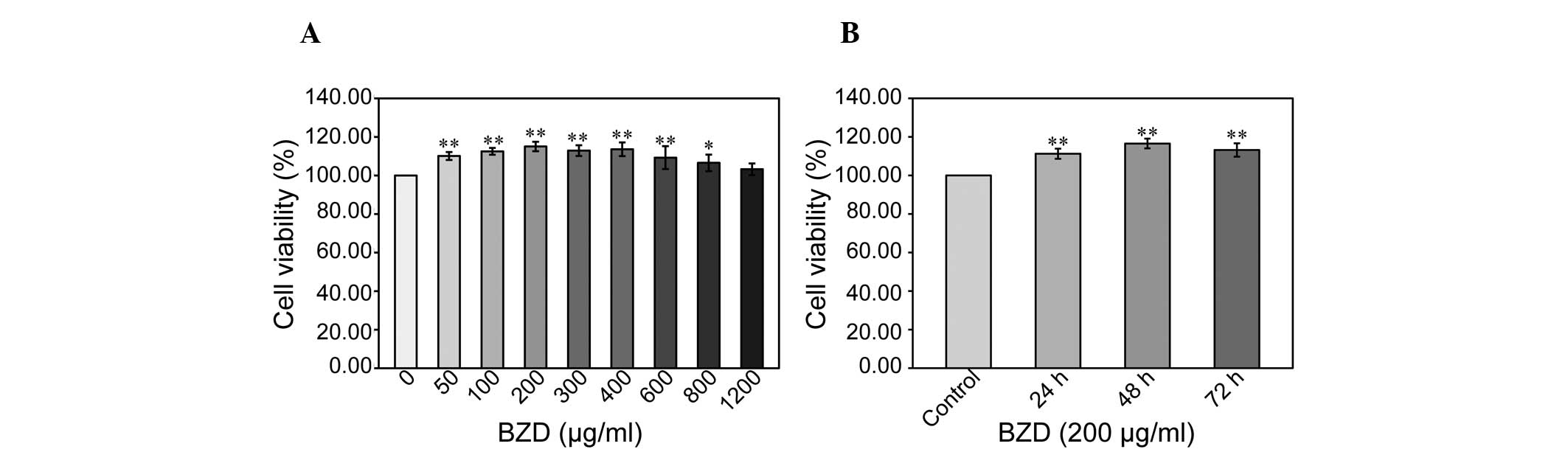
BZD enhances chondrocyte viability and
promotes cell cycle progression
To explore the effect of BZD on chondrocyte
viability, the cell viability of chondrocytes treated with BZD at
different concentrations and for different lengths of time was
examined using an MTT assay. The cells were treated with BZD
concentrations of 50 μg/ml (cell viability, 110.10±2.07%), 100
μg/ml (cell viability, 112.51±1.76%), 200 μg/ml (cell viability,
115.03±2.45%), 300 μg/ml (cell viability, 112.91±2.76%), 400 μg/ml
(cell viability, 113.60±3.53%), 600 μg/ml (cell viability,
109.25±5.96%), 800 μg/ml (cell viability, 106.55±4.32%) and 1,200
μg/ml (cell viability, 103.25±3.03%) for 48 h. A dose-dependent
increase in cell viability was observed following BZD treatment
compared with untreated cells (100±0.00%) (P<0.05 or P<0.01)
(Fig. 2A). Cell viability was
gradually enhanced in chondrocytes treated with 200 μg/ml BZD for
24, 48 and 72 h (Fig. 2B),
indicating that BZD promotes chondrocyte viability in a dose- and
time-dependent manner.
