Introduction
Ulcerative colitis (UC) is a relapsing and remitting
disease characterized by acute non-infectious inflammation of the
colorectal mucosa. The rectal mucosa is invariably affected. The
incidence of UC is between 1.2 and 20.3 per 100,000 individuals per
year, and its prevalence is between 7.6 and 246.0 per 100,000
individuals per year (1). Although
the etiology of UC has yet to be fully elucidated, it has been
suggested that environmental factors, such as gut microbiota,
stimulate the inappropriate activation of mucosal immunity, thus
leading to inflammation (2).
Intestinal homeostasis is dependent on a controlled innate immune
response to the microbiota, which are recognized by Toll-like
receptors (TLRs) on epithelial and immune cells (3).
TLRs comprise an important family of type I
transmembrane receptors that allow immune cells to recognize
pathogens and trigger inflammatory responses, and are expressed not
only in a variety of immune cells but also in non-immune cells,
such as fibroblasts and epithelial cells (4). TLRs are constitutively or inducibly
expressed by a number of different cell types in the
gastrointestinal tract, including intestinal epithelial cells and
monocytes/macrophages, dendritic cells of the lamina propria and
myofibroblasts, endothelial cells and adipocytes of the intestinal
submucosa. When genetic predisposition or environmental stimuli
impair mucosal or commensal homeostasis, certain intestinal
diseases, such as Crohn’s disease (CD), may develop (4–6).
Aberrant TLR signaling can induce tissue damage and
barrier destruction through the overproduction of cytokines and
chemokines and the loss of the commensal-mediated responses of
colonic epithelial progenitors (7); however, although previous studies
(8–10) have investigated the role of TLRs in
inflammatory bowel disease (IBD), the expression of TLRs and their
role in microbial recognition in innate immunity have not, to the
best of our knowledge, been extensively evaluated in the colonic
mucosa of patients with UC. The aim of the present study was to
determine the alterations in the expression of the main conductors
of the innate immune response, including TLR1, TLR2, TLR3, TLR4 and
TLR9, in the colonic mucosa of patients with UC and normal
controls. The protein and mRNA levels of TLRs 1–4 and TLR9 were
evaluated by immunohistochemical techniques and reverse
transcription-quantitative polymerase chain reaction analysis,
respectively.
Materials and methods
Clinical samples
Following the provision of informed consent,
unrelated patients with UC (n=30) were recruited from the
Outpatient Clinic at the Department of Gastroenterology of The
Second Affiliated Hospital of Harbin Medical University (Harbin,
China). The patients all fulfilled the diagnostic requirements of
UC according to the Chinese Medical Association criteria. Unrelated
healthy individuals with a normal colonoscopy (n=30) were randomly
recruited from the same hospital. Two colonic biopsy specimens were
collected from each participant for routine histological
examination. One sample was fixed in 10% formalin for
immunohistochemistry. The other sample was immediately frozen in
liquid nitrogen and stored at −80°C for later RNA extraction.
All subjects were of Chinese Han descent, and the
patients and controls were matched for age and gender. This study
was approved by the Ethical Review Committee of Research in Harbin
Medical University.
Immunohistochemistry
Samples fixed in 10% formalin were subsequently
embedded in paraffin, and sections of 4-mm thickness were cut from
the formalin-fixed samples. The sectioned tissue was deparaffinized
in xylene and then rehydrated in a graded ethyl alcohol series. For
increased specificity and sensitivity, tissues were microwaved for
10 min for antigen retrieval. Following cooling and rinsing in
distilled water, endogenous peroxide activity was blocked with 3%
H2O2 for 10 min, and the samples were then
rinsed in 0.01 mol/l phosphate-buffered saline (PBS, pH 7.4) for 10
min. The sections were subsequently preincubated with a protein
blocking solution (ZSGB-BIO, Beijing, China) for 10 min, prior to
incubation with the primary polyclonal antibodies against TLR1
(cat. no. sc-30000; rabbit polyclonal), TLR2 (cat. no. sc-10739;
rabbit polyclonal), TLR3 (cat. no. sc-32232; mose monoclonal), TLR4
(cat. no. sc-10741; rabbit polyclonal) and TLR9 (cat. no. sc-25468;
rabbit polyclonal) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) at dilutions of 1:300, 1:70, 1:300, 1:100 and 1:50,
respectively, at 4°C overnight in a humid chamber. The slides were
then washed three times in PBS and incubated with secondary
biotinylated antibody (ZSGB-BIO) for 15 min at room temperature.
