Introduction
Chronic rhinosinusitis (CRS) is characterized by
mucosal inflammation of the nose and paranasal sinuses and often
taken as an umbrella term for a heterogeneous group of sinus
diseases. CRS has been divided into CRS without nasal polyps
(CRSsNP) and with nasal polyps (CRSwNP) on the basis of clinical
presentation (1,2). However, controversy exists as to
whether they represent the different stages of one disorder or
separate entities due to their distinct histomorphology,
inflammatory and remodeling profiles (3).
Over the last decades, mounting evidence has
suggested that the etiology and pathophysiology of CRS is complex
and multifactorial (4). Of the
multiple etiological hypotheses, including bacterial, fungal,
microbial biofilm, superantigen and immune barrier hypotheses
(4–6), an exogenous pathogen is probably
essential for the development and persistence of mucosal
inflammation. Staphylococcus aureus enterotoxins (SEs),
secreted by Staphylococcus aureus (S. aureus), the
most common colonizer of nasal passages and sinuses, are broadly
classified as superantigens (7).
It has been speculated that SEs may aggravate inflammation severity
in airway diseases, such as CRS, asthma and allergic rhinitis
(8–10).
Although a causal relationship of S. aureus
in patients with CRS has not been established, SEs might skew the
cytokine response towards a Th2 phenotype inducing both
eosinophilia and the production of polyclonal IgE (11), and thus contribute to, at least in
some cases, the development of CRSwNP (8,12,13).
By contrast, the evidence for the effect of SEs in CRSsNP patients
is so far relatively lacking (14). Recent studies have shown that the
inflammatory phenotypes in Chinese patients with CRS are
inconsistent with those in Caucasian subjects despite their similar
histomorphological pattern (15–17).
Further understanding the role of SEs in Chinese subjects with CRS
may help to provide insight into the mechanistic basis underlying
CRS. In the present study, the serum levels of total IgE, specific
IgE to SEA, SEB, and SEC, and eosinophil cationic protein (ECP)
were investigated in Chinese patients with CRSsNP and CRSwNP.
Materials and methods
Study subjects
A total of 70 patients undergoing endoscopic sinus
surgery for CRS were enrolled consecutively at the Department of
Otorhinolaryngology, the First Affiliated Hospital, Nanjing Medical
University (Nanjing, China). The diagnosis of CRS was based on
medical history, clinical symptoms, endoscopic examination, and
sinus CT scanning according to the European Position Paper on
Rhinosinusitis and Nasal Polyps 2007 as well as the Chinese CRS
guidelines (1,2). Thirty of the patients were classified
as CRSsNP, and the other 40 patients were CRSwNP. The study also
involved 30 healthy volunteers with no sinonasal diseases as
control subjects. Atopic status was evaluated by screening for
specific IgE to common aeroallergens (Phadiatop; Phadia AB,
Uppsala, Sweden). Subjects who had taken glucocorticoids within 4
weeks, H1-antihistamines or leukotriene modifiers within 2 weeks,
and/or had asthma, atopic dermatitis or Samter’s triad were
excluded. The characteristics of the CRS patients and healthy
controls are shown in Table I.
This study was approved by the ethics committee of Nanjing Medical
University, and all participants gave their written informed
consent.
 | Table ICharacteristics and laboratory data of
the CRS patients and healthy controls. |
Table I
Characteristics and laboratory data of
the CRS patients and healthy controls.
| Variable | CRSsNP (n=30) | CRSwNP (n=40) | Control (n=30) | P-valuea |
|---|
| Gender,
male:female | 16:14 | 28:12 | 15:15 | >0.05 |
| Age, years | 45.2±15.6 | 46.1±12.6 | 40.6±10.2 | >0.05 |
| Atopy | 2/30 | 4/40 | 1/30 | >0.05 |
| Total IgE, kU/l | 121.5
(64.6–298.5)b | 114.5
(54.4–242.3)b | 12.0 (5.14–38.1) | <0.001 |
| ECP, μg/l | 8.18
(4.53–22)c | 9.31
(5.46–36.4)b | 2.71
(<2–6.27) | <0.001 |
| SE-specific IgE,
positive number (%) |
| SEA, grade
I/II/III | 2/2/0 (13.3) | 4/0/0 (10.0) | 1/0/0 (3.33) | >0.05 |
| SEB, grade
I/II/III | 5/1/1 (23.3) | 3/8/0 (27.5)d | 1/0/0 (3.33) | 0.025 (0.018) |
| SEC, grade
I/II/III | 1/4/1 (20.0) | 4/5/1 (25.0) | 2/0/0 (6.67) | >0.05 |
| Positive SEs | 9/30 | 13/40d | 2/30 | 0.028 |
Measurement of total IgE, specific IgE
and ECP in serum
Peripheral blood (3 ml) was collected from each
subject. After centrifugation at 100 × g for 10 min, the serum was
separated and stored at −70°C until further analysis. The levels of
total IgE, specific IgE to SEA, SEB and SEC, and ECP in sera were
measured using ImmunoCAP assays (Phadia AB) according to the
manufacture’s recommendations. The detection limits were set at
<2 kU/l for total IgE and 2 μg/l for ECP. A concentration of
specific IgE ≥0.35 kUA/l was considered as positive, and the levels
were expressed as the following grades: 0, <0.35 kUA/l; I,
0.35–0.69 kUA/l; II, 0.7–3.49 kUA/l; III, 3.5–17.49 kUA/l. IV,
17.5–49.9 kUA/l; V, 50–100 kUA/l; VI, >100 kUA/l.
Statistical analysis
Statistical analyses were performed using SAS
software version 9.1.3 (SAS Institute, Cary, NC, USA). Specific IgE
to SEA, SEB, and SEC were analyzed as ordinal data. Total IgE and
ECP are presented as median and interquartile range. Data were
first compared within different groups by the Kruskal-Wallis H
test. The Mann-Whitney U test with the Bonferroni’s post hoc test
was then applied to evaluate the statistical differences
between-group comparison. Differences in proportions among groups
were compared with the χ2 test or Fishers’ exact test.
Correlations were calculated using the Spearman test. A P-value
<0.05 was considered statistically significant.
Results
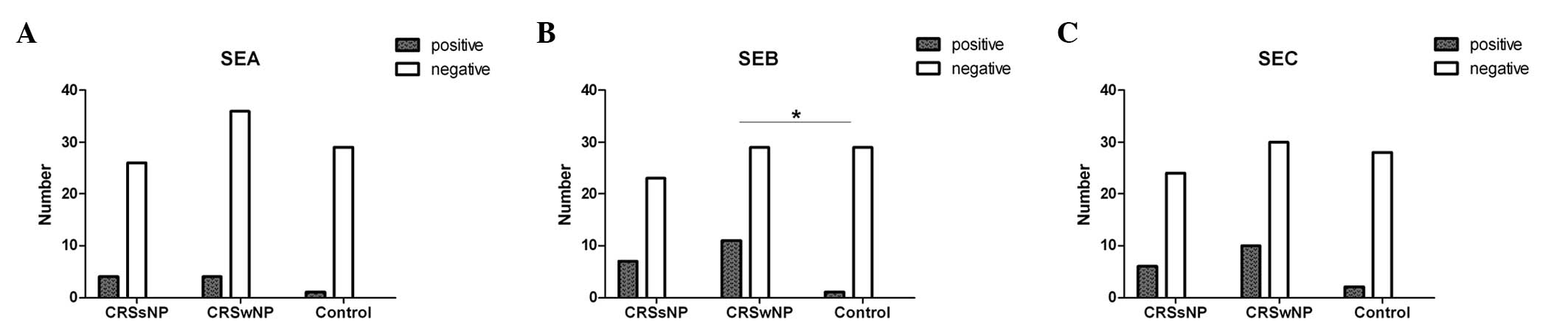
Serum SE-specific IgE in the CRS and
control groups
As shown in Table
I, specific IgE against at least one subtype of SEs was
detected in the serum from 9 of 30 (30.0%) subjects with CRSsNP, 13
of 40 (32.5%) with CRSwNP and 2 of 30 (6.7%) controls, respectively
(level range, grades I-III). Of these, only the positive rate of
SEB-specific IgE was significantly higher in the CRSwNP group than
that in the control group (P=0.027; Fig. 1). Furthermore, the serum level of
specific IgE to SEB, rather than that to SEA and SEC, was elevated
markedly in the CRSwNP group compared with that in the control
group (P=0.021). The positive rate and level of SEB-specific IgE in
CRSsNP group showed an increasing trend but did not reach
significance (P=0.06 and P=0.069, respectively).
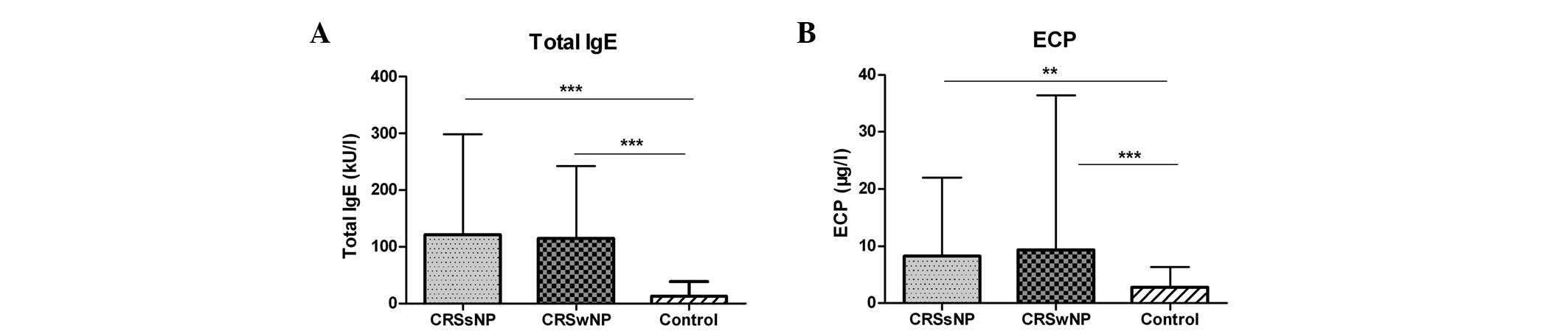
Serum total IgE and ECP in the CRS and
control groups
As shown in Fig. 2,
the serum levels of total IgE were significantly higher in the
CRSsNP and CRSwNP groups than those in the control group (both
P<0.001). Also, the serum levels of ECP were elevated markedly
in the CRSsNP group (P=0.002) and in the CRSwNP group (P<0.001)
compared with those in the control group. However, no significant
differences were observed between the two CRS groups.
Correlation between SE-specific IgE and
total IgE/ECP in the CRS groups
In the CRSsNP group, serum total IgE was positively
correlated with specific IgE to SEA (r=0.470, P=0.009), to SEB
(r=0.393, P=0.032) and to SEC (r=0.397, P=0.03). In the CRSwNP
group, serum total IgE was also positively correlated with specific
IgE to SEB (r=0.581, P<0.001) and to SEC (r=0.501, P=0.001), but
not to SEA. There was no correlation between the serum levels of
ECP and specific IgE to SEs in either group of CRS. The results are
summarized in Table II.
 | Table IICorrelation between SE-specific IgE
and total IgE/ECP in sera from CRS patients. |
Table II
Correlation between SE-specific IgE
and total IgE/ECP in sera from CRS patients.
| Variable | SEA | SEB | SEC |
|---|
| CRSsNP |
| Total IgE | r=0.470, P=0.009 | r=0.393, P=0.032 | r=0.397, P=0.03 |
| ECP | r=0.069,
P=0.718 | r=−0.157,
P=0.407 | r=−0.045,
P=0.812 |
| CRSwNP |
| Total IgE | r=0.240,
P=0.135 | r=0.581,
P<0.001 | r=0.501,
P=0.001 |
| ECP | r=−0.088,
P=0.590 | r=−0.134,
P=0.411 | r=−0.303,
P=0.057 |
Discussion
The SEs have been described as superantigens due to
their ability to bridge major histocompatibility (MHC) class II
molecules and directly activate polyclonal T cells and B cells in a
nonspecific manner (18,19). To date, there are >20 distinct
SEs including SEA through V and toxic shock syndrome toxin-1
(TSST-1), but only a few of them have been well researched
(7).
In the present study, the serum specific IgE
antibodies against three common staphylococcal superantigens (SEA,
SEB and SEC) were detected in patients with CRSsNP and CRSwNP, and
healthy controls. The results demonstrated that the positive rate
and level of serum specific IgE to SEB in CRSwNP patients were
significantly higher in comparison with those in the controls. In
line with earlier findings (20–22),
the results of the present study have revealed that specific IgE to
SEs can be detected in serum, and may have an effect on CRSwNP. SEs
are known to have the ability to cross airway epithelial barriers
in an immunologically intact form (23,24),
possibly via inducing extensive inflammation that brings about an
increase in epithelial permeability and a reduction in tight
junction proteins (7). In
addition, as reported in an in vivo study in mice by Hamad
et al (25), SEB is more
efficient at traversing the epithelial barrier and entering the
blood than SEA, although both rapidly reached functional levels in
the serum following oral administration. This may account for the
discrepancy of serum levels among different specific IgE against
SEs.
Previous studies have suggested that SEB stimulation
highly increases the expression of TNF-α, IFN-γ, IL-2, IL-4, IL-5,
IL-13, and IL-17 (11,26). These cytokines are capable of
promoting the recruitment of neutrophils or eosinophils by means of
induction of chemokines and granulopoiesis factors. SEB is likely
to be able to induce multiple T-effector cell cytokines, leading to
a mixed inflammation pattern involving the infiltration of
neutrophilic and eosinophilic granulocytes. Furthermore, a mixed
Th1/Th2/Th17 pattern has been confirmed in Chinese CRSsNP and
CRSwNP patients (15).
Accordingly, SEB may play a comparable role in the two CRS entities
of Chinese patients. However, a direct association was not observed
between SEB and CRSsNP in the present study, although specific IgE
against SEB tended to be higher in CRSsNP patients than in the
controls.
Significant increases of total IgE and ECP levels in
the sera from the two CRS groups were observed in the present
study, and an analysis of the correlation among total IgE, ECP and
specific IgE to SEs was conducted. Total IgE had a positive
correlation with specific IgE to SEB as well as to SEC in CRSwNP,
and these correlations were stronger than those in CRSsNP. Kowalski
et al (9) found that total
IgE had a strong correlation with specific IgE to SEs in serum from
asthma patients that was independent of atopic status, and these
two factors significantly correlated with asthma severity markers.
Accordingly, total IgE may be directly promoted by specific IgE to
SEs; in addition, they both have predictive roles in the
development and persistence of airway inflammation. By contrast,
inconsistent with findings in Caucasian patients (20), no significant correlation was
detected between ECP and specific IgE to SEs in the CRSwNP or
CRSsNP groups in the present study. Patients with asthma and atopic
dermatitis were excluded, and the prevalence of allergic rhinitis
was not different among groups. The increased levels of ECP, which
indicate an intense activation of eosinophilic inflammation in the
sera from the two CRS groups seem to be unassociated with atopic
status in this study. However, various effects of unknown factors
on peripheral blood should be taken into account, and the
relationships require further study in local tissue. In view of the
considerable differences in inflammatory pattern between Chinese
and Caucasian patients, SEs might have different effect on
sinonasal inflammation via varying immune system responses.
