Introduction
The dried root of Salvia miltiorrhiza Bunge
(S. miltiorrhiza; Radix Salvia Miltiorrhizae), known as
Danshen in Chinese, Dansam in Korean and Tansen in Japanese, is one
of the most commonly used substances in traditional Chinese
medicine (TCM), and is also used in other Asian countries,
including Korea and Japan (1).
S. miltiorrhiza has been used in the treatment of a variety
of conditions, including cardiovascular disease, cerebrovascular
disease, diabetic vascular complications (1), liver dysfunction (2) and renal disease (3). Furthermore, Wen et al
previously demonstrated that S. miltiorrhiza is effective in
the treatment of gastrointestinal inflammatory disease (4). The active chemical constituents of
S. miltiorrhiza root have been studied extensively. These
active components, the majority of which have been identified and
purified, are generally divided into two major groups;
water-soluble phenolic compounds and lipophilic diterpene quinones
(5). The lipid-soluble compounds,
usually extracted using alcohol solvents, are rich in abietanoids
and diterpene quinones (tanshinones). Numerous diterpenoid
tanshinones have been extracted from S. miltiorrhiza,
including dihydrotanshinone I, cryptotanshinone and tanshinones I
and IIA, which are the three most commonly studied. These
tanshinones have been reported to exhibit antioxidative (6–9),
anti-inflammatory (6,10,11),
anti-allergic (12) and
anti-cancer effects (13,14).
In China, S. miltiorrhiza has been used as a
medicine or dietary supplement for improving health. The oral
administration of this medicinal herb appears to exhibit negligible
effects on the pharmacokinetics of therapeutic agents, such as
docetaxel or clopidogrel, indicating the potential benefits of
combining S. miltiorrhiza with standard therapeutics
(15). Furthermore, the
combination of S. miltiorrhiza and its active constituents
with other TCM or chemotherapeutic substances has been observed to
result in more notable anti-cancer effects compared with either
agent alone (16,17). However, the safety of orally
administered S. miltiorrhiza as a nutritional or dietary
supplement is not known and may be a potential disadvantage to its
use as a therapeutic.
The first step in the utilization of medicinal
plants as dietary supplements, health foods/nutritional supplements
or pharmaceuticals is the extraction of the bioactive constituents
from the plant materials. The active components of plant materials
are commonly extracted using a solvent. Numerous solvents,
including methanol, ethanol, acetone and ethyl acetate, have
previously been used to prepare extracts from plant materials,
either alone or in combination and at varying aqueous dilutions.
Ethanol is considered to be a particularly effective solvent, and
its use is permitted by the food industry for the preparation of
dietary supplements or functional foods, as it is safe for human
consumption (18). Numerous S.
miltiorrhiza preparations are currently available, including
lipophilic and hydrophilic extracts. The ethanol extract of S.
miltiorrhiza, which is rich in lipophilic constituents, is the
most commonly used in Chinese clinics (19).
The present study aimed to compare the effects of a
number of ethanol extracts of S. miltiorrhiza, obtained
using ethanol at various aqueous dilutions as a solvent, with an
extract obtained using acetone, one of the most common solvents for
hydrophobic compounds, on the viability of cancer cells. In
addition, the cytotoxic effects of the bioactive constituents
indicated to be present in the most active fraction of S.
miltiorrhiza were assessed in five human cancer cell lines.
Materials and methods
Reagents
Dihydrotanshinone I, cryptotanshinone and tanshinone
I were purchased from Sigma-Aldrich (St. Louis, MO, USA). These
reference tanshinones were dissolved in dimethylsulfoxide (DMSO) at
a 100 μM concentration and stored at −20°C prior to the
experiments, and further dilutions were performed in the culture