The streptavidin-peroxidase method (ZSGB-BIO)was used to detect the
antigen-antibody complexes, and diaminobenzidine (DAB) was used as
the chromogen substrate. Peroxidase signals were visualized
following 3 min of treatment with a DAB substrate-chromogen system
(ZSGB-BIO). Finally, the sections were stained lightly with
hematoxylin. For the negative control, PBS was used in place of the
primary antibody. All sections were coded and independently
examined by two investigators. The staining intensity was scored as
negative or weak (−), moderate (+), strong (++) or very strong
(+++).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The expression rates of the TLR genes were analyzed
through RT-qPCR analysis. Total RNA was extracted from fresh frozen
tissue using TRIzol™ reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA) in accordance with the manufacturer’s
instructions. Total RNA (1 μg) was subjected to reverse
transcription using a Reverse Transcription system (Promega Biotech
Co., Ltd., Beijing, China), according to the manufacturer’s
instructions. All primers (Sangon Biotech Co., Ltd., Shanghai,
China) used in this study are shown in Table I. For the qPCR, 2 μl cDNA, 1 μl
primers and 10 μl qPCR iQ™ SYBR® Green supermix
(Bio-Rad, Hercules, CA, USA) was mixed to obtain a final volume of
20 μl. The reaction was followed by 40 cycles at 95°C for 30 sec,
60°C for 30 sec and 72°C for 30 sec. All reactions were performed
in duplicate. Amplification of the expected single products was
confirmed by dynamic melting curves and by electrophoresis on 1.5%
agarose gels stained with ethidium bromide. Fluorescence data were
automatically collected and analyzed by iCycler iQ Optical Software
(version 3.0a; Bio-Rad). The expression of TLRs 1–4 and TLR9 was
examined and normalized to a constitutive gene (GAPDH), and
relative induction was calculated using the 2(−ΔΔCt)
method (11).
 | Table IQuantitative polymerase chain reaction
primers used for the detection of TLRs. |
Table I
Quantitative polymerase chain reaction
