Introduction
The number of individuals with diabetes has been
increasing rapidly worldwide. Poorly controlled diabetes leads to
numerous complications (1,2), including macroangiopathy, which is a
major cause of mortality in patients with diabetes (3). One independent risk factor for
macroangiopathy in patients with diabetes is postprandial
hyperglycemia, which has been proposed to be caused by the
induction of oxidative stress (5–9). In
order to prevent the onset and/or progression of macroangiopathy in
patients with diabetes, it is important to suppress hyperglycemia
after meals. Two non-drug treatments for this purpose include the
intake of food with a low glycemic index (GI) and postprandial
exercise. These treatments have been investigated separately in
several studies; however, to the best of our knowledge, they have
not been investigated as a combined treatment. In the present
study, the effectiveness of the combination of low-GI food intake
and postprandial exercise for the suppression of hyperglycemia was
investigated through the monitoring of blood glucose levels,
oxidative stress and antioxidative activity.
Materials and methods
Subjects and treatment
Nine male and four female individuals (range, 20–29
years old) who had no abnormal glucose tolerance in medical
check-ups for the prior year and who had received no drug therapy
were enrolled in the present study (Table I). Female subjects did not undergo
the present study during their menstrual periods.
 | Table ISubject characteristics. |
Table I
Subject characteristics.
| Parameter | Total (n=13) | Male (n=9) | Female (n=4) |
|---|
| Age (years) | 22.8±1.7 | 23.0±2.0 | 22.3±1.0 |
| Height (cm) | 168.2±9.0 | 172.3±6.8 | 158.8±5.5 |
| Weight (kg) | 60.6±8.3 | 63.9±7.6 | 53.3±4.1 |
| BMI
(kg/m2) | 21.4±1.5 | 21.5±1.7 | 21.1±0.8 |
For a test meal, cooked rice was prepared. The
quantity of saccharide in each test meal was set at 50 g through
adjusting the quantity of cooked rice provided in the meal. As a
low-GI meal, a vegetable salad of shredded cabbage dressed with
olive oil, vinegar and salt was prepared (Table II).
 | Table IINutrient composition and quantity of
the test foods. |
Table II
Nutrient composition and quantity of
the test foods.
| Food | Weight (g) | Protein (g) | Fat (g) | Carbohydrate (g) | Energy (kcal) |
|---|
| White rice | 147.0 | 3.1 | 0.6 | 50.0 | 223.2 |
| Cabbage | 60.0 | 0.8 | 0.1 | 3.1 | 13.8 |
| Olive oil | 10.0 | 0.0 | 10.0 | 0.0 | 92.1 |
| Vinegar | 10.0 | 0.0 | 0.0 | 0.2 | 25.0 |
| Total | 227.0 | 3.9 | 10.7 | 53.3 | 354.1 |
For the postprandial exercise, step aerobics at a
speed of 80 steps per min was performed. The following four
experiments were randomly assigned to the 13 subjects, which were
conducted using the crossover method: i) consumption of the cooked
rice only (control; n=13); ii) consumption of the vegetable salad
first and then the cooked rice (LGI; n=13); iii) consumption of the
cooked rice and performing the exercise 30 min later (EX; n=13);
and iv) consumption of the vegetable salad first, followed by the
cooked rice and performing the exercise 30 min later (MIX; n=13).
The day prior to the test, food and drink other than water was
prohibited after 9:00 p.m. The starting time of consuming the
cooked rice or the vegetable salad (whichever was first) was
defined as 0 min. When the vegetable salad was consumed first, the
cooked rice was consumed 10 min later. The test meals were consumed
at the individual’s own pace. All participants provided written
informed consent, and the study protocol was approved by the Ethics
Committee of Josai University (Sakado, Japan).
Blood sample collection and analysis of
glucose and insulin levels
Blood samples were taken from the cutaneous vein of
the fingertip using a puncture device for self exsanguination eight
times: 10 min prior to the meal, then 20, 30, 45, 60, 90, 120 and
180 min following the first meal. Blood was collected using a
capillary tube and blood plasma was obtained through
centrifugation.
Blood glucose levels were measured using a
self-administered blood glucose measuring device (Glutest Neo
Super®; Sanwa Kagaku Kenkyusho Co. Ltd., Aichi, Japan).