medium. MTT was obtained from Amresco LLC (Solon, OH, USA) and
RPMI-1640 medium, Dulbecco’s modified Eagle’s medium (DMEM), fetal
bovine serum (FBS) and penicillin-streptomycin were purchased from
HyClone (GE Healthcare, Logan, UT, USA).
Preparation of crude S. miltiorrhiza
fractions
Extraction was conducted at room temperature by
placing 50 g powdered S. miltiorrhiza root (Hangzhou
Botanical Technology Co., Ltd., Hangzhou, China) in a 500-ml
conical flask and adding 250 ml acetone or 100, 70 or 30% aqueous
ethanol (v/v). In addition, an extraction using 30% aqueous ethanol
at 4°C was conducted. The mouth of the conical flask was covered
with aluminum foil and the contents left for 4 h, allowing
extraction of the active components into the solvent. Next, the
extracts were separated from the residues by filtering the
extraction mixture through Whatman No. 1 filter paper (GE
Healthcare Life Sciences, Pittsburgh, PA, USA), and the solvent was
removed using a rotary vacuum evaporator (Centra Evaporator;
Bioneer, Daejon, Korea). Finally, the dried crude extracts were
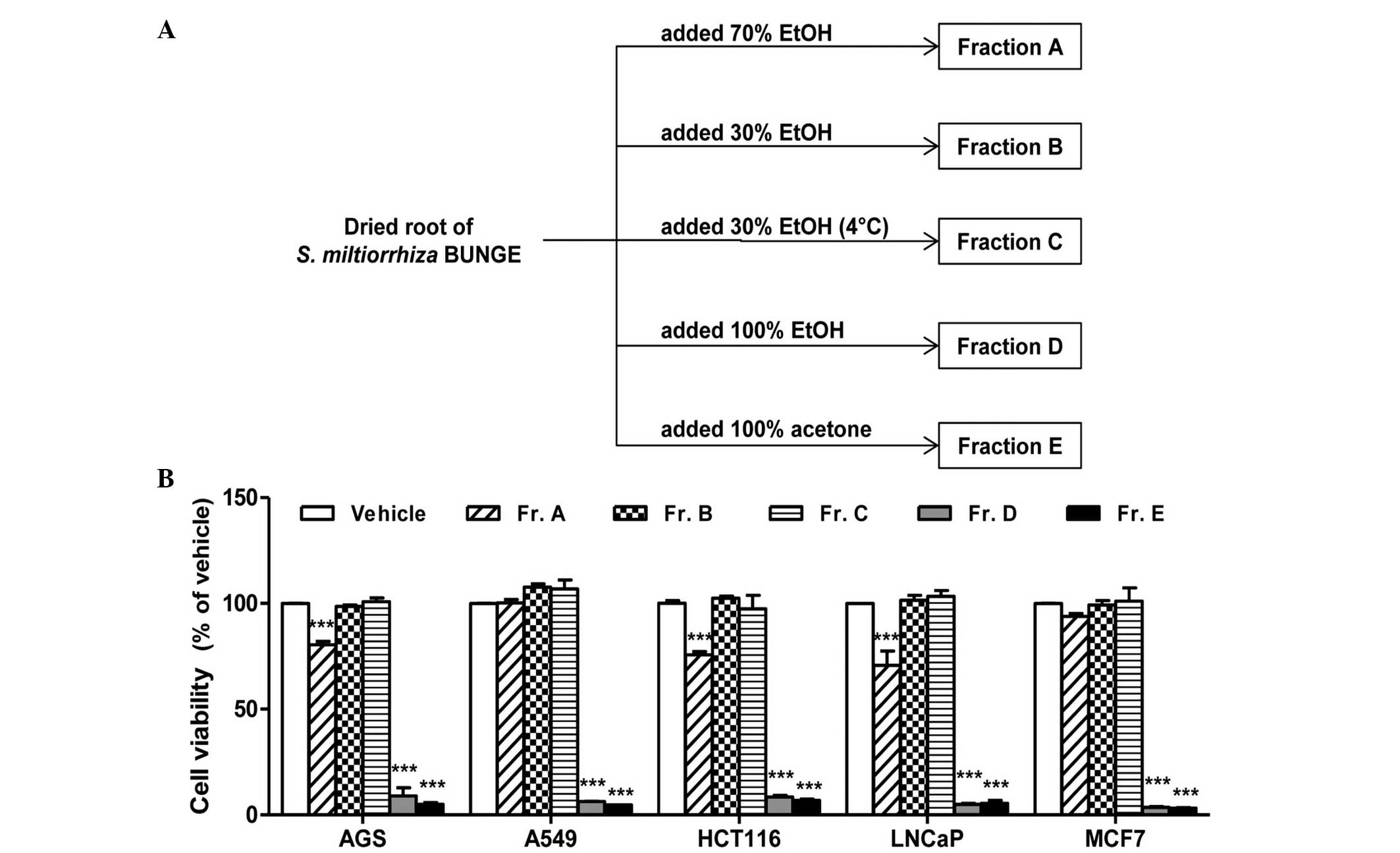
collected and used for further experiments, subsequently labeled
fractions A–E (Fig. 1A). The total
extract yields were calculated as the percentage weight of extract
per 100 g S. miltiorrhiza root on a dry basis. These
indicated that S. miltiorrhiza root extracts obtained using
30% ethanol gave the highest total extract yield (14.5%), followed
by 30% ethanol at 4°C (11.7%), 70% ethanol (8.2%), 100% ethanol
(0.65%) and acetone (0.58%).
Cell culture
Human gastric adenocarcinoma (AGS), prostate
carcinoma (LNCaP), breast adenocarcinoma (MCF7), colorectal
carcinoma (HCT116) and lung adenocarcinoma (A549) cells were
purchased from the American Type Culture Collection (Manassas, VA,
USA). The AGS, LNCaP and A549 cells were cultured in RPMI-1640
medium with 10% FBS. HCT116 and MCF7 cells were cultured in DMEM
with 10% FBS. In addition, the media contained penicillin (100
U/ml) and streptomycin (100 μg/ml). Cells were maintained in a
humidified incubator with 5% CO2 at 37°C. Cells were
subcultured every two days, and cells in the logarithmic growth
phase were used for experiments.
Thin-layer chromatography (TLC)
assay
TLC plates (5×10 cm) were prepared by cutting the
commercially available sheets (TLC Silica gel 60 F254; Merck,
Darmstadt, Germany). The fractions extracted from S.
miltiorrhiza were transferred and the plates were eluted in a
closed chamber with a mobile phase consisting of methylene
chloride, methanol and water at a ratio of 25:8:5, respectively.
Pure tanshinone reagents, including cryptotanshinone,
dihydrotanshinone I and tanshinone I were used as controls.
Cell cytotoxicity assay
Cells were seeded in 48-well plates at a
concentration of 2×104 cells/well and incubated for 24 h
at 37°C to allow the cells to adhere to the bottoms of the plates.
Cell culture media were removed by aspiration, replaced with fresh
media containing an extreme concentration (250 μg/ml) of each
fraction, and incubated for 24 h in order to compare the cytotoxic
effects of the fractions. Next, the cells were further incubated in
the dark with MTT reagent (0.5 mg/ml) for 2 h at 37°C.
Subsequently, the MTT reagent-containing culture media was
aspirated from each well, and DMSO was added to dissolve the
formazan precipitate. The absorbance of each sample was measured
using a Multiskan EX microplate reader (Thermo Fisher Scientific,
Vantaa, Finland) at a wavelength of 540 nm. Following analysis of
the results to determine an appropriate concentration range, the
assay was repeated using 0, 5, 10, 20 or 50 μg/ml S.
miltiorrhiza extract or 0, 1, 5 or 10 μM tanshinone reagent
Observation of cellular morphology
Following the cell viability assay, cells treated
with the three tanshinone reagents were examined for any
morphological alterations. Cells were photographed using a Zeiss
Axiovert 100 microscope (magnification, ×400; Carl Zeiss Microscopy
GmbH, Jena, Germany).