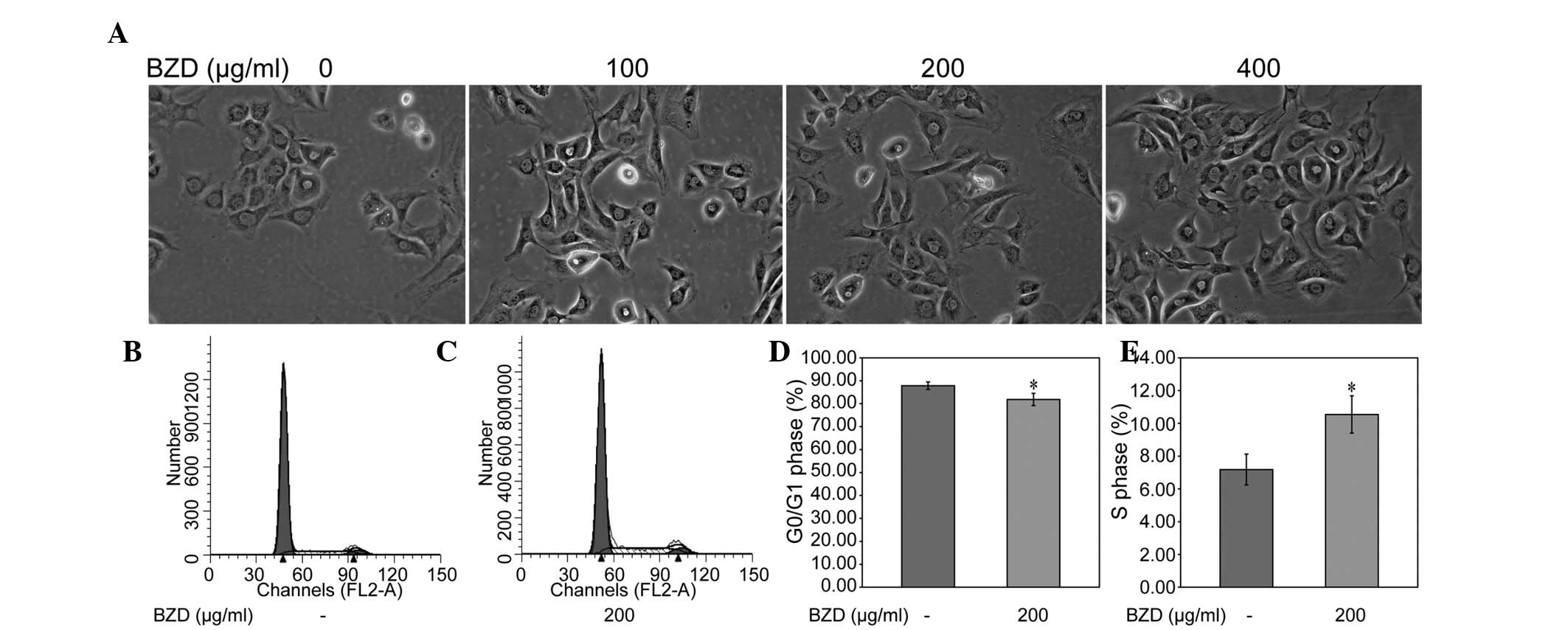
The morphology of the treated chondrocytes was
observed by inverted microscopy. The results showed that the
treated chondrocytes underwent morphological changes, including in
cell shape and size, compared with the untreated chondrocytes. A
few cells were additionally observed to contain two nuclei
(Fig. 3). To investigate whether
BZD promoted the chondrocyte viability by stimulating cell cycle
progression, the cell cycle of the chondrocytes treated with BZD
was examined using FACS analysis. The percentage proportion of
G0/G1 cells was significantly lower, and the percentage proportion
of S cells was significantly higher, in treated cells compared with
that in untreated cells (P<0.05) (Fig. 3B–E), indicating that BZD promotes
chondrocyte viability by stimulating cell cycle progression.