primers used for the detection of TLRs.
| Gene | Primer sequence
(5′-3′) | Amplicon size
(bp) |
|---|
| TLR1 | | 102 |
| Sense |
AGGTCTTGCTGGTCTTAGGAGA | |
| Antisense |
TGTTTGTGGGGAACACAATGTG | |
| TLR2 | | 160 |
| Sense |
TACTTTGTGGATGGTGTGGGTC | |
| Antisense |
GCTTTTTACAGCTTCTGTGAGC | |
| TLR3 | | 196 |
| Sense |
AACTCAGAAGATTACCAGCCGC | |
| Antisense |
TCAGTCAAATTCGTGCAGAAGG | |
| TLR4 | | 110 |
| Sense |
TCCATTTCAGCTCTGCCTTCAC | |
| Antisense |
ACACCACAACAATCACCTTTCG | |
| TLR9 | | 154 |
| Sense |
CTGCGACCACGCTCCCAACCCC | |
| Antisense |
TCCCAGCCCACGGAACCAACTG | |
| GAPDH | | 213 |
| Sense |
AAGAAGGTGGTGAAGCAGGC | |
| Antisense |
TCCACCACCCAGTTGCTGTA | |
Statistical analysis
All data are presented as the mean ± standard
deviation and were analyzed using SPSS 16.0 statistical software
(SPSS, Inc., Chicago, IL, USA). Statistical differences between
groups were analyzed with non-parametric methods (Mann-Whitney
U-test for unpaired data). The χ2 test was used to
estimate mRNA values. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of TLR proteins in mucosal
biopsies
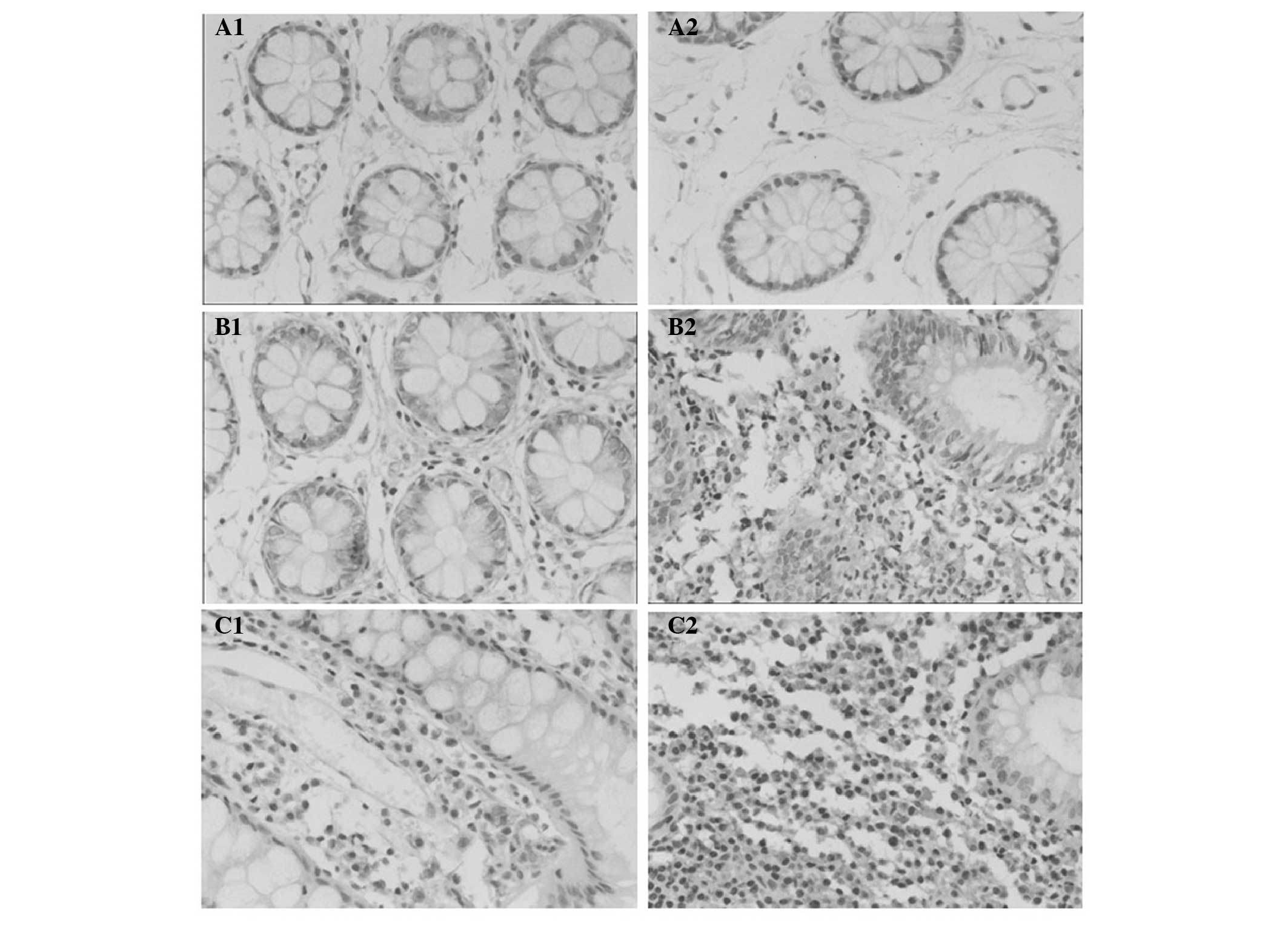
Immunohistochemical assessment for TLRs in the
mucosal biopsies was performed using anti-TLR antibodies.
Representative staining patterns for the TLRs are shown in Fig. 1. Positive staining for TLRs 1–4 and
TLR9 were generally observed in the cytoplasm of epithelial cells,
and TLR3 was also expressed on the cell membrane. In the stromal
non-epithelial tissue, a weak positive reaction was additionally
occasionally observed in the endothelium of the small vessels. The
expression of TLR2, TLR4 and TLR9 was significantly stronger in the
cytoplasm of epithelial cells from the patients with UC than those
from the normal controls (Table
II). The differences in the expression levels of TLR1 and TLR3
in the colonic mucosa between patients with UC and normal controls
were not statistically significant (Table II).
 | Table IITLR protein detection by
immunohistochemistry. |
Table II
TLR protein detection by
immunohistochemistry.
| Index of staining
pattern | | |
|---|
|
| | |
|---|
| Protein | Negative/weak
(−) | Moderate (+) | Strong (++) | Very strong
(+++) | Mean rank | P-value |
|---|
| TLR1 | | | | | | 0.076 |
| Control | 2 | 9 | 18 | 1 | 34.95 | |
| UC | 0 | 19 | 11 | 0 | 26.95 | |
| TLR2 | | | | | | 0.025 |
| Control | 4 | 11 | 15 | 0 | 25.85 | |
| UC | 2 | 9 | 16 | 3 | 35.15 | |
| TLR3 | | | | | | 0.205 |
| Control | 0 | 10 | 18 | 2 | 33.03 | |
| UC | 2 | 12 | 15 | 1 | 27.97 | |
| TLR4 | | | | | | 0.030 |
| Control | 2 | 12 | 15 | 1 | 26.12 | |
| UC | 0 | 9 | 19 | 2 | 34.88 | |
| TLR9 | | | | | | 0.031 |
| Control | 6 | 18 | 6 | 0 | 26.10 | |
| UC | 3 | 13 | 14 | 0 | 34.90 | |
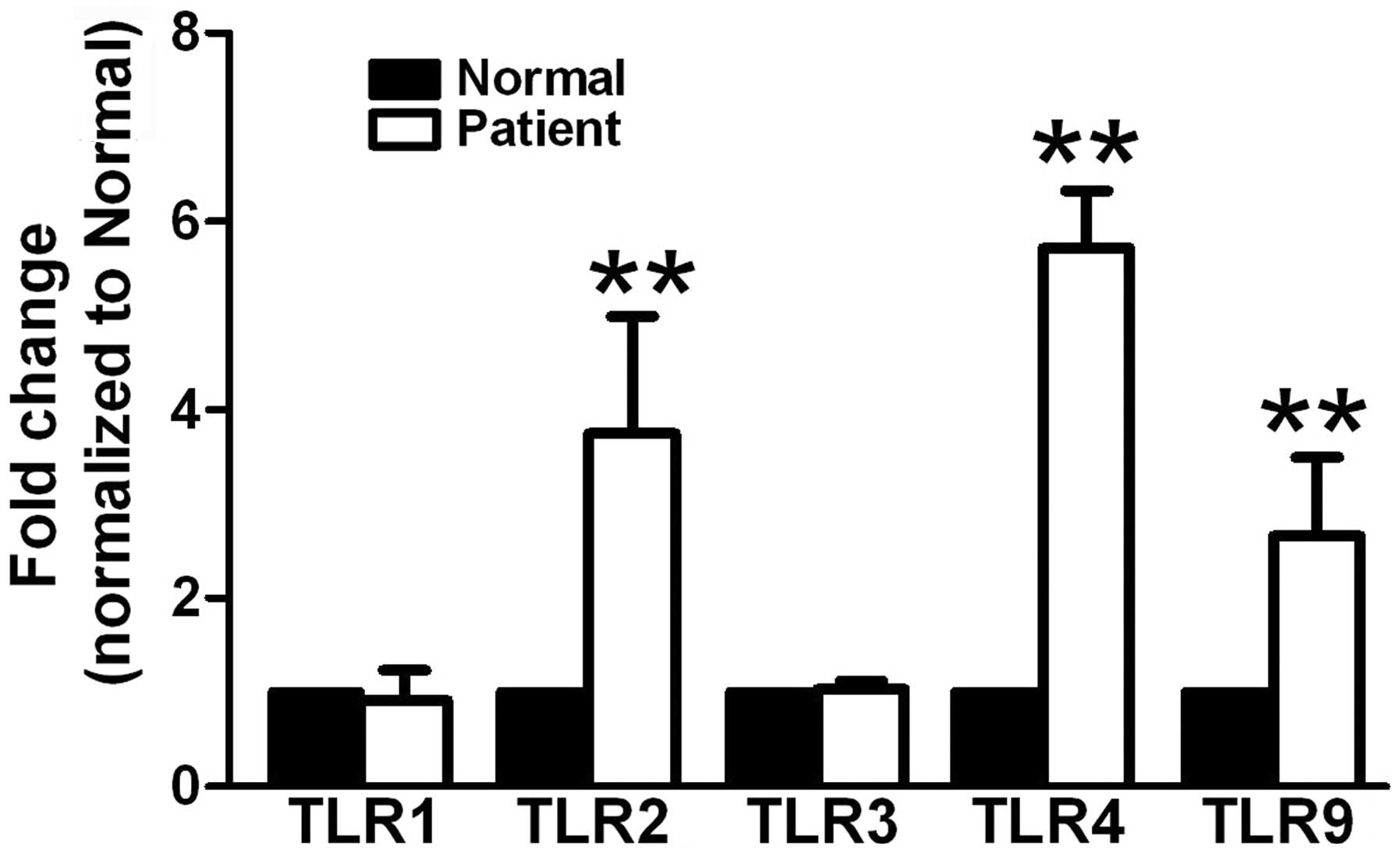
Levels of TLR mRNA in mucosal biopsies of
patients with UC and normal controls
Similar to the results for protein levels, the mRNA
expression levels of TLR2, TLR4 and TLR9, but not TLR1 and TLR3,
were significantly higher in the patients with UC than those in the
normal controls (Fig. 2).
Discussion
UC comprises a group of chronic inflammatory
disorders involving the mucosa of the colon. Despite a lack of
extensive investigation into the role of the adaptive immune
response in UC, evidence suggests an association between innate
immunity and the pathology of the disease (12). The findings of numerous studies
have indicated that the surface epithelium plays a critical role as
the front line of the mucosal innate immune system in the
gastrointestinal tract (13–15).
The dysregulation of innate and adaptive intestinal
immune responses to bacterial microbiota is believed to be the
hallmark of IBD pathogenesis. In the present study it has been
demonstrated that intestinal epithelial cells constitutively
express several functional TLRs, which recognize a variety of
distinct microbial components. These TLRs not only play a key role
in microbial recognition in innate immunity but also participate in
the activation of adaptive immune responses (16). Following the recognition of
pathogen-associated molecular patterns by TLRs, signal transduction
is initiated. This signaling pathway results in the activation of a
number of transcription factors, including activator protein 1,
nuclear factor-κB, ETS domain-containing protein Elk-1, cyclic
adenosine monophosphate-response element-binding protein and signal
transducers and activators of transcription. As a result of the
activation of the TLRs, major histocompatibility complex and
costimulatory molecules are upregulated and proinflammatory
cytokines and chemokines are expressed (17–19).
In the gastrointestinal tract, the polarized
expression of TLRs in apical and basolateral compartments has been
suggested to be a key factor underlying the discrimination between
pathogenic bacteria invading the epithelium and nonpathogenic
microbes facing the luminal surface (20–22).
TLR ligation on the epithelial cells of the intestine by bacterial
products induces epithelial cell proliferation, the secretion of
immunoglobulin A into the gut and the expression of antimicrobial
peptides (23).
In the present study, it was found that the gene
expression of TLR2 and TLR4 was significantly upregulated at the
mRNA and protein levels in the inflamed mucosa as compared with the
normal controls. By contrast, TLR1 and TLR3 levels were not found
to be significantly altered in the patients with UC. These results
corroborate those previously reported for TLR2 and TLR4 gene
expression in IBD (5,6,17–19).
Using immunofluorescence histochemistry, Cario and Podolsky
(17) found that TLR3 expression
was significantly downregulated in intestinal epithelial cells in
active CD but not in UC. By contrast, significant upregulation of
TLR4 was found in both UC and CD (17). In a study by Hausmann et al
(18), the induction of TLR2, TLR4
and TLR5 mRNA was observed in inflammation-stimulated macrophages
in the colonic mucosa of patients with IBD.
In the present study it was additionally found that
TLR9 gene expression was upregulated in the mucosa of patients with
UC as compared with the healthy controls, although in a previous
study it was reported that TLR9 expression was downregulated in
inflamed colonic mucosa from patients with IBD (24). In normal circumstances, TLR9
combines with unmethylated CpG motifs of the pathogen during
bacterial or viral infection, thus stimulating the maturation and
activation of dendritic cells and B cells and the elimination of
pathogens by the secretion of cytokines and specific antibodies
(25). In a study by Hall et
al (26) it was reported that,
in response to commensal bacterial DNA, dendritic cells signaled
via TLR9 to inhibit the differentiation of regulatory T cells in
the gut. The activation of TLR9 has been shown to result in the
maturation of dendritic cells and the release of type 1 T-helper
cell cytokines, such as interleukin (IL)-6, IL-12, IL-10 and tumor
necrosis factor-α (27–29). Consistent with the present results,
previous studies have analyzed TLR9 expression in cells (30,31),
and found little TLR9 protein expression in isolated colonic
epithelial cells, as assessed by western blot analysis. Gut
inflammation with leukocyte infiltration may therefore play a role
in the upregulation of TLR9 expression in the colonic mucosa of
patients with UC.