Statistical analysis
Results are expressed as the mean ± standard error
of the mean of three separate experiments. Statistical analyses
were performed with GraphPad Prism 5 software for Windows (GraphPad
Software, Inc., San Diego, CA, USA). The data were subjected to
one-way analysis of variance, followed by Dunnett’s multiple
comparison tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
Influence of solvent and extraction
method on the cytotoxic activity of S. miltiorrhiza extracts
The composition and properties of the active
constituents in an extract/fraction of plant material vary in
response to extraction conditions, including the solvent,
extraction time and temperature. Thus, the selection of the
extraction method depends on the physical and chemical
characteristics of the constituents being investigated. In the
present study, cell viability was measured in the presence of an
extreme concentration of each fraction (250 μg/ml), in order to
compare the cytotoxic effects of the fractions. Fractions D (100%
ethanol) and E (100% acetone) exhibited the most notable cytotoxic
effect in all five cell lines compared with fractions A–C (Fig. 1B). Among the three fractions
extracted with aqueous ethanol solvent (fractions A–C), only
fraction A (70% ethanol) produced a moderate reduction in cell
viability; however, this effect was limited to the AGS, HCT116 and
LNCaP cells. In addition fractions B and C, which were extracted
using identical solvents but at different extraction temperatures,
exhibited no significant differences in cytotoxicity.
Effect of ethanolic S. miltiorrhiza
extracts on cell viability
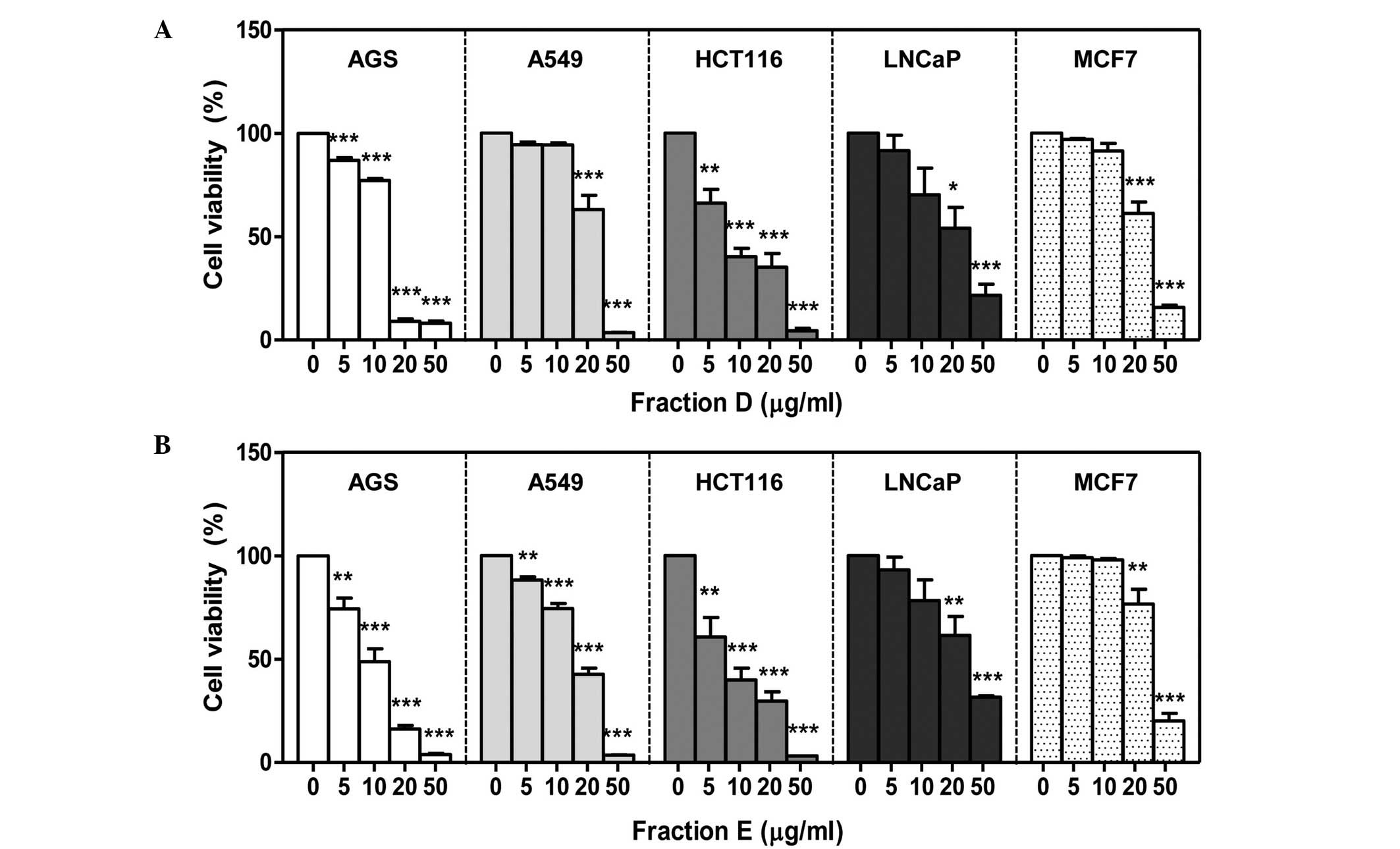
Next, the concentration-dependent effects of the two
most active fractions (D and E) on cancer cell viability were
investigated. No differences were observed in the results of the
MTT assays conducted using concentrations of fractions D and E
ranging from 0 to 250 μg/ml. Thus, 50 μg/ml was the highest
concentration investigated for determining the cytotoxic effects of
fractions D and E (data not shown). Cells were treated with various
concentrations of fractions D or E and reductions of cell viability
were identified using an MTT assay. All five tumor cell lines
exhibited reductions in cell viability following a 24-h treatment
with fractions D or E (Fig. 2).
AGS and HCT116 cells were the most affected by the S.
miltiorrhiza extracts. The IC50 values (inhibitory
concentration that reduces cell viability by 50%) of fractions D
and E in the HCT116 cells were 10.22 and 8.70 μg/ml, respectively
(Table I). Compared with the other
four cell cancer cell lines, LNCaP cells exhibited lower
sensitivity to the cytotoxic effects of fractions D and E. In
addition, although the IC50 values of fractions D and E
in the MCF7 cells were comparable with those in LNCaP cells, a
significant cytotoxic effect was only observed in MCF7 cells at
concentrations of 20 and 50 μg/ml (Table I; Fig.
2).
 | Table ICytotoxic effect of extract fractions
D and E on five human cancer cell lines (IC50,
μg/ml). |
Table I
Cytotoxic effect of extract fractions
D and E on five human cancer cell lines (IC50,
μg/ml).
| Fraction | AGS | A549 | HCT116 | LNCaP | MCF7 |
|---|
| D | 10.44 | 15.72 | 10.22 | 22.65 | 22.15 |
| E | 9.22 | 13.64 | 8.70 | 29.86 | 25.50 |
Confirmation of the presence of the
reference tanshinones in the active fractions of S.
miltiorrhiza
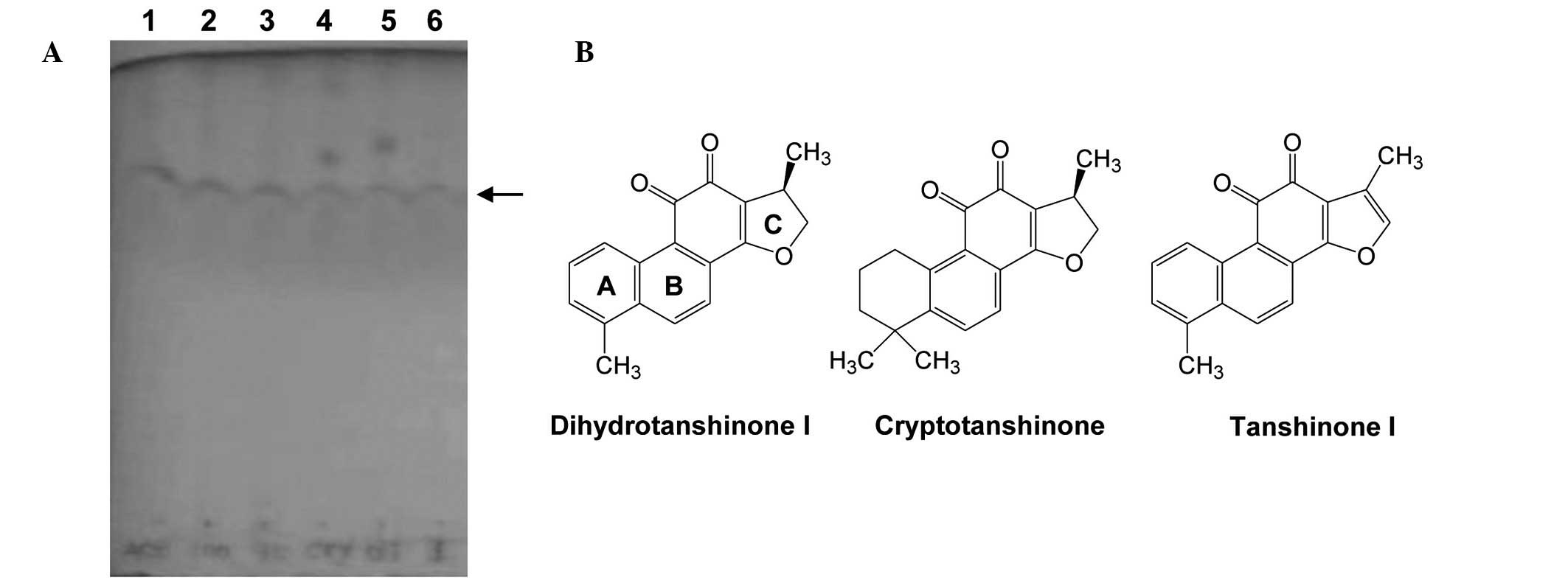
Qiu et al previously reported that the
ethanol extract of S. miltiorrhiza is rich in lipophilic
constituents, including cryptotanshinone, tanshinone I and IIA, and
dihydrotanshinone I (19). The
present study investigated whether the ethanol- and
acetone-extracted fractions of S. miltiorrhiza contained
lipophilic tanshinones that were able to produce a reduction in
cell viability. The presence of tanshinones in the fractions was
initially confirmed by TLC assays, using purified dihydrotanshinone
I, cryptotanshinone and tanshinone I as reference materials.
Fractions D and E presented bands at similar positions (Fig. 3A). Furthermore, it was observed
that the TLC bands of fractions D and E were similar to those of
the reference tanshinones (structures presented in Fig. 3B). These results suggest that
dihydrotanshinone I, cryptotanshinone and tanshinone I may be the
primary constituents of the potent cytotoxic fractions of S.
miltiorrhiza root.
Effects of reference tanshinones on the
viability of human cancer cells
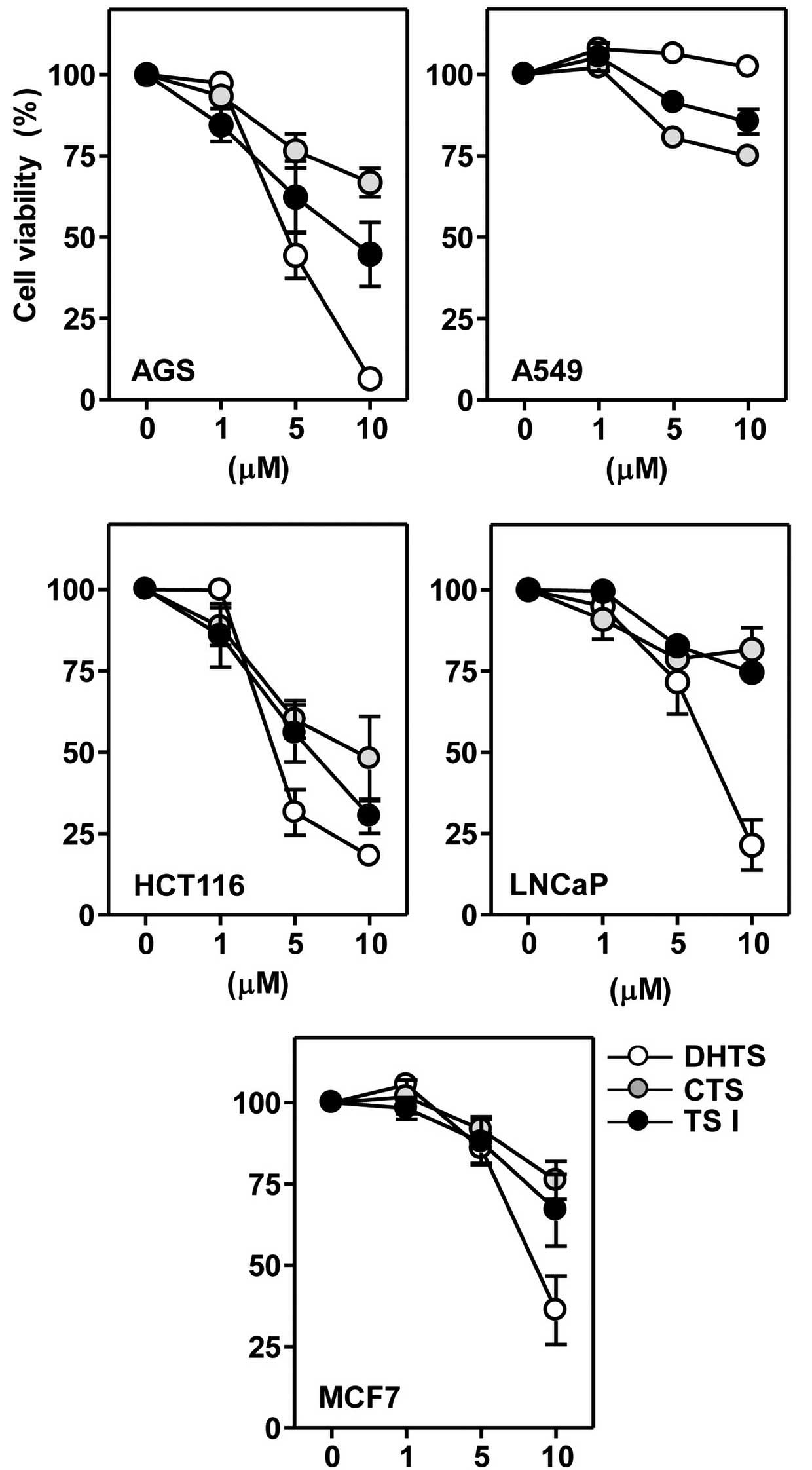