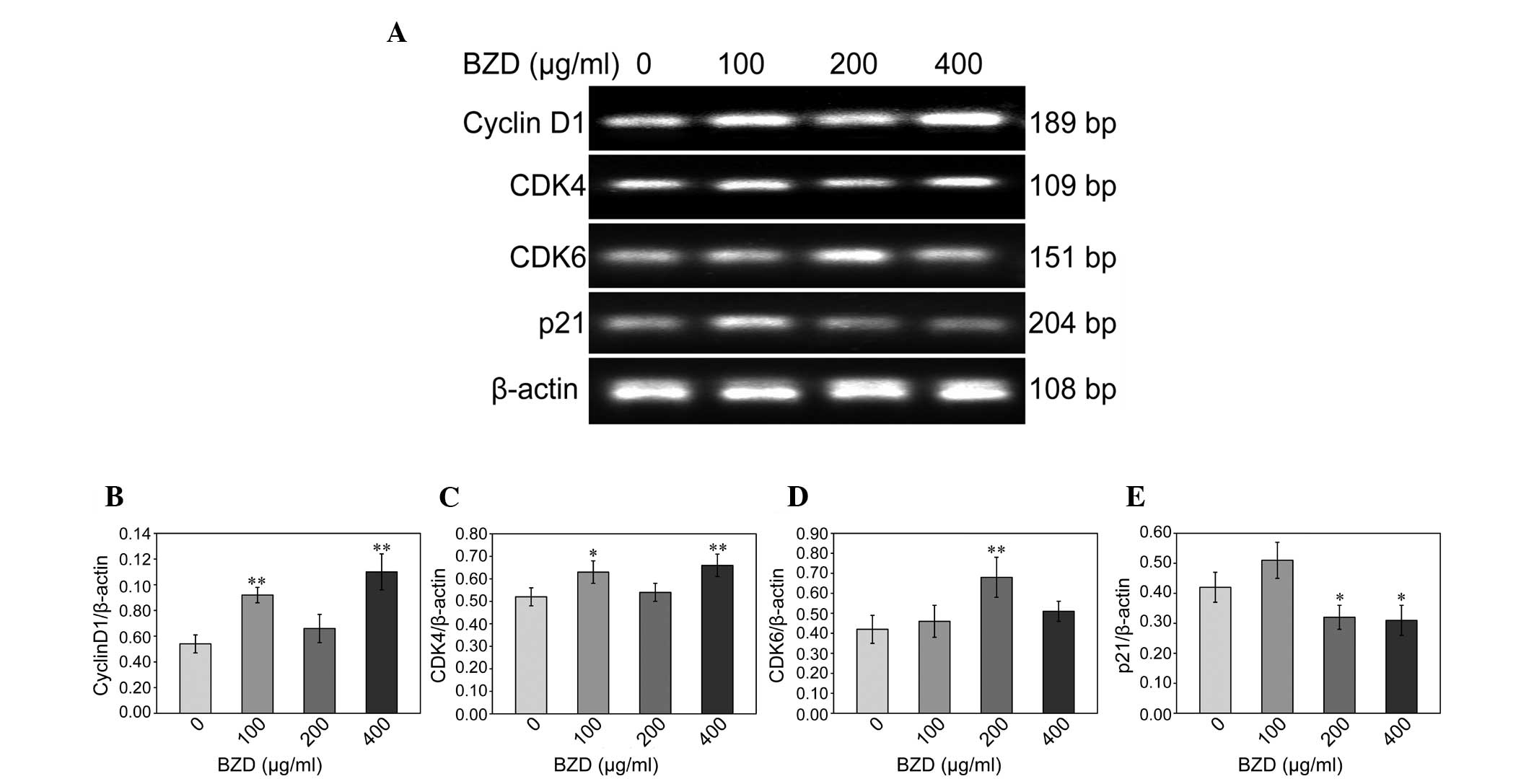
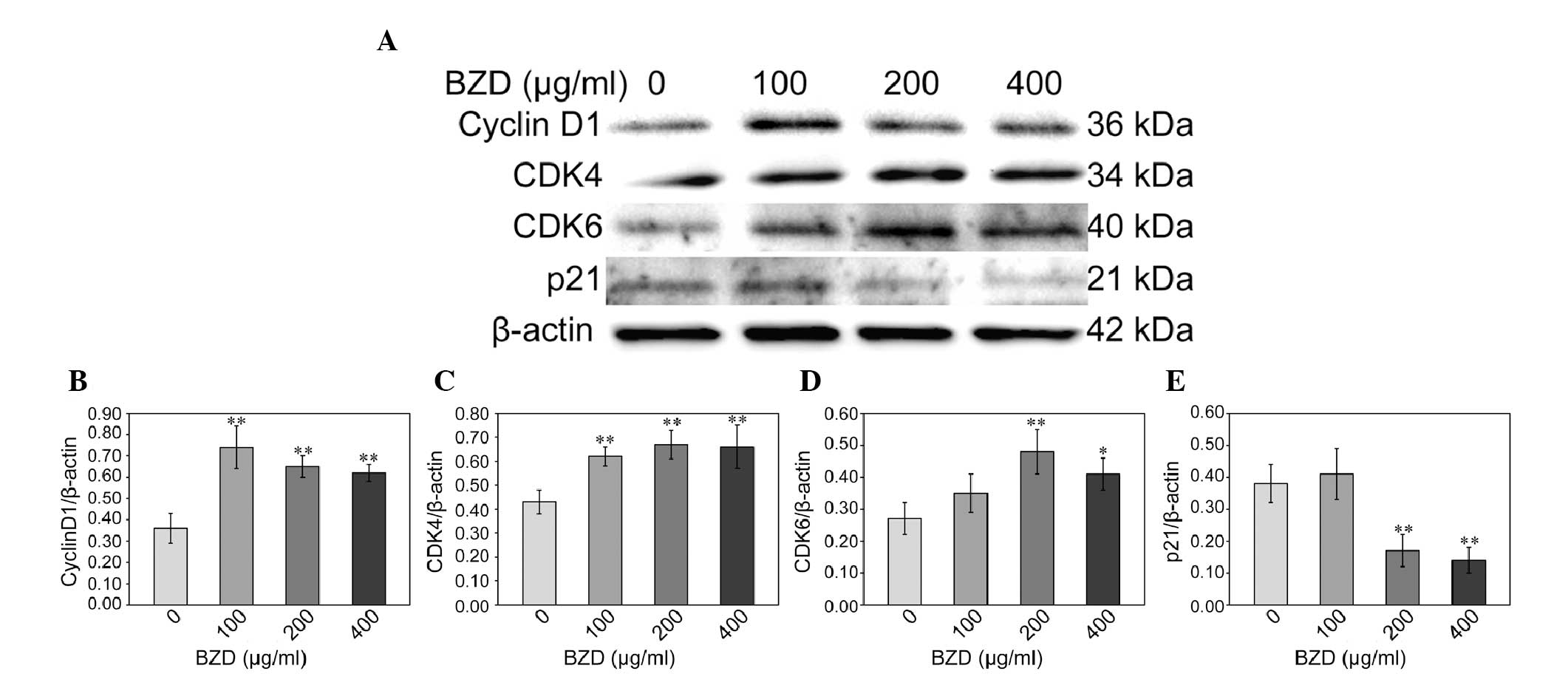
BZD upregulates the expression of cyclin
D1, CDK4 and CDK6 and downregulates the expression of p21
To gain an insight into the effect of BZD on the
cell cycle of chondrocytes, the mRNA and protein expression of
cyclin D1, CDK4, CDK6 and p21 was analyzed using RT-PCR and western
blotting. Compared with the untreated cells, the mRNA expression of
cyclin D1, CDK4 and CDK6 in the BZD-treated chondrocytes was
significantly upregulated (P<0.01 or P<0.05), while the mRNA
expression of p21 was significantly downregulated (P<0.05)
(Fig. 4). The protein levels of
cyclin D1, CDK4, CDK6 and p21 were similar to their respective mRNA
expression (Fig. 5).
Discussion
OA, belonging to the category of Gu Bi (bone
impediment) in TCM, is based on a deficiency of the liver and
kidney (16,17). BZD can nourish the kidney, soothe
the liver, promote blood circulation and dispel wind, and acts
against the pathogenesis of OA, with kidneys dominating bones and
the liver governing tendons, according to the theory. It was found
in the present study that BZD promoted chondrocyte viability in a
dose- and time-dependent manner by stimulating cell cycle
progression, indicating that BZD is a potential therapeutic agent
for the treatment of OA.
Alternations in gene and protein expression in
articular chondrocytes are likely to play an important role in the
pathological process of OA. Cartilage has minimal reparative
capacity, and the degradation of articular cartilage can have
severe consequences. Chondrocytes produce and maintain the ECM,
which is responsible for providing the appropriate function to
articular cartilage (18,19). For this reason, the
differentiation, proliferation and apoptosis of chondrocytes are
crucial to the maintenance of the cartilage function, and the
functional changes of chondrocytes play important roles
contributing to the degeneration of the joint cartilage. Previous
studies have focused on the promotion of chondrocyte proliferation
as an efficient treatment to delay the progression of cartilage
degradation (4,15); thus, the effect of BZD on the
proliferation of chondrocytes was investigated in the present
study.
The MTT data showed that BZD promoted chondrocyte
viability in a dose- and time-dependent manner; flow cytometry
detected changes in the cell cycle with a greater sensitivity than
MTT. The results showed that the percentage of chondrocytes in the
G0/G1 phase was significantly decreased, and the percentage of
chondrocytes in the S phase was significantly increased, in treated
versus untreated cells. This indicates that BZD promotes the cell
cycle progression of chondrocytes in vitro, thus enhancing
chondrocyte proliferation.
The cell cycle refers to the series of events that
take place in a cell that leads to its division and duplication
(replication), and results in the production of two daughter cells
(20). Cell cycle regulation can
be divided into exogenous and endogenous regulation. The former is
primarily caused by cytokines and external stimuli, while the
latter is mainly realized through a complicated network by cyclin,
CDKs and CDKIs (21). There are
two restriction points in the cell cycle. One begins at the initial
synthesis of DNA, which is the G1/S restriction point; the other
starts at the beginning of mitosis, i.e. the G2/S restriction
point. The G1/S restriction point is more important since it is key
to cell proliferation whether cells can pass through it (22). Cyclin D1 is the positive regulatory
factor for the switch of the G1/S phase in the cell cycle and
combines with CDK4 to promote the cell cycle process. In addition,
p21 competes with cyclin D1 in the combination with CDK4 to prevent
Rb from being phosphorylated, leading to a block at the G1 phase.
Cyclin D1, CDK4 and p21, therefore, are all key proteins of cell
cycle signals at the G1 phase, and the G1/S restriction point
determines whether cells will divide or activate the mechanism for
apoptosis (23,24). The effect of BZD on the mRNA and
protein expression of cyclin D1, CDK4, CDK6 and p21 was also
studied and the results showed that BZD enhances cyclin D1, CDK4
and CDK6 expression and reduces p21 expression in chondrocytes.
In conclusion, the present data demonstrated that
BZD can promote chondrocyte proliferation by upregulating the
expression of the positive regulators cyclin D1, CDK4 and CDK6 and
downregulating the expression of the negative regulator p21. Based
on these results, future experiments are necessary to focus on
investigating the effect of BZD on the chondrocyte function in OA
in vivo.
Acknowledgements
This study was supported by the Key Project of
Fujian Provincial Department of Science and Technology (grant no.
2012Y0046) and the Young Talent Scientific Research Project of
Fujian Province Universities (grant no. JA12165).
References
|
1
|
Juhl C, Christensen R, Roos EM, Zhang W
and Lund H: Impact of exercise type and dose on pain and disability
in knee osteoarthritis: a systematic review and meta-regression
analysis of randomized controlled trials. Arthritis Rheumatol.
66:622–636. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang W, Nuki G, Moskowitz RW, et al:
OARSI recommendations for the management of hip and knee
osteoarthritis: part III: Changes in evidence following systematic
cumulative update of research published through January 2009.
Osteoarthritis Cartilage. 18:476–499. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tonge DP, Pearson MJ and Jones SW: The
hallmarks of osteoarthritis and the potential to develop
personalised disease-modifying pharmacological therapeutics.
Osteoarthritis Cartilage. 22:609–621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li X, Ye H, Yu F, et al: Millimeter wave
treatment promotes chondrocyte proliferation via G1/S cell cycle
transition. Int J Mol Med. 29:823–831. 2012.PubMed/NCBI
|
|
5
|
Kurimchak A, Haines DS, Garriga J, et al:
Activation of p107 by fibroblast growth factor, which is essential
for chondrocyte cell cycle exit, is mediated by the protein
phosphatase 2A/B55α holoenzyme. Mol Cell Biol. 33:3330–3342. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee HP, Chen YL, Shen HC, Lo WH and Hu YC:
Baculovirus transduction of rat articular chondrocytes: roles of
cell cycle. J Gene Med. 9:33–43. 2007. View
Article : Google Scholar
|
|
7
|
Panda DK, Miao D, Lefebvre V, Hendy GN and
Goltzman D: The transcription factor SOX9 regulates cell cycle and
differentiation genes in chondrocytic CFK2 cells. J Biol Chem.
276:41229–41236. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lambert RG, Hutchings EJ, Grace MG,
Jhangri GS, Conner-Spady B and Maksymowych WP: Steroid injection
for osteoarthritis of the hip: a randomized, double-blind,
placebo-controlled trial. Arthritis Rheum. 56:2278–2287. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang S, Dubé CE, Eaton CB, McAlindon TE
and Lapane KL: Longitudinal use of complementary and alternative
medicine among older adults with radiographic knee osteoarthritis.
Clin Ther. 35:1690–1702. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li XH, Wu MX, Ye HZ, et al: Experimental
study on the suppression of sodium nitroprussiate-induced
chondrocyte apoptosis by Tougu Xiaotong capsule-containing serum.
Clin J Integr Med. 17:436–443. 2011. View Article : Google Scholar
|
|
11
|
Li X, Lang W, Ye H, et al: Tougu Xiaotong
capsule inhibits the tidemark replication and cartilage degradation
of papain-induced osteoarthritis by the regulation of chondrocyte
autophagy. Int J Mol Med. 31:1349–1356. 2013.PubMed/NCBI
|
|
12
|
Zhou GS, Li XF and Guan GH: Effects of
Bushen Zhuangjin Decoction containing serum on the apoptosis of
chondrocytes induced by mechanics stimulus. Zhongguo Zhong Xi Yi
Jie He Za Zhi. 32:789–792. 2012.(In Chinese). PubMed/NCBI
|
|
13
|
Li X, Liang W, Dang C, et al: Empirical
study on Bushen Zhuangjin Decoction inhibiting inflammatory
cytokine expression experiments to delay the degeneration of
articular cartilage. Feng Shi Bing Yu Guan Jie Yan. 3:20–25.
2014.(In Chinese).
|
|
14
|
Cheng J, Li X, Ye H and Liu X: Review of
the application of computer aided drug molecular docking design
technology in filtering the effective components of Bushen
Zhuangjin Decoction for prevention and cure of osteoarthritis.
Zhong Yi Zheng Gu. 25:56–67. 2013.(In Chinese).
|
|
15
|
Li H, Li X, Liu G, et al: Bauhinia
championi (Benth.) Benth polysaccharides upregulate Wnt/β-catenin
signaling in chondrocytes. Int J Mol Med. 32:1329–1336.
2013.PubMed/NCBI
|
|
16
|
Yuan PW, Liu DY, Chu XD, Hao YQ, Zhu C and
Qu Q: Effects of preventive administration of juanbi capsules on
TNF-alpha, IL-1 and IL-6 contents of joint fluid in the rabbit with
knee osteoarthritis. J Tradit Chin Med. 30:254–258. 2010.
View Article : Google Scholar
|
|
17
|
Li XH, Liang WN and Liu XX: Clinical
observation on curative effect of dissolving phlegm-stasis on 50
cases of knee osteoarthritis. J Tradit Chin Med. 30:108–112. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ohshika S, Ishibashi Y, Kon A, Kusumi T,
Kijima H and Toh S: Potential of exogenous cartilage proteoglycan
as a new material for cartilage regeneration. Int Orthop.
36:869–877. 2012. View Article : Google Scholar :
|
|
19
|
Hoshiba T, Yamada T, Lu H, Kawazoe N and
Chen G: Maintenance of cartilaginous gene expression on
extracellular matrix derived from serially passaged chondrocytes
during in vitro chondrocyte expansion. J Biomed Mater Res A.
100:694–702. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Beier F: Cell-cycle control and the
cartilage growth plate. J Cell Physiol. 202:1–8. 2005. View Article : Google Scholar
|
|
21
|
Kamel S, Kruger C, Salbaum JM and Kappen
C: Morpholino-mediated knockdown in primary chondrocytes implicates
Hoxc8 in regulation of cell cycle progression. Bone. 44:708–716.
2009. View Article : Google Scholar :
|
|
22
|
Löwenheim H, Reichl J, Winter H, et al: In
vitro expansion of human nasoseptal chondrocytes reveals distinct
expression profiles of G1 cell cycle inhibitors for replicative,
quiescent, and senescent culture stages. Tissue Eng. 11:64–75.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu F, Li X, Cai L, et al: Achyranthes
bidentata polysaccharides induce chondrocyte proliferation via the
promotion of the G1/S cell cycle transition. Mol Med Rep.
7:935–940. 2013.PubMed/NCBI
|
|
24
|
Hwang SG, Song SM, Kim JR, Park CS, Song
WK and Chun JS: Regulation of type II collagen expression by
cyclin-dependent kinase 6, cyclin D1, and p21 in articular
chondrocytes. IUBMB Life. 59:90–98. 2007. View Article : Google Scholar : PubMed/NCBI
|