Plasma insulin values in 25 μl plasma were measured using an
insulin measuring kit (YK060 Insulin ELISA kit®;
Yanaihara Institute Inc., Shizuoka, Japan). Levels of derivatives
of reactive oxidative metabolites (d-ROM; an oxidative stress
marker) and the biological antioxidant potential (BAP; a marker of
antioxidative activity ) were measured in 30 μl plasma using a free
radical analyzer (FREE Carpe Diem; Wismerll, Tokyo, Japan) at 0,
60, 120 and 180 min. Four of the 13 subjects provided insufficient
sample for measuring d-ROM and BAP.
Statistical analysis
Sequential blood glucose, insulin, d-ROM and BAP
levels recorded for the subjects following the consumption of the
test meal were set as the Δblood sugar, Δinsulin level, Δd-ROM
level and ΔBAP level by subtraction of the level at 0 min. ΔBAP was
divided by Δd-ROM, then divided by 7.541. This result was
designated as the Δmodified BAP/d-ROM ratio, which was considered
to be an indicator of antioxidant capacity. The sequential changes
in blood glucose and plasma insulin levels were assessed in each
group. P<0.05 was considered to indicate a statistically
significant difference between groups. The data were analyzed using
Statcel2 software (OMS Publishing Inc., Saitama, Japan). The