Next, it was determined whether the reference
tanshinones alone were able to affect the viability of cancer
cells. Cells were incubated with 1, 5 or 10 μM concentrations of
the three tanshinones for 24 h. All three of the examined
tanshinones produced reductions in cell viability in a
concentration-dependent manner; however, the extent of these
effects varied (Fig. 4). Table II presents the IC50
values of dihydrotanshinone I, tanshinone I and cryptotanshinone in
various cancer cell lines. The IC50 values of the
tanshinones in A549 cells were not determinable. In all five cancer
cell lines, the IC50 value and cytotoxic potency of the
three tanshinones descended in the following order:
Dihydrotanshinone I > tanshinone I > cryptotanshinone
(Table II). Among the three
tanshinones investigated, dihydrotanshinone I reduced the cell
viability with similar potency in the majority of cancer cell
lines, with the exception of A549.
 | Table IICytotoxic effect of tanshinones from
Salvia miltiorrhiza (Bunge) on five human cancer cell lines
(IC50, μM). |
Table II
Cytotoxic effect of tanshinones from
Salvia miltiorrhiza (Bunge) on five human cancer cell lines
(IC50, μM).
| Reagent | AGS | A549 | HCT116 | LNCaP | MCF7 |
|---|
| DHTS | 3.35 | >10 | 3.87 | 5.29 | 8.02 |
| CTS | >10 | >10 | 8.84 | >10 | >10 |
| TS I | 8.21 | >10 | 5.82 | >10 | >10 |
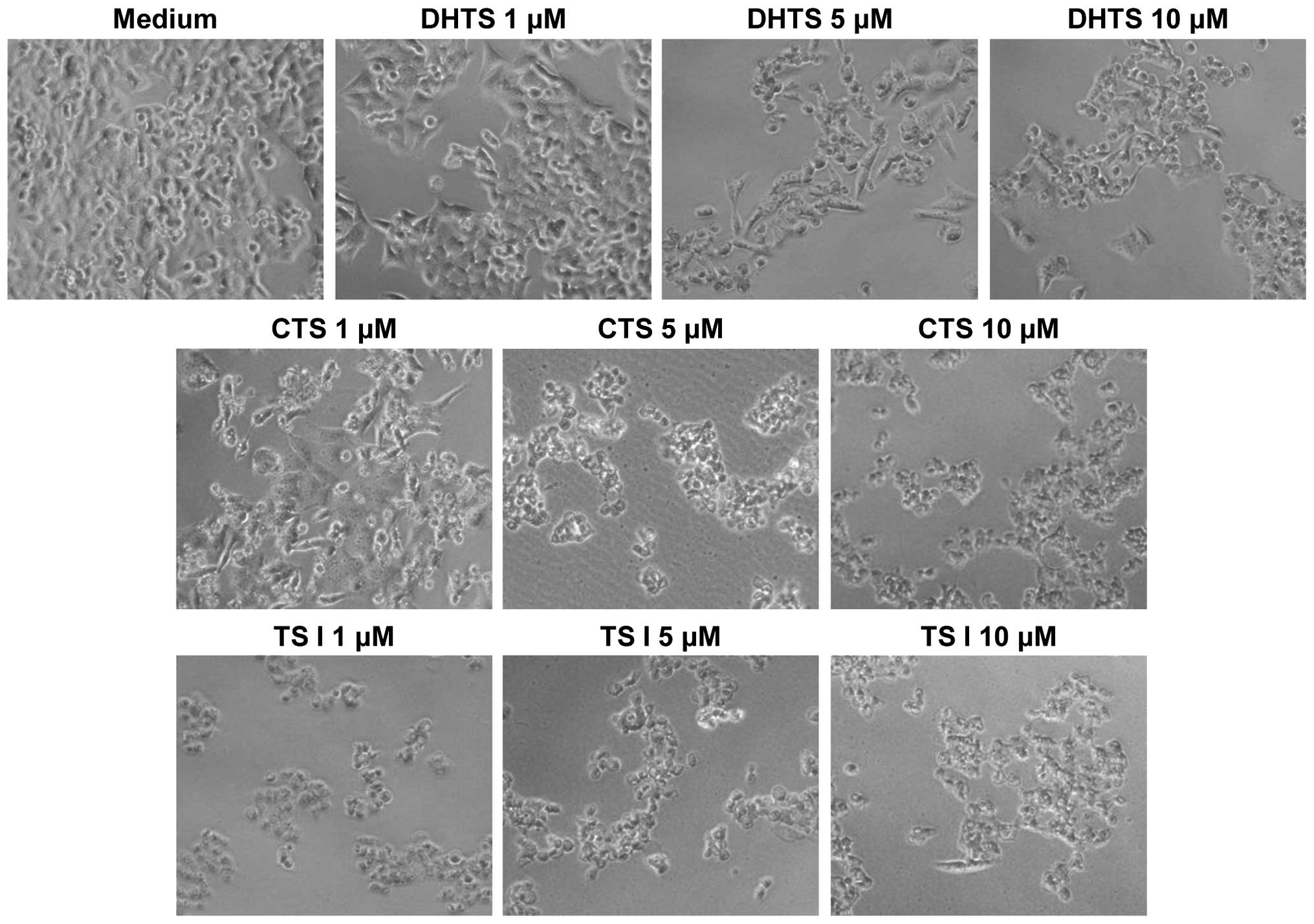
Tanshinone-induced alteration of cellular
morphology
The effects of the reference tanshinones on cellular
morphology were observed directly using an optical microscope.