It may be speculated that S. aureus and SEB
formed in the sinonasal tissues might be responsible for
participating and amplifying the development of mucosal
inflammation, and further be a source of persistent inflammation
due to the capacity of S. aureus for residing in the
nonphagocytic eukaryotic cells and escaping from immune
surveillance (27). However,
direct evidence for this and the exact molecular mechanisms by
which S. aureus and SEs exert their effect in CRS require
exploration in future studies.
The limitation of the present study is that the
levels of specific IgE to SEs in the local tissues, sinonasal
mucosa and polyps were not measured due to the lack of sufficient
samples from control subjects. In further studies, it is
recommended that tissue samples should also be analyzed and
comparative and correlation analysis with serum expression
conducted to elucidate the role of SEs (particularly SEB) in the
development and severity of CRS comprehensively.
In summary, the positive rate and level of
SEB-specific IgE were significantly higher in the serum from the
Chinese CRSwNP patients than in that from the healthy controls. In
addition, the presence of total IgE correlated positively with that
of SEB-specific IgE. It is suggested that SEB may play a role in
the pathogenesis of CRSwNP in Chinese patients.
Acknowledgements
This study was supported by grants from the Priority
Academic Program Development of Jiangsu Higher Education
Institutions (PAPD 2010–2013), the Health Promotion Project of
Jiangsu Province (XK200719 and RC2011071) and the Health Ministry
Special Fund (201202005), China.
References
|
1
|
Fokkens W, Lund V and Mullol J; European
Position Paper on Rhinosinusitis and Nasal Polyps group. European
position paper on rhinosinusitis and nasal polyps 2007. Rhinol
Suppl. (20): 1–136. 2007.PubMed/NCBI
|
|
2
|
No authors listed. Guidelines for
diagnosis and treatment of chronic rhinitis and nasal sinusitis
(2008, Nanchang). Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
44:6–7. 2009.(In Chinese).
|
|
3
|
Van Crombruggen K, Zhang N, Gevaert P,
Tomassen P and Bachert C: Pathogenesis of chronic rhinosinusitis:
inflammation. J Allergy Clin Immunol. 128:728–732. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tan BK, Schleimer RP and Kern RC:
Perspectives on the etiology of chronic rhinosinusitis. Curr Opin
Otolaryngol Head Neck Surg. 18:21–26. 2010. View Article : Google Scholar
|
|
5
|
Foreman A, Boase S, Psaltis A and Wormald
PJ: Role of bacterial and fungal biofilms in chronic
rhinosinusitis. Curr Allergy Asthma Rep. 12:127–135. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van Drunen CM, Mjösberg JM, Segboer CL,
Cornet ME and Fokkens WJ: Role of innate immunity in the
pathogenesis of chronic rhinosinusitis: progress and new avenues.
Curr Allergy Asthma Rep. 12:120–126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pinchuk IV, Beswick EJ and Reyes VE:
Staphylococcal enterotoxins. Toxins (Basel). 2:2177–2197. 2010.
View Article : Google Scholar
|
|
8
|
Guven M, Karabay O, Akidil O, Yilmaz MS
and Yildirim M: Detection of staphylococcal exotoxins in
antrochoanal polyps and chronic rhinosinusitis with nasal polyps.
Otolaryngol Head Neck Surg. 148:302–307. 2013. View Article : Google Scholar
|
|
9
|
Kowalski ML, Cieślak M, Pérez-Novo CA,
Makowska JS and Bachert C: Clinical and immunological determinants
of severe/refractory asthma (SRA): association with Staphylococcal
superantigen-specific IgE antibodies. Allergy. 66:32–38. 2011.
View Article : Google Scholar
|
|
10
|
Liu JN, Shin YS, Yoo HS, et al: The
prevalence of serum specific IgE to superantigens in asthma and
allergic rhinitis patients. Allergy Asthma Immunol Res. 6:263–266.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Patou J, Gevaert P, Van Zele T, et al:
Staphylococcus aureus enterotoxin B, protein A, and lipoteichoic
acid stimulations in nasal polyps. J Allergy Clin Immunol.
121:110–115. 2008. View Article : Google Scholar
|
|
12
|
Sejima T, Holtappels G, Kikuchi H,
Imayoshi S, Ichimura K and Bachert C: Cytokine profiles in Japanese
patients with chronic rhinosinusitis. Allergol Int. 61:115–122.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seiberling KA, Conley DB, Tripathi A, et
al: Superantigens and chronic rhinosinusitis: detection of
staphylococcal exotoxins in nasal polyps. Laryngoscope.
115:1580–1585. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hsu J and Peters AT: Pathophysiology of
chronic rhinosinusitis with nasal polyp. Am J Rhinol Allergy.
25:285–290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao PP, Li HB, Wang BF, et al: Distinct
immunopathologic characteristics of various types of chronic
rhinosinusitis in adult Chinese. J Allergy Clin Immunol.
124:478–484. 484.e1–2. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang N, Van Zele T, Perez-Novo C, et al:
Different types of T-effector cells orchestrate mucosal
inflammation in chronic sinus disease. J Allergy Clin Immunol.
122:961–968. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi J, Fan Y, Xu R, et al: Characterizing
T-cell phenotypes in nasal polyposis in Chinese patients. J
Investig Allergol Clin Immunol. 19:276–282. 2009.PubMed/NCBI
|
|
18
|
Bachert C and Zhang N: Chronic
rhinosinusitis and asthma: novel understanding of the role of IgE
‘above atopy’. J Intern Med. 272:133–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fraser JD and Proft T: The bacterial
superantigen and superantigen-like proteins. Immunol Rev.
225:226–243. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bachert C, Zhang N, Holtappels G, et al:
Presence of IL-5 protein and IgE antibodies to staphylococcal
enterotoxins in nasal polyps is associated with comorbid asthma. J
Allergy Clin Immunol. 126:962–968. 968.e1–6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Conley DB, Tripathi A, Ditto AM, et al:
Chronic sinusitis with nasal polyps: staphylococcal exotoxin
immunoglobulin E and cellular inflammation. Am J Rhinol.
18:273–278. 2004.PubMed/NCBI
|
|
22
|
Tripathi A, Conley DB, Grammer LC, et al:
Immunoglobulin E to staphylococcal and streptococcal toxins in
patients with chronic sinusitis/nasal polyposis. Laryngoscope.
114:1822–1826. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Soong G, Martin FJ, Chun J, Cohen TS, Ahn
DS and Prince A: Staphylococcus aureus protein A mediates invasion
across airway epithelial cells through activation of RhoA GTPase
signaling and proteolytic activity. J Biol Chem. 286:35891–35898.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Parker D and Prince A: Immunopathogenesis
of Staphylococcus aureus pulmonary infection. Semin Immunopathol.
34:281–297. 2012. View Article : Google Scholar
|
|
25
|
Hamad AR, Marrack P and Kappler JW:
Transcytosis of staphylococcal superantigen toxins. J Exp Med.
185:1447–1454. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grumann D, Scharf SS, Holtfreter S, Kohler
C, Steil L, Engelmann S, Hecker M, Völker U and Bröker BM: Immune
cell activation by enterotoxin gene cluster (egc)-encoded and
non-egc superantigens from Staphylococcus aureus. J Immunol.
181:5054–5061. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Corriveau MN, Zhang N, Holtappels G, Van
Roy N and Bachert C: Detection of Staphylococcus aureus in nasal
tissue with peptide nucleic acid-fluorescence in situ
hybridization. Am J Rhinol Allergy. 23:461–465. 2009. View Article : Google Scholar : PubMed/NCBI
|