Introduction
Despite treatment with effective antibiotics,
Streptococcus pneumoniae (SP) meningitis remains a serious
infectious disease of the central nervous system (CNS) with
mortality rates between 16 and 37% (1) and neurological sequelae in up to 30% of
survivors (2). Therefore, further
study of the pathological mechanisms of SP meningitis and the
identification of new means of intervention has a great clinical
significance. SP-induced meningitis can cause disruption of the
blood-brain barrier (BBB), and most severely it can result in brain
damage, which affects the prognosis of patients with SP meningitis.
Emerging studies have demonstrated that necrosis and apoptosis of
brain neurons occur while brain damage is taking place.
Neuron-specific enolase (NSE) and S100B have been confirmed to be
specific markers of brain damage (3). B7 homolog 3 (B7-H3) is a newly
identified member of the B7 superfamily, which serves a key
function in the regulation of immune responses (4). Our previous study (5) found an abnormally high expression level
of soluble B7-H3 (sB7H3) protein in children with bacterial
meningitis. In a murine model of SP-induced meningitis, we also
demonstrated that B7-H3 further augmented the inflammatory
response, exacerbated BBB disruption, and aggravated the
pathological injury associated with the disease (6). In order to investigate the impact of
B7-H3 on brain damage during SP-induced meningitis, the present
study examined the effect of B7-H3 on neuronal necrosis and
apoptosis and the protein expression of NSE and S100B in brain
tissues in a murine model of SP-induced meningitis.
Materials and methods
SP-induced meningitis in mice
Pyrogen-free, 8–10-week-old BALB/c mice were
purchased from SLAC (Shanghai, China). The mice were housed in
barrier cages under controlled environmental conditions (12/12 h
light/dark cycle, 55±5% humidity, 23°C) in the Institute of Medical
Biotechnology, Soochow University (Suzhou, China) and had free
access to standard laboratory chow and water. All animal procedures
were carried out in the Institute of Medical Biotechnology, Soochow
University. The institutional animal care and use committee of
Soochow University Academic Medical Center approved all experiments
and all animal studies were conducted with ethical approval granted
by the Soochow University Ethics Committee. In addition, the study
was conducted in accordance with the recommendations of the Guide
for the Care and Use of Laboratory Animals of the National
Institutes of Health. Age- and weight-matched BALB/c mice were
anesthetized by the intraperitoneal injection of 0.4 ml 1.8%
Avertin. Pneumococcal meningitis was induced by the
intracerebroventricular injection of 15 µl sterile normal saline
(NS) containing 0.5×104 CFU/ml serotype 3 SP (American
Type Culture Collection, Manassas, VA, USA) into the lateral
ventricle as described previously (2). Black ink (#440; Shanghai Fine
Stationery, Co., Ltd., Shanghai, China) injection at the base of
the skull was used to confirm that injected fluids were distributed
throughout the cerebrospinal fluid.
Grouping and evaluation of clinical
disease status
Mice were randomized into one of the following four
experimental groups, and each mouse received an
intracerebroventricular injection of 15 µl in total: i) Control
group, injected with 15 µl normal saline (NS); ii) B7-H3 group,
injected with 15 µl NS containing 3.3 µg recombinant mouse B7-H3
(R&D Systems, Minneapolis, MN, USA); iii) SP group, injected
with 15 µl NS containing 0.5×104 CFU/ml SP; and iv) SP
plus B7-H3 group, injected with 5 µl NS containing
1.5×104 CFU/ml SP and 10 µl NS containing 3.3 µg B7-H3.
Mice were weighed, put into cages, allowed to wake up, and
evaluated clinically at 18, 48 and 72 h post SP infection. The
clinical disease status was examined by body weight loss and
spontaneous motor activity. The following scores were used to
assess spontaneous motor activity of mice as described previously
(7): 5 points, normal motor activity
and turned upright in <5 sec when put on its back; 4 points,
decreased spontaneous activity, but turned upright in <5 sec; 3
points, turned upright in >5 sec; 2 points, did not turn
upright; and 1 point, did not move or coma. At 18, 48 and 72 h post
SP infection, mice were sacrificed by cervical dislocation. The
brain of each animal was removed, and half of the brain was fixed
immediately with 4% formalin for histopathological analysis and
immunohistochemical staining.