Untreated HCT116 cells were homogeneously distributed on a cultured
field, exhibiting a uniform polygonal shape (Fig. 5). Following incubation with the
three tanshinones, various morphological changes were observed.
Exposure of the cells to tanshinones transformed the shapes of the
cells from polygonal to circular, and resulted in cell shrinkage.
Furthermore, a reduction in cell number was observed and numerous
floating cells were detected. Notably, these morphological
alterations were observed at tanshinone concentrations as low as 1
μM. Similar modifications in morphology were observed in the AGS,
LNCaP and MCF7 cells following incubation with the tanshinones
(data not shown).
Discussion
The primary objective of the present study was to
determine whether extracts of S. miltiorrhiza obtained with
different solvents differ in their ability to induce cytotoxicity
in five human cancer cell lines. Tanshinones, including
cryptotanshinone, tanshinone I and IIA, and dihydrotanshinone I,
have been reported to be the primary constituents of the ethanol
extract of S. miltiorrhiza, and to exhibit the most marked
cytotoxic effects (19). In
addition, the present study investigated the possibility that these
tanshinones were responsible for the cytotoxic activity of the
ethanol extract of S. miltiorrhiza.
The results of the present study indicate that S.
miltiorrhiza extract, obtained by extraction with 100% ethanol,
effectively inhibits cancer cell growth. Solvent-based extraction
methods may be advantageous compared with other methods of
extracting active components from plant material due to their low
cost and simplicity. Traditionally, numerous biologically active
compounds have been extracted from plant materials using organic
solvents, such as hexane, ether, acetonitrile, benzene and ethanol,
at various dilutions (20,21). However, these solvents may exert
toxic effects in human patients, limiting their therapeutic
potential (22). Thus, the solvent
must be separable from the final extract, particularly if the
product is intended to be used in food applications (21). Ethanol is a typical solvent used
for plant extraction and is safe for human consumption. Ethanol is
considered to be ‘generally recognized as safe’ by the US Food and
Drug Administration designation for food additives, functional
foods and dietary supplements (23). In the Republic of Korea
particularly, ethanol is recommended for the preparation of
food-grade extracts according to ‘Regulation on approval of
functional food ingredients for health functional food’ (24). The results of the present study
suggest that the ethanol extract of S. miltiorrhiza may be
used as a functional food ingredient or as a dietary supplement for
the enhancement of health.
In the present study, fraction D (100% ethanol)
exhibited the most potent cytotoxic activity, comparable to that of
the acetone extract (fraction E; Fig.
1B). It is widely accepted that the specific components
responsible for the anti-cancer activity of S. miltiorrhiza
are diterpenoids with a furano-1,2- or a furano-1,4-naphthoquinone
skeleton, otherwise known as tanshinones. A previous study
demonstrated that the ethanol extract of S. miltiorrhiza
root contained 729.6 μg dihydrotanshinone I, 352.4 μg
cryptotanshinone, 88.95 μg tanshinone I and 649.9 μg tanshinone IIA
per gram of dried root (14). This
may explain why fraction D produced the most notable reduction in
the growth of cancer cells. However, further research is required
in order to identify the chemical compositions of the individual
S. miltiorrhiza extracts.
In the present study, differences in the efficacy of
the extracts obtained using ethanol as a solvent at 30, 70 and
100%, suggested that the concentration of ethanol used affected the
cytotoxic activity of the resulting extract. The 100% ethanol
extract of S. miltiorrhiza exhibited the most marked
cytotoxic effect, followed by 70 and 30%. Previous studies have
reported that the hydrophilic compounds present in S.
miltiorrhiza exhibit anti-cancer activity in a number of tumor
cell types (25–27). However, in the present study, the
30% ethanol extract (fraction B) was unable to induce toxic effects
in any of the five cancer cell lines. It remains unclear why the
30% ethanol extract of S. miltiorrhiza was only minimally
toxic to the cancer cells. The possibilities include the potency or
selectivity of hydrophilic compounds in the extract. For example,
protocatechualdehyde in the aqueous fraction of S.
miltiorrhiza reduced cell growth of HCT116 cells by 21% at 50
μM (28), while the
IC50 values of the tanshinones in the present study were
<10 μM in the same cell lines. The concentration of biologically
active hydrophilic compounds in the 30% ethanol extract is another
possible reason for the lack of observed cytotoxicity. Thus, there
is a requirement for further studies to elucidate the chemical
composition and concentrations of the active constituents of the
ethanol extracts of S. miltiorrhiza.
To date, ~1,700 studies have been published on S.
miltiorrhiza, <200 in association with cancer and more than
half concerning tanshinones. Among the numerous compounds
previously identified in S. miltiorrhiza, tanshinones have
become increasingly studied due to their relatively high abundance
and their ability to exert a cytotoxic effect by inhibiting growth
and inducing apoptosis. In the present study, dihydrotanshinone I
exhibited the most notable cytotoxicity against the human cancer
cell lines, followed by tanshinone I and cryptotanshinone. These
results are consistent with previous reports, which indicated that
dihydrotanshinone I possesses the most marked cytotoxic effect
among the three tanshinones examined in numerous human cancer cell
lines (14,29,30).
As these three tanshinones possess similar chemical structures, the
limited variations in these structures may be responsible for the
differences in their cytotoxic effects. As presented in Fig. 3B, the only structural differences
among the three reference tanshinones are in aromatic rings A and
C. A previous report indicated that the structure of ring A may
contribute to the cytotoxic effect, resulting in the increased
cytotoxic potential of dihydrotanshinone I and tanshinone I
compared with cryptotanshinone, in terms of growth inhibition
(29).
There are a limited number of studies regarding the
effects of the active components of S. miltiorrhiza in
humans. In China, S. miltiorrhiza is used extensively in
various TCM preparations, and ~80,000,000 kg of crude S.
miltiorrhiza extract is consumed as a drug every year (31). The long historical use of this
plant as a TCM indicates that S. miltiorrhiza produces
limited side-effects and may be safe for human consumption. Studies
of the anti-cancer potential of S. miltiorrhiza began in the
early 1990s, although it has been used for >2,000 years for
medicinal purposes (32,33). Studies published in the two
subsequent decades have reported that S. miltiorrhiza
extracts and tanshinones possess broad-range growth inhibitory
activity against various cancer cell lines (34,35).
Furthermore, two reports have indicated that combinations of S.
miltiorrhiza extracts with other medicinal plants modulate
immunological functions in patients (36,37).
Capsules containing S. miltiorrhiza combined with the
mushroom Trametes versicolor (known as yunzhi in China)
appeared to alleviate lymphopenia to patients with nasopharyngeal
carcinoma, when administered orally for 16 weeks during the course
of radiotherapy (36). In patients
with breast cancer, regular oral consumption of these capsules was
observed to promote cell-mediated and humoral immunological
functions, and thus exhibited an anti-cancer effect (37). No serious adverse side-effects were
observed in these studies. Based on these previous studies, we
hypothesize that S. miltiorrhiza may be used safely as an
adjunct treatment. Thus, previous studies suggest that the
consumption of the ethanol extract of S. miltiorrhiza as an
ingredient in functional foods need not be limited to healthy
individuals but may aid cancer patients and enhance the efficacy of
standard cancer therapy.
In conclusion, the present study indicates that the
ethanol extract of S. miltiorrhiza inhibits the growth of
cancer cells. Furthermore, the tanshinone components in this
extract appear to be capable of effectively reducing cancer cell
viability. These results support the use of the ethanol extract of
S. miltiorrhiza as a novel, efficacious and safe candidate
for dietary supplements or as an ingredient in functional foods.
However, future studies, involving clinically relevant animal
models, are required to further elucidate the potential of S.
miltiorrhiza extract as a therapeutic agent.
Acknowledgements
The present study was supported by the 2014 Post-Doc
Development Program of Pusan National University (Busan, South
Korea). The authors thank Aging Tissue Bank (Busan, South Korea)
for providing research materials. The present study was also
supported by the Research and Development Programme of the Ministry
of Trade, Industry and Energy (MOTIE)/Korea Institute for
Advancement of Technology (no. N0000697; Establishment of
Infrastructure for Anti-Aging Industry Support) and the Research
and Development Programme of the MOTIE/Korea Evaluation Institute
of Industry Technology (no. 10040391; Development of Functional
Food Materials and Devices for the Prevention of Aging-associated
Muscle Function Decrease).
References
|
1
|
Cheng TO: Cardiovascular effects of
Danshen. Int J Cardiol. 121:9–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wasser S, Ho JM, Ang HK and Tan CE: Salvia
miltiorrhiza reduces experimentally-induced hepatic fibrosis in
rats. J Hepatol. 29:760–771. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ahn YM, Kim SK, Lee SH, et al:
Renoprotective effect of Tanshinone IIA, an active component of
Salvia miltiorrhiza, on rats with chronic kidney disease. Phytother
Res. 24:1886–1892. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wen XD, Wang CZ, Yu C, et al: Salvia
miltiorrhiza (dan shen) significantly ameliorates colon
inflammation in dextran sulfate sodium induced colitis. Am J Chin
Med. 41:1097–1108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li YG, Song L, Liu M, Hu ZB and Wang ZT:
Advancement in analysis of Salviae miltiorrhizae Radix et Rhizoma
(Danshen). J Chromatogr A. 1216:1941–1953. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tao S, Zheng Y, Lau A, et al: Tanshinone I
activates the Nrf2-dependent antioxidant response and protects
against As(III)-induced lung inflammation in vitro and in vivo.
Antioxid Redox Signal. 19:1647–1661. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park EJ, Zhao YZ, Kim YC and Sohn DH:
Preventive effects of a purified extract isolated from Salvia
miltiorrhiza enriched with tanshinone I, tanshinone IIA and
cryptotanshinone on hepatocyte injury in vitro and in vivo. Food
Chem Toxicol. 47:2742–2748. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tao S, Justiniano R, Zhang DD and Wondrak
GT: The Nrf2-inducers tanshinone I and dihydrotanshinone protect
human skin cells and reconstructed human skin against solar
simulated UV. Redox Biol. 1:532–541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mahesh R, Jung HW, Kim GW, Kim YS and Park
YK: Cryptotanshinone from Salviae miltiorrhizae radix inhibits
sodium-nitroprusside-induced apoptosis in neuro-2a cells. Phytother
Res. 26:1211–1219. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jeon SJ, Son KH, Kim YS, Choi YH and Kim
HP: Inhibition of prostaglandin and nitric oxide production in
lipopolysaccharide-treated RAW 264.7 cells by tanshinones from the
roots of Salvia miltiorrhiza bunge. Arch Pharm Res. 31:758–763.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Trinh HT, Chae SJ, Joh EH, Son KH, Jeon SJ
and Kim DH: Tanshinones isolated from the rhizome of Salvia
miltiorrhiza inhibit passive cutaneous anaphylaxis reaction in
mice. J Ethnopharmacol. 132:344–348. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X, Morris-Natschke SL and Lee KH: New
developments in the chemistry and biology of the bioactive
constituents of Tanshen. Med Res Rev. 27:133–148. 2007. View Article : Google Scholar
|
|
13
|
Zhang Y, Jiang P, Ye M, Kim SH, Jiang C
and Lu J: Tanshinones: sources, pharmacokinetics and anti-cancer
activities. Int J Mol Sci. 13:13621–13666. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee WY, Chiu LC and Yeung JH: Cytotoxicity
of major tanshinones isolated from Danshen (Salvia miltiorrhiza) on
HepG2 cells in relation to glutathione perturbation. Food Chem
Toxicol. 46:328–338. 2008. View Article : Google Scholar
|
|
15
|
Lee JH, Shin YJ, Kim HJ, Oh JH, Jang YP
and Lee YJ: Danshen extract does not alter pharmacokinetics of
docetaxel and clopidogrel, reflecting its negligible potential in
P-glycoprotein- and cytochrome P4503A-mediated herb-drug
interactions. Int J Pharm. 410:68–74. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao Y, Hao Y, Ji H, et al: Combination
effects of salvianolic acid B with low-dose celecoxib on inhibition
of head and neck squamous cell carcinoma growth in vitro and in
vivo. Cancer Prev Res (Phila). 3:787–796. 2010. View Article : Google Scholar
|
|
17
|
Franek KJ, Zhou Z, Zhang WD and Chen WY:
In vitro studies of baicalin alone or in combination with Salvia
miltiorrhiza extract as a potential anti-cancer agent. Int J Oncol.