In conclusion, the results of the present study have
shown overexpression of TLR2, TLR4 and TLR9 in the colonic mucosa
of patients with UC, which may be important in the biological
pathogenesis of UC. The purpose of this receptor upregulation may
be to increase the antigenic stimulation of inflammatory and immune
pathways activated by ligand binding. Further studies are necessary
to provide an enhanced understanding of the function of these TLRs
in the mucosa from patients with UC.
Acknowledgements
This study was supported by the Natural Science
Foundation of Heilongjiang Province (no. D201178).
References
|
1
|
Loftus EV Jr: Clinical epidemiology of
inflammatory bowel disease: Incidence, prevalence, and
environmental influences. Gastroenterology. 126:1504–1517. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xavier RJ and Podolsky DK: Unravelling the
pathogenesis of inflammatory bowel disease. Nature. 448:427–434.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abreu MT: Toll-like receptor signalling in
the intestinal epithelium: how bacterial recognition shapes
intestinal function. Nat Rev Immunol. 10:131–144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Akira S and Takeda K: Toll-like receptor
signalling. Nat Rev Immunol. 4:499–511. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Szebeni B, Veres G, Dezsofi A, Rusai K,
Vannay A, Bokodi G, Vásárhelyi B, Korponay-Szabó IR, Tulassay T and
Arató A: Increased mucosal expression of Toll-like receptor (TLR)2
and TLR4 in coeliac disease. J Pediatr Gastroenterol Nutr.
45:187–193. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Frolova L, Drastich P, Rossmann P,
Klimesova K and Tlaskalova-Hogenova H: Expression of Toll-like
receptor 2 (TLR2), TLR4, and CD14 in biopsy samples of patients
with inflammatory bowel diseases: upregulated expression of TLR2 in
terminal ileum of patients with ulcerative colitis. J Histochem
Cytochem. 56:267–274. 2008. View Article : Google Scholar
|
|
7
|
Chen LW, Chang WJ, Chen PH, Liu WC and Hsu
CM: TLR ligand decreases mesenteric ishcemia and reperfusion
injury-induced gut damage through TNF-alpha signaling. Shock.
30:563–570. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berkowitz D, Peri R, Lavy A and Kessel A:
Increased Toll-like receptor 9 expression by B cells from
inflammatory bowel disease patients. Hum Immunol. 74:1519–1523.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Erridge C, Duncan SH, Bereswill S and
Heimesaat MM: The induction of colitis and ileitis in mice is
associated with marked increases in intestinal concentrations of
stimulants of TLRs 2, 4, and 5. PLoS One. 5:e91252010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Paiva NM, Ayrizono ML, Milanski M,
Coope A, Oliveira LM, Fagundes JJ, Velloso LA, Coy CS and Leal RF:
Differential expression of TLR2, TLR4 and JNK in mucosa of ileal
pouches for ulcerative colitis. Is there a role for bacterial
antigen pathway in asymptomatic patients? Int J Clin Exp Med.
4:179–186. 2011.PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
12
|
Geremia A, Biancheri P, Allan P, Corazza
GR and Sabatino A: Innate and adaptive immunity in inflammatory
bowel disease. Autoimmun Rev. 13:3–10. 2014. View Article : Google Scholar
|
|
13
|
Mayer L, Eisenhardt D, Salomon P, Bauer W,
Plous R and Piccinini L: Expression of class II molecules on
intestinal epithelial cells in humans. Gastroenterology. 100:3–12.
1991.PubMed/NCBI
|
|
14
|
Gomez CR, Boehmer ED and Kovacs EJ: The
aging innate immune system. Curr Opin Immunol. 17:457–462. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lavelle EC, Murphy C, O’Neill LA and
Creagh EM: The role of TLRs, NLRs, and RLRs in mucosal innate
immunity and homeostasis. Mucosal Immunol. 3:17–28. 2010.
View Article : Google Scholar
|
|
16
|
Cario E, Rosenberg IM, Brandwein SL, Beck
PL, Reinecker HC and Podolsky DK: Lipopoly-saccharide activates
distinct signaling pathways in intestinal epithelial cell lines
expressing Toll-like receptors. J Immunol. 164:966–972. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cario E and Podolsky DK: Differential
alteration in intestinal epithelial cell expression of toll-like
receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect
Immun. 68:7010–7017. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hausmann M, Kiessling S, Mestermann S,
Webb G, Spöttl T, Andus T, Schölmerich J, Herfarth H, Ray K, Falk W
and Rogler G: Toll-like receptors 2 and 4 are up-regulated during
intestinal inflammation. Gastroenterology. 122:1987–2000. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Toiyama Y, Araki T, Yoshiyama S, Hiro J,
Miki C and Kusunoki M: The expression patterns of Toll-like
receptors in the ileal pouch mucosa of postoperative ulcerative
colitis patients. Surg Today. 36:287–290. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gewirtz AT, Navas TA, Lyons S, Godowski PJ
and Madara JL: Cutting edge: bacterial flagellin activates
basolaterally expressed TLR5 to induce epithelial proinflammatory
gene expression. J Immunol. 167:1882–1885. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gewirtz AT, Simon PO Jr, Schmitt CK,
Taylor LJ, Hagedorn CH, O’Brien AD, Neish AS and Madara JL:
Salmonella typhimurium translocates flagellin across intestinal
epithelia, inducing a proinflammatory response. J Clin Invest.
107:99–109. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xavier RJ and Podolsky DK: Microbiology.
How to get along - friendly microbes in a hostile world. Science.
289:1483–1484. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shaykhiev R, Behr J and Bals R: Microbial
patterns signaling via Toll-like receptors 2 and 5 contribute to
epithelial repair, growth and survival. PLoS One. 3:e13932008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pedersen G, Andresen L, Matthiessen MW,
Rask-Madsen J and Brynskov J: Expression of Toll-like receptor 9
and response to bacterial CpG oligodeoxynucleotides in human
intestinal epithelium. Clin Exp Immunol. 141:298–306. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Asselin-Paturel C and Trinchieri G:
Production of type I interferons: plasmacytoid dendritic cells and
beyond. J Exp Med. 202:461–465. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hall JA, Bouladoux N, Sun CM, Wohlfert EA,
Blank RB, Zhu Q, Grigg ME, Berzofsky JA and Belkaid Y: Commensal
DNA limits regulatory T cell conversion and is a natural adjuvant
of intestinal immune responses. Immunity. 29:637–649. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee J, Rachmilewitz D and Raz E:
Homeostatic effects of TLR9 signaling in experimental colitis. Ann
NY Acad Sci. 1072:351–355. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ewaschuk JB, Backer JL, Churchill TA,
Obermeier F, Krause DO and Madsen KL: Surface expression of
Toll-like receptor 9 is upregulated on intestinal epithelial cells
in response to pathogenic bacterial DNA. Infect Immun.
75:2572–2579. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ghadimi D, Vrese M, Heller KJ and
Schrezenmeir J: Effect of natural commensal-origin DNA on toll-like
receptor 9 (TLR9) signaling cascade, chemokine IL-8 expression, and
barrier integritiy of polarized intestinal epithelial cells.
Inflamm Bowel Dis. 16:410–427. 2010. View Article : Google Scholar
|
|
30
|
Lee J, Mo JH, Katakura K, Alkalay I,
Rucker AN, Liu YT, Lee HK, Shen C, Cojocaru G, Shenouda S, Kagnoff
M, Eckmann L, Ben-Neriah Y and Raz E: Maintenance of colonic
homeostasis by distinctive apical TLR9 signalling in intestinal
epithelial cells. Nat Cell Biol. 8:1327–1336. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Akhtar M, Watson JL, Nazli A and McKay DM:
Bacterial DNA evokes epithelial IL-8 production by a
MAPK-dependent, NF-kappaB-independent pathway. FASEB J.
17:1319–1321. 2003.PubMed/NCBI
|