results were assessed with Tukey Kramer test.
Results
Changes in blood glucose and insulin
levels
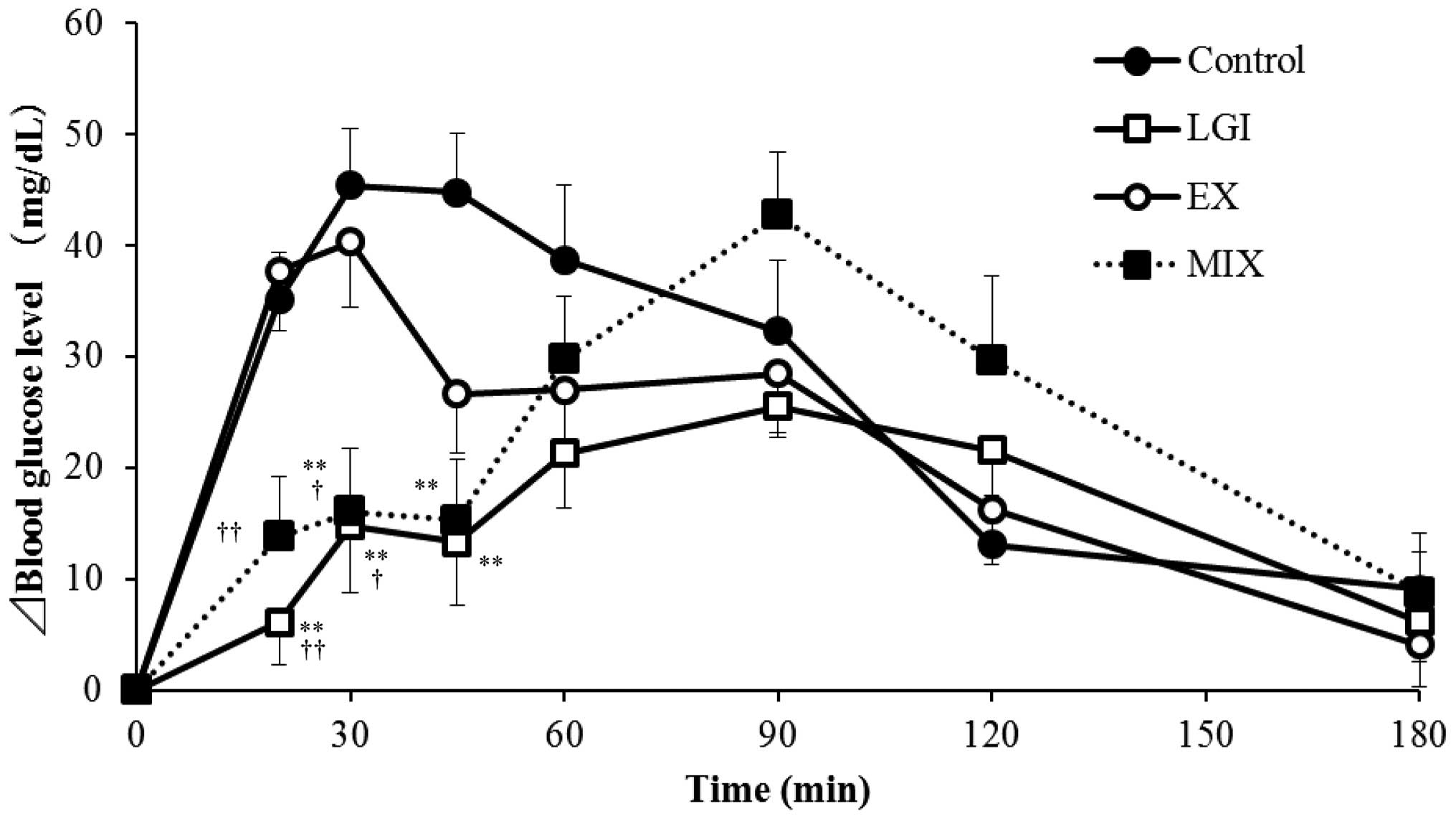
The changes in blood glucose levels in each group
are shown in Fig. 1. The blood
glucose levels in the individuals in the control group were found
to increase immediately following the meal and reached a peak at 30
min following the meal, then decreased. In the LGI group, the blood
glucose level was observed to increase for up to 45 min following
the meal, but it was significantly suppressed compared with that in
the control group. In the EX group, the increase in blood glucose
levels between 45 and 60 min following the meal was suppressed
compared with that in the control group. In the MIX group, the
changes in blood glucose levels up to 45 min following the meal
were comparable with those in the LGI group; however, the blood
sugar level at the 60 min time-point was increased compared with
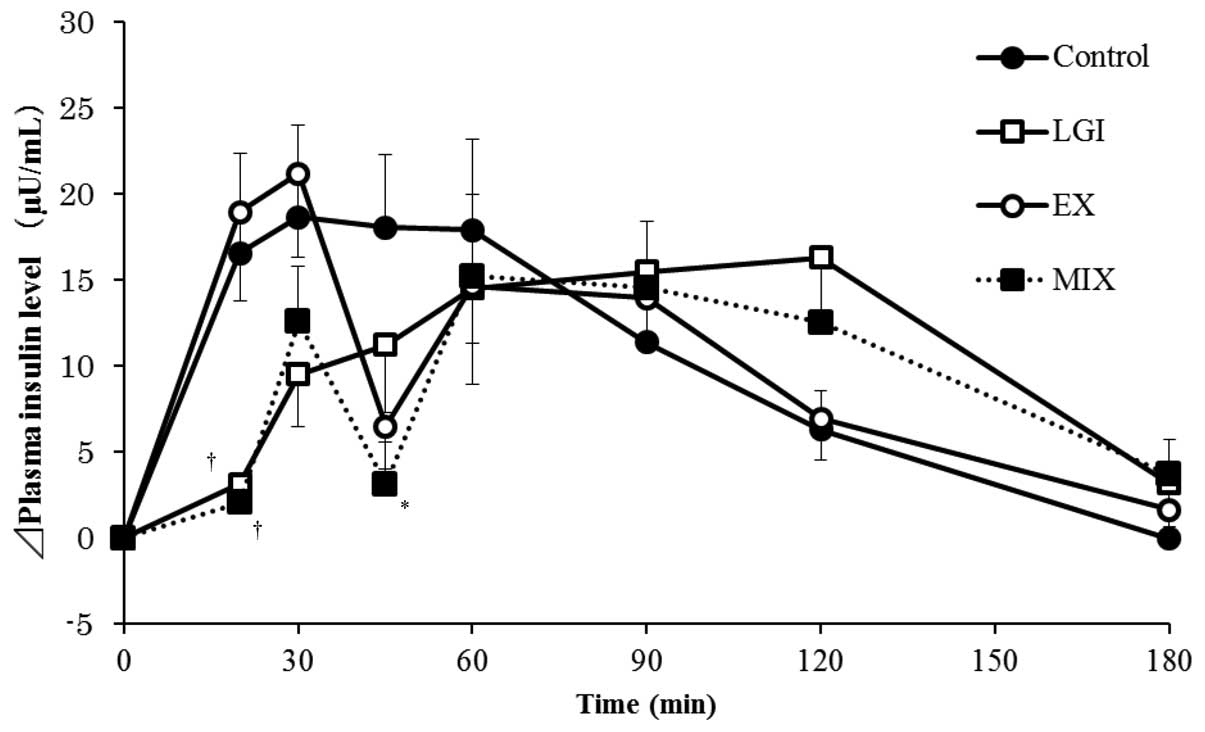
that in the LGI group. The sequential changes in insulin values
following the meal in each group were similar to those in the blood
glucose levels (Fig. 2). However,
the insulin values in the EX and MIX groups were found to be
decreased 45 min following the meal compared with those in the
control group.
Time course of antioxidant potential
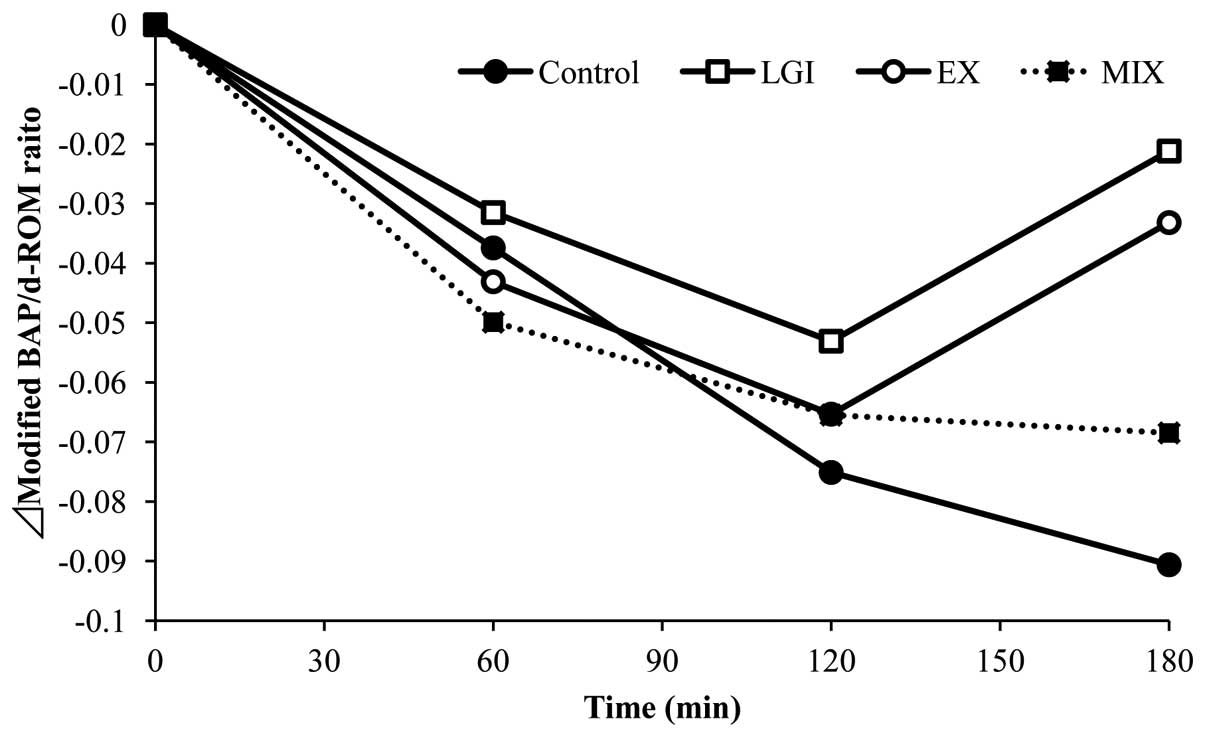
The antioxidant potential of each of the test groups
is shown in Fig. 3. The Δmodified
BAP/d-ROM ratio in the control group was observed to decrease over
time. By contrast, the reduction in this ratio was suppressed in
the LGI group compared with that in the control group. Moreover, at
180 min, the Δmodified BAP/d-ROM ratio in the control group was
found to exhibit the greatest reduction amongst all the groups,
while the Δmodified BAP/d-ROM ratio in the LGI and EX groups was
increased compared with that at 120 min. The Δmodified BAP/d-ROM
ratio in MIX group was not observed to increase at 180 min.
Discussion
The results indicate that the combination of low GI
food consumption and post-meal exercise affects postprandial blood
glucose. Blood glucose data revealed that combining low-GI food
intake and 10 min of exercise 30 min following a meal increases
postprandial blood glucose levels compared with those observed
following only low-GI food consumption. Regarding the effect of
low-GI food on postprandial blood glucose, the low-GI meal was
found to have a suppressive effect on the rapid increase in
postprandial blood glucose levels. A meta-analysis of studies of
this effect revealed that this benefit of low-GI food reduces
several cardiovascular risk factors (10).
In the present study, a vegetable salad was used as
the low-GI food. The data from the treatment and control groups
revealed that consuming the salad prior to the cooked rice
effectively helped control postprandial blood glucose levels, as
observed in a previous study (11). It has been reported that uncooked
cabbage, olive oil and vinegar (the components of the salad used in
the present study) each suppress hyperglycemia following a meal.
The water-soluble dietary fiber in cabbage has high viscosity,
which slows down the rate of gastric emptying and this delay has
been proposed to suppress the rapid elevation in glycemia (12). The insoluble dietary fiber in
cabbage has also been reported to improve the insulin resistance of
the body (13). Olive oil contains
a high quantity of monounsaturated fatty acids that enhance insulin
sensitivity and lower postprandial blood glucose through
stimulating the secretion of glucagon-like peptide-1 (14). Vinegar has also been proposed to
lower the gastric emptying rate and thus slow down digestion,
suppressing hyperglycemia following a meal (15). These characteristics of low-GI
foods are supported by the findings of the present study.
Regarding the effect of post-meal exercise on
postprandial blood glucose, Larsen et al (16) observed that performing moderate
exercise 45 min after breakfast lowered blood glucose and/or
insulin levels in patients with diabetes. The underlying mechanism
may involve an increase in glucose consumption in the skeletal
muscle (16). Viollet et al
(17) reported that adenosine
monophosphate-activated protein (AMP) kinase is activated due to
the increase in the AMP/ATP ratio upon exercise in the skeletal
muscle, and glucose transporter type 4 becomes transferred to the
cell membrane. However, it has also been reported that if an
individual exercises in a fasting state, the glucose supply from
the liver is stimulated as catecholamine secretion is increased,
which is associated with sympathetic nerve stimulation;
furthermore, insulin secretion, which suppresses gluconeogenesis in
the liver, is decreased (18).
In the present study, the EX group exhibited an
inhibited postprandial blood glucose increase at 45 and 60 min
following the meal compared with the glucose level in the control
group. The MIX group exhibited a greater increase in postprandial
blood glucose level compared with that of the LGI group. In light
of the aforementioned mechanism, the reason why the MIX group
showed a greater increase in postprandial blood sugar level than
the LGI group may be that the exercise was performed when the blood
sugar level was not sufficiently high; thus, glucose was supplied
from the liver.
An increase in blood glucose levels has been
proposed to lead to oxidative stress. Ceriello et al
(19) observed that patients with
diabetes exhibit a significant post-meal reduction in antioxidant
levels and an increase in the levels of malondialdehyde, a marker
of oxidative stress. In the present study, the antioxidant capacity
of the control group continued to decrease until 180 min after the
meal, whereas the antioxidant capacity in the LGI and EX groups was
beginning to increase at 180 min following the meal. A previous
study indicated that the oxidation of LDL is prevented by the
polyphenols contained in olive oil (20), and the exercise-induced reduction
in the manifestation of NADPH oxidase (21) is thought to contribute to this.
It is well established that a controlled diet and
exercise are essential therapies for the treatment of diabetes.
Thus, the consumption of low-GI food combined with post-meal
exercise may be used as a treatment option for patients with
diabetes. The findings of the present study support the use of
dietary counseling regarding low-GI food. However, the individuals
employed in the present study were healthy, with no diabetes; thus,
the data obtained should be investigated in further studies of
patients with diabetes.
Acknowledgements
The present study was supported by a grant (no.
B-22002) from the Kao Research Council for the Study of Healthcare
Science.
References
|
1
|
Huxley R, Barzi F and Woodward M: Excess
risk of fatal coronary heart disease associated with diabetes in
men and women: meta-analysis of 37 prospective cohort studies. BMJ.
332:73–78. 2006. View Article : Google Scholar :
|
|
2
|
Haffner SM, Lehto S, Rönnemaa T, Pyörälä K
and Laakso M: Mortality from coronary heart disease in subjects
with type 2 diabetes and in nondiabetic subjects with and without
prior myocardial infarction. N Engl J Med. 339:229–234. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Niskanen L, Turpeinen A, Penttilä I and
Uusitupa MI: Hyperglycemia and compositional lipoprotein
abnormalities as predictors of cardiovascular mortality in type 2
diabetes: a 15-year follow-up from the time of diagnosis. Diabetes
Care. 21:1861–1969. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
DECODE Study Group, the European Diabetes
Epidemiology Group. Glucose tolerance and cardiovascular mortality:
comparison of fasting and 2-hour diagnostic criteria. Arch Intern
Med. 161:397–405. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ceriello A, Falleti E, Motz E, et al:
Hyperglycemia-induced circulating ICAM-1 increase in diabetes
mellitus: the possible role of oxidative stress. Horm Metab Res.
30:146–149. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nappo F, Esposito K, Cioffi M, et al:
Postprandial endothelial activation in healthy subjects and in type
2 diabetic patients: role of fat and carbohydrate meals. J Am Coll
Cardiol. 39:1145–50. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Esposito K, Nappo F, Marfella R, et al:
Inflammatory cytokine concentrations are acutely increased by
hyperglycemia in humans: role of oxidative stress. Circulation.
106:2067–2072. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marfella R, Quagliaro L, Nappo F, Ceriello
A and Giugliano D: Acute hyperglycemia induces an oxidative stress
in healthy subjects. J Clin Invest. 108:635–636. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Williams SB, Goldfine AB, Timimi FK, et
al: Acute hyperglycemia attenuates endothelium-dependent
vasodilation in humans in vivo. Circulation. 97:1695–1701. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Opperman AM, Venter CS, Oosthuizen W,
Thompson RL and Vorster HH: Meta-analysis of the health effects of
using the glycaemic index in meal-planning. Br J Nutr. 92:367–381.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kanamoto I, Inoue Y, Moriuchi T, et al:
Effect of differences in low glycemic index food intake sequence on
plasma glucose profile. J Jpn Diabetes Soc. 53:96–101. 2010.
|
|
12
|
Lu ZX, Walker KZ, Muir JG, Mascara T and
O’Dea K: Arabinoxylan fiber, a byproduct of wheat flour processing,
reduces the postprandial glucose response in normoglycemic
subjects. Am J Clin Nutr. 71:1123–1128. 2000.PubMed/NCBI
|
|
13
|
Weickert MO, Möhlig M, Schöfl C, et al:
Cereal fiber improves whole-body insulin sensitivity in overweight
and obese women. Diabetes Care. 29:775–780. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Paniagua JA, de la Sacristana AG, Sánchez
E, et al: A MUFA-rich diet improves posprandial glucose, lipid and
GLP-1 responses in insulin-resistant subjects. J Am Coll Nutr.
26:434–444. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ostman E, Granfeldt Y, Persson L and
Björck I: Vinegar supplementation lowers glucose and insulin
responses and increases satiety after a bread meal in healthy
subjects. Eur J Clin Nutr. 59:983–988. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Larsen JJ, Dela F, Kjaer M and Galbo H:
The effect of moderate exercise on postprandial glucose homeostasis
in NIDDM patients. Diabetologia. 40:447–453. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Viollet B, Lantier L, Devin-Leclerc J, et
al: Targeting the AMPK pathway for the treatment of Type 2
diabetes. Front Biosci (Landmark Ed). 14:3380–3400. 2009.
View Article : Google Scholar
|
|
18
|
Poirier P, Tremblay A, Catellier C, et al:
Impact of time interval from the last meal on glucose response to
exercise in subjects with type 2 diabetes. J Clin Endocrinol Metab.
85:2860–2864. 2000.PubMed/NCBI
|
|
19
|
Ceriello A, Bortolotti N, Motz E, et al:
Meal-generated oxidative stress in type 2 diabetic patients.
Diabetes Care. 21:1529–1533. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Castañer O, Covas MI, Khymenets O,
Nyyssonen K, Konstantinidou V, Zunft HF, de la Torre R,
Muñoz-Aguayo D, Vila J and Fitó M: Protection of LDL from oxidation
by olive oil polyphenols is associated with a downregulation of
CD40-ligand expression and its downstream products in vivo in
humans. Am J Clin Nutr. 95:1238–1244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kojda G and Hambrecht R: Molecular
mechanisms of vascular adaptations to exercise: Physical activity
as an effective antioxidant therapy? Cardiovasc Res. 67:187–197.
2005. View Article : Google Scholar : PubMed/NCBI
|