26:217–224. 2005.
|
|
18
|
Wang SX, Hunter W and Plant A: Isolation
and purification of functional total RNA from woody branches and
needles of Sitka and white spruce. Biotechniques. 28:292–296.
2000.PubMed/NCBI
|
|
19
|
Qiu F, Jiang J, Ma Y, et al: Opposite
effects of single-dose and multidose administration of the ethanol
extract of Danshen on CYP3A in healthy volunteers. Evid Based
Complement Alternat Med. 2013:7307342013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Plaza M, Santoyo S, Jaime L, et al:
Screening for bioactive compounds from algae. J Pharm Biomed Anal.
51:450–455. 2010. View Article : Google Scholar
|
|
21
|
Starmans DAJ and Nijhuis HH: Extraction of
secondary metabolites from plant material: A review. Trends Food
Sci Techol. 7:191–197. 1996. View Article : Google Scholar
|
|
22
|
Li BB, Smith B and Hossain MM: Extraction
of phenolics from citrus peels I. Solvent extraction method. Sep
Purif Technol. 48:182–188. 2006. View Article : Google Scholar
|
|
23
|
United States Food and Drug
Administration. Generally Recognized as Safe (GRAS). http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/uri.
Accessed October 5, 2014
|
|
24
|
Korean Food and Drug Administration.
Regulation on approval of functional food ingredients for health
functional food. http://www.foodnara.go.kr/hfoodi/industry/main/sub.jsp?Mode=view&boardID=s_0502_bbs&num=96&tpage=3&keyfield=&key=&bCate=uri.
Accessed October 5, 2014
|
|
25
|
Chang JY, Chang CY, Kuo CC, Chen LT, Wein
YS and Kuo YH: Salvinal, a novel microtubule inhibitor isolated
from Salvia miltiorrhizae Bunge (Danshen), with antimitotic
activity in multidrug-sensitive and -resistant human tumor cells.
Mol Pharmacol. 65:77–84. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bi L, Chen J, Yuan X, Jiang Z and Chen W:
Salvianolic acid A positively regulates PTEN protein level and
inhibits growth of A549 lung cancer cells. Biomed Rep. 1:213–217.
2013.
|
|
27
|
Yang Y, Ge PJ, Jiang L, Li FL and Zhu QY:
Modulation of growth and angiogenic potential of oral squamous
carcinoma cells in vitro using salvianolic acid B. BMC Complement
Altern Med. 11:542011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jeong JB and Lee SH: Protocatechualdehyde
possesses anti-cancer activity through downregulating cyclin D1 and
HDAC2 in human colorectal cancer cells. Biochem Biophys Res Commun.
430:381–386. 2013. View Article : Google Scholar
|
|
29
|
Li H, Zhang Q, Chu T, et al:
Growth-inhibitory and apoptosis-inducing effects of tanshinones on
hematological malignancy cells and their structure-activity
relationship. Anticancer Drugs. 23:846–855. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee WY, Cheung CC, Liu KW, Fung KP, Wong
J, Lai PB and Yeung JH: Cytotoxic effects of tanshinones from
Salvia miltiorrhiza on doxorubicin-resistant human liver cancer
cells. J Nat Prod. 73:854–859. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu W, Zhu Y, Zhang L, Yang R and Zhou Y:
Extraction, preliminary structural characterization, and
antioxidant activities of polysaccharides from Salvia miltiorrhiza
Bunge. Carbohydr Polym. 87:1348–1353. 2012. View Article : Google Scholar
|
|
32
|
Zhou L, Zuo Z and Chow MS: Danshen: an
overview of its chemistry, pharmacology, pharmacokinetics, and
clinical use. J Clin Pharmacol. 45:1345–1359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu WL, Chang WL and Chen CF: Cytotoxic
activities of tanshinones against human carcinoma cell lines. Am J
Chin Med. 19:207–216. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gong Y, Li Y, Lu Y, et al: Bioactive
tanshinones in Salvia miltiorrhiza inhibit the growth of prostate
cancer cells in vitro and in mice. Int J Cancer. 129:1042–1052.
2011. View Article : Google Scholar :
|
|
35
|
Liu L, Jia J, Zeng G, et al: Studies on
immunoregulatory and anti-tumor activities of a polysaccharide from
Salvia miltiorrhiza Bunge. Carbohydr Polym. 92:479–483. 2013.
View Article : Google Scholar
|
|
36
|
Bao YX, Wong CK, Leung SF, et al: Clinical
studies of immunomodulatory activities of Yunzhi-Danshen in
patients with nasopharyngeal carcinoma. J Altern Complement Med.
12:771–776. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wong CK, Bao YX, Wong EL, Leung PC, Fung
KP and Lam CW: Immunomodulatory activities of Yunzhi and Danshen in
post-treatment breast cancer patients. Am J Chin Med. 33:381–395.
2005. View Article : Google Scholar : PubMed/NCBI
|