Histopathology
Brain samples fixed with 4% formalin were routinely
processed, and embedded in paraffin. Sections (4 µm) were cut using
a microtome (Leica Biosystems GmbH, Wetzlar, Germany), and neuronal
loss and cell apoptosis in the cortex were evaluated by Nissl and
terminal deoxynucleotidyl transferase-mediated dUTP nick
end-labeling (TUNEL) staining (8).
Normal neurons were identified by the presence of Nissl bodies in
the cytoplasm, loose chromatin and prominent nucleoli. Injured
neurons were identified by the loss of Nissl bodies and cavitation
around the nucleus. TUNEL staining using an in situ
apoptosis detection kit was performed to investigate the apoptosis
according to the manufacturer's instructions (Merck, Darmstadt,
Germany).
Immunohistochemical analysis
Brain tissue sections (4 µm) were deparaffinized and
rehydrated with graded ethanol. For immunohistochemical staining of
NSE and S100B, goat anti-NSE (ab90473) and anti-S100B (ab41458)
polyclonal antibodies (Abcam, Cambridge, MA, USA) were used at a
dilution of 1:100, according to the manufacturer's instructions.
Sections incubated with isotype-matched antibodies were used as the
negative control.
Statistical analysis
All data are presented as the mean ± standard
deviation. Levene's test was conducted for equal variances, one-way
analysis of variance (ANOVA) for multi-group analysis, and the
t-test for all other analyses. SPSS software, version 18.0
(SPSS, Inc., Chicago, IL, USA) was used to conduct the analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
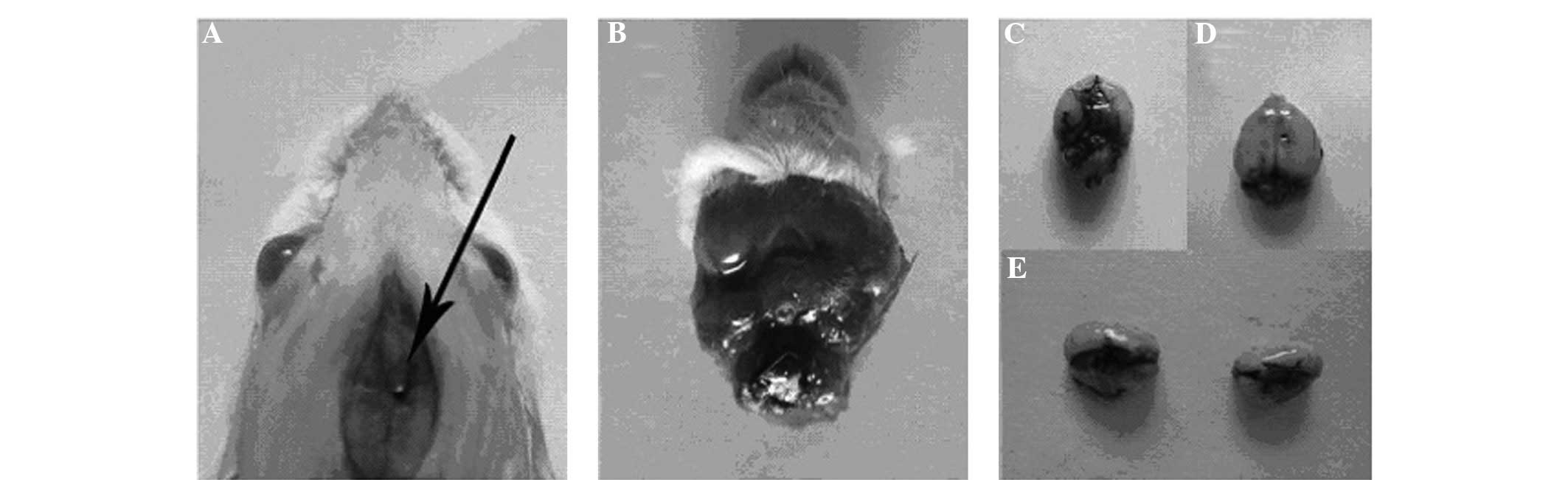
Mouse model of SP meningitis
As evidenced by black ink staining, fluid injected
at the base of the skull rapidly distributed throughout the
cerebrospinal fluid (CSF; Fig.
1).
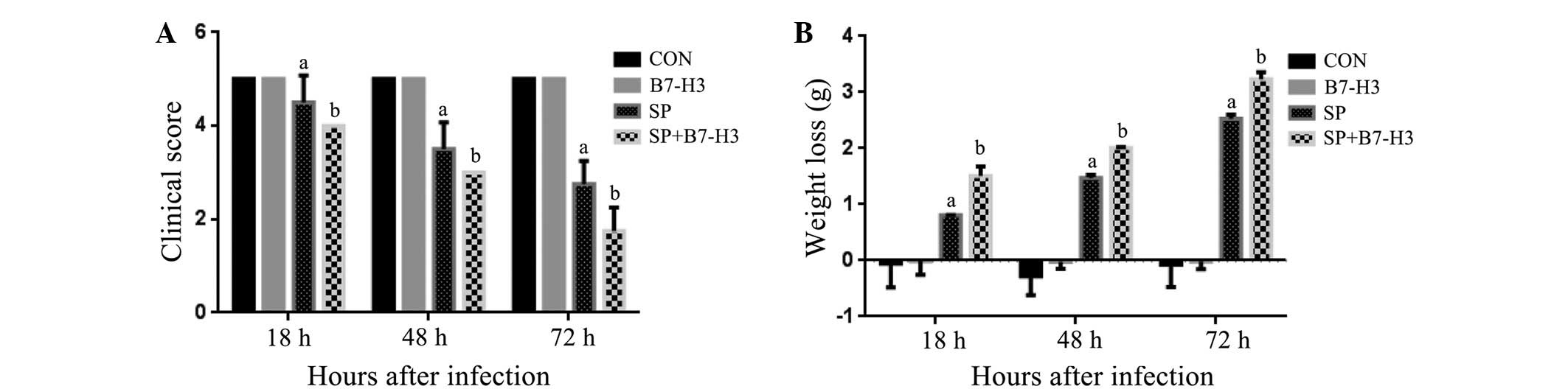
Assessment of the clinical disease
status of mice with SP meningitis
Within 18 and 72 h post SP inoculation, all infected
mice displayed signs of disease represented by decreased
spontaneous motor activity (Fig. 2A)
and body weight loss (Fig. 2B)
compared with NS-injected mice. Moreover, the combined treatment of
mice with SP and B7-H3 further exaggerated the severity of the
disease with significantly decreased spontaneous motor activity and
increased body weight loss (P<0.05 in comparison with that in
the mice challenged with SP alone; Fig.
2). B7-H3 alone did not affect the clinical disease status
(Fig. 2).
Nissl staining
To determine whether B7-H3 exacerbates the neuronal
injury in SP-induced meningitis, brain tissue cytoarchitecture was
evaluated using Nissl staining. The results revealed marked damage
in terms of cell death in brain tissues between 18 and 72 h after
SP infection. The majority of the damaged neurons exhibited
shrinkage with condensed nuclei and sparse Nissl bodies (Fig. 3). Moreover, more damaged neurons were
observed in the SP plus B7-H3 group compared with the SP group
(Fig. 3). Quantitative data
demonstrated that the numbers of Nissl-positive cells were
significantly decreased in the SP group at 18, 48 and 72 h post SP
infection, and this effect was further augmented by B7-H3
administration (P<0.05 vs. SP alone; Fig. 3).
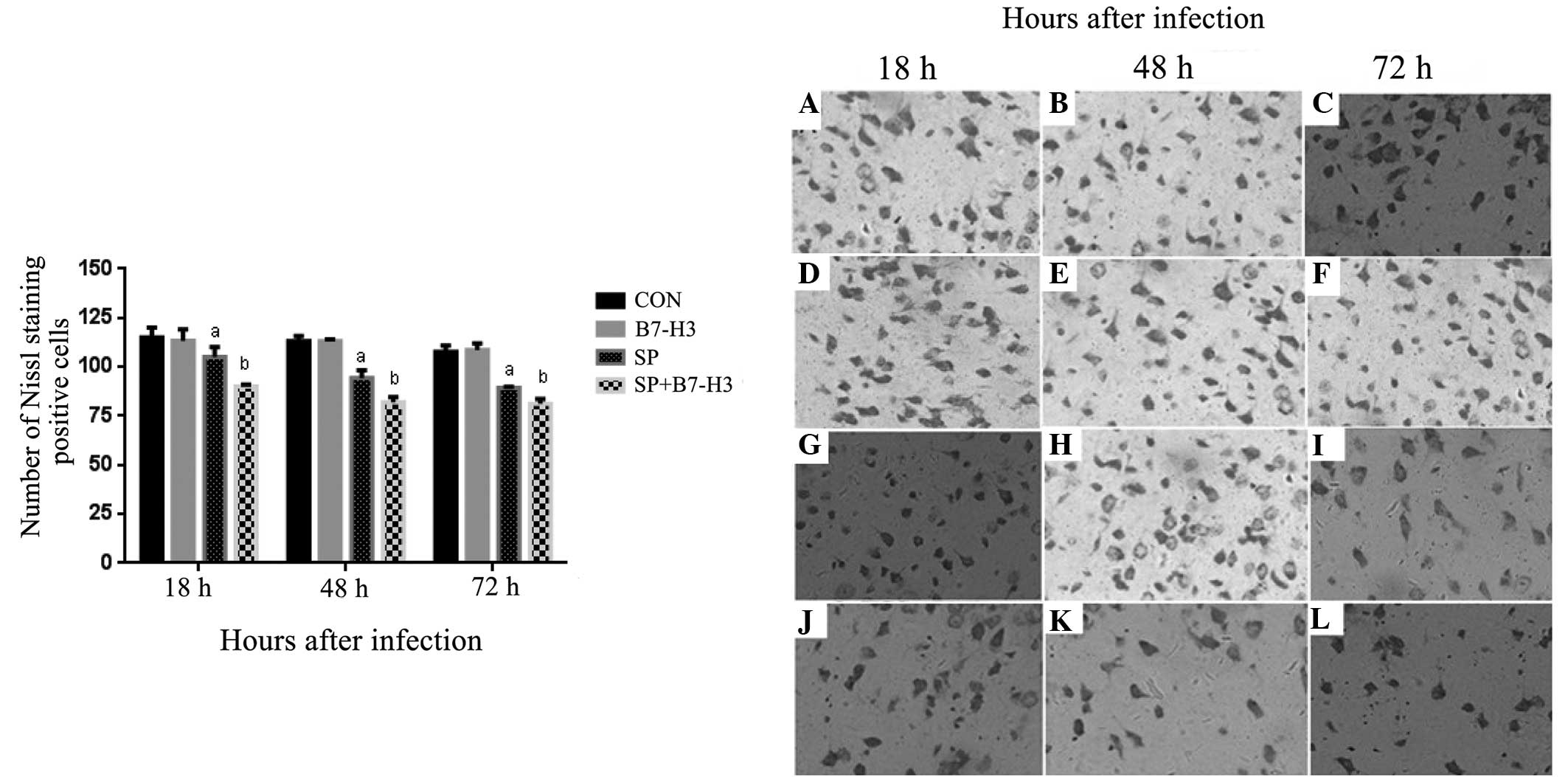 | Figure 3.B7-H3 promotes neuronal loss and
injury in brain tissues during Streptococcus
pneumoniae-infected meningitis. BALB/c mice were challenged
with NS as the control, B7-H3, live S. pneumoniae or live
S. pneumoniae plus B7-H3 via intracerebroventricular
injection. Brains were harvested from (A–C) NS-treated, (D–F)
B7-H3-treated, (G–I) S. pneumoniae-infected and (J–L) S.
pneumoniae plus B7-H3-infected mice, and brain sections were
subjected to Nissl staining as described in Materials and methods.
Representative immunohistochemical analyses are shown
(magnification, x400). B7-H3 treatment led to significantly reduced
Nissl staining of positive neurons or a greater number of injured
neurons identified by the loss of Nissl substance, shrinkage with
condensed nuclei and sparse Nissl bodies at (J) 18 (K) 48 and (L)
72 h after S. pneumoniae infection compared with that
observed for S. pneumoniae alone (G–I, respectively). Data
in the bar graph are expressed as mean ± standard deviation of 6
mice per time-point and represent 3 separate experiments.
aP<0.05 vs. mice that received NS,
bP<0.05 vs. mice that received S. pneumoniae
alone. B7-H3, B7 homolog 3; NS, normal saline; CON, control; SP,
Streptococcus pneumoniae. |
TUNEL staining
Apoptotic death was examined using TUNEL staining.
The number of apoptotic cells in the brain tissues was
significantly increased in the SP group at 18, 48 and 72 h after SP
inoculation compared with that in the control group (P<0.05;
Fig. 4). Importantly, a
substantially greater number of apoptotic cells were observed in
the brain tissues of the mice challenged with SP plus B7-H3 than in
the mice challenged with SP alone at all measured time-points
(P<0.05; Fig. 4), indicating that
challenge with B7-H3 exacerbates SP-induced apoptotic cell death in
the brain.
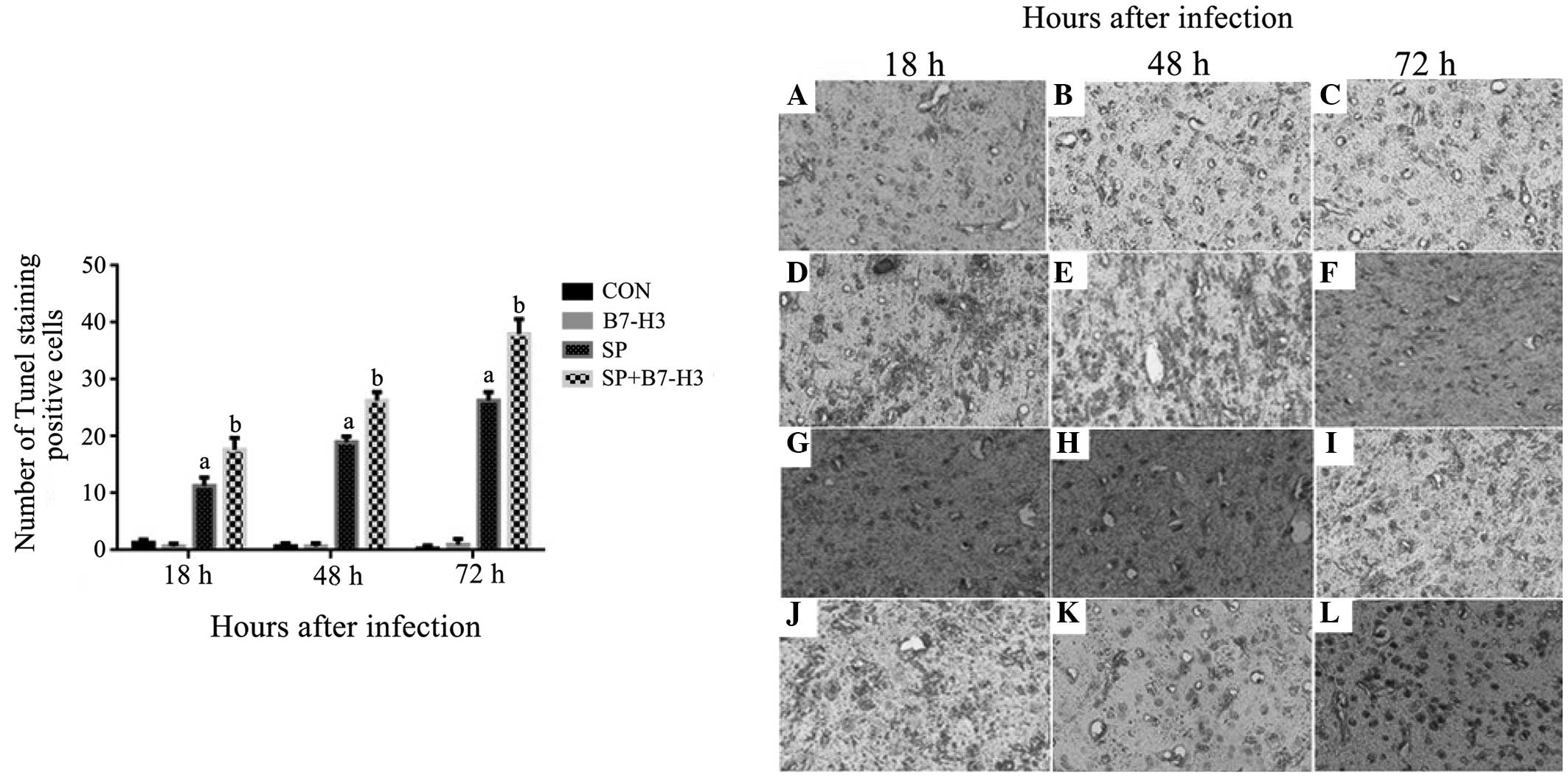 | Figure 4.B7-H3 promotes neuronal loss and
injury in brain tissues during Streptococcus
pneumoniae-infected meningitis. BALB/c mice were challenged
with NS as the control, B7-H3, live S. pneumoniae or live
S. pneumoniae plus B7-H3 via intracerebroventricular
injection. Brains were harvested from (A–C) NS-treated, (D–F)
B7-H3-treated (G–I) S. pneumoniae-infected and (J–L) S.
pneumoniae plus B7-H3-infected mice, and brain sections were
subjected to TUNEL staining as described in materials and methods.
Representative immunohistochemical analyses are shown
(magnification, x200). S. pneumoniae plus B7-H3 treatment
led to significantly increased apoptosis at (J) 18, (K) 48 and (L)
72 h after S. pneumoniae infection compared with S.
pneumoniae infection alone (G–I, respectively). The data in the
bar chart are expressed as mean ± standard deviation of 6 mice per
time-point and represent 3 separate experiments.
aP<0.05 vs. mice that received NS,
bP<0.05 vs. mice that received S. pneumoniae
alone. B7-H3, B7 homolog 3; NS, normal saline; CON, control; SP,
Streptococcus pneumoniae; TUNEL, terminal deoxynucleotidyl
transferase-mediated dUTP nick end-labeling. |
Immunohistochemical analysis of NSE
and S100B expression
Having considered that NSE and S100B are biomarkers
of brain injury in cerebral infections (9), protein expression of NSE and S100B was
assessed in the brain tissues of SP-infected mice by immunochemical
staining. While significantly decreased NSE-positive cells in brain
tissues were observed at 18, 48 and 72 h after SP infection in the
mice challenged with SP alone compared with the control group
(P<0.05; Fig. 5), mice receiving
a combination of SP and B7-H3 displayed further significant
reductions in NSE-positive cells at 18, 48 and 72 h after SP
infection (P<0.05 vs. mice challenged with SP alone; Fig. 5). SP infection led to increased
protein expression of S100B at 18, 48 and 72 h after infection
(Fig. 6). Notably, a substantial
further accumulation of S100B protein in the brain was evident in
mice challenged with a combination of SP and B7-H3 at all measured
time-points (P<0.05 vs. mice challenged with SP alone) (Fig. 6).
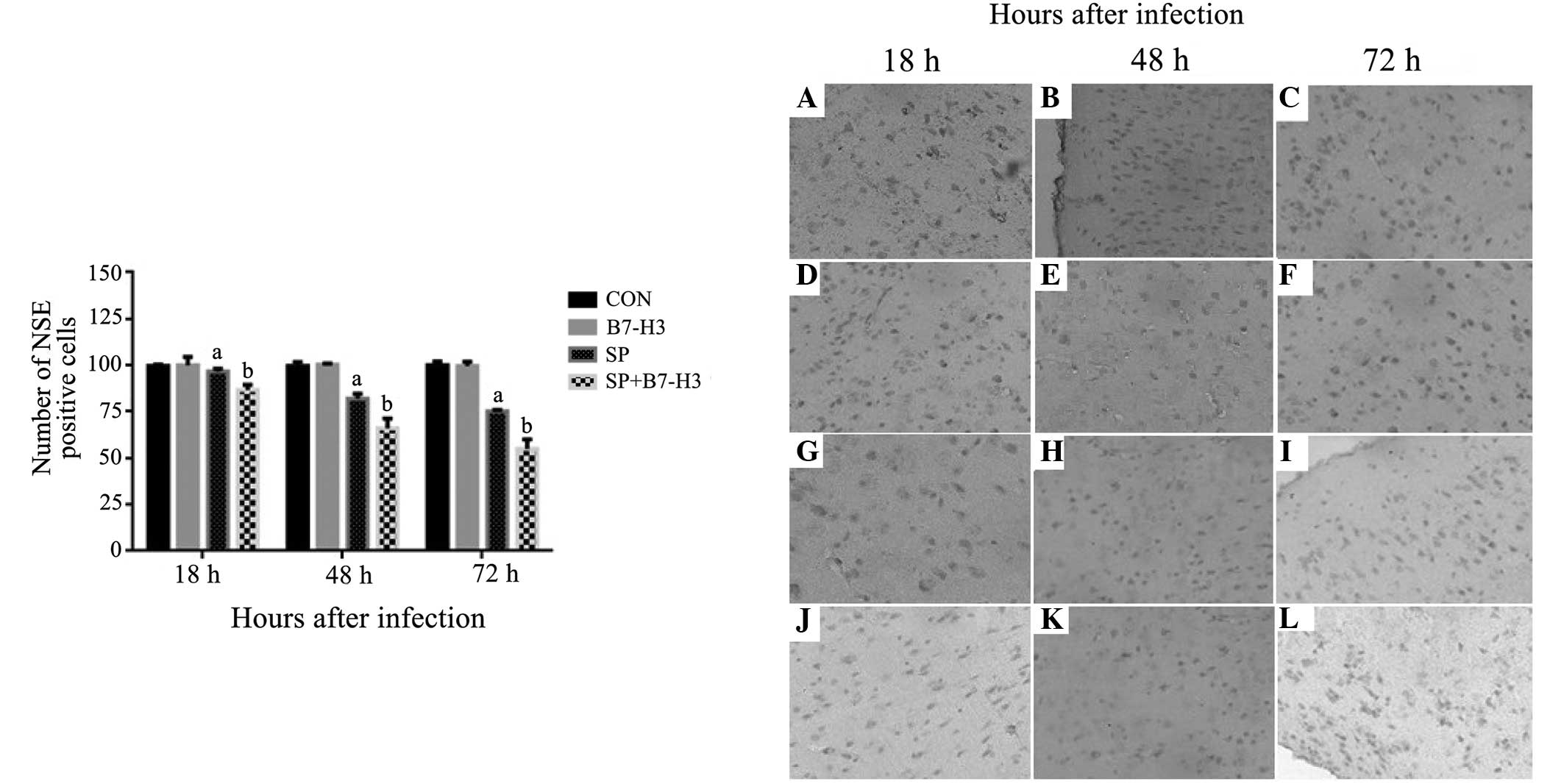 | Figure 5.Efect of B7-H3 on the protein
expression of NSE in the brain tissues of Streptococcus
pneumoniae-infected mice. BALB/c mice were challenged with NS
as the control, B7-H3, live S. pneumoniae or live S.
pneumoniae plus B7-H3 via intracerebroventricular injection.
Brains were harvested from the mice challenged with (A–C) NS, (D–F)
B7-H3, (G–I) S. pneumoniae and (J–L) S. pneumoniae
plus B7-H3 and the expression of NSE in the brain tissue was
assessed by immunohistochemistry as described in materials and
methods. Representative immunohistochemical analyses are shown
(magnification, x200). The number of NSE-positive cells differed
significantly between the S. pneumoniae plus B7-H3 group at
(J) 18, (K) 48 and (L) 72 h and the S. pneumoniae group at
these time-points (G–I, respectively); there was no significant
difference in the number of S100B-positive cells between the
control group at (A) 18, (B) 48 and (C) 72 h and the B7-H3 group at
these time-points (D–F, respectively). The data in the bar graph
are expressed as the mean ± standard deviation of 6 mice per
time-point and represent 3 separate experiments.
aP<0.05 vs. mice that received NS,
bP<0.05 vs. mice that received S. pneumoniae
alone. B7-H3, B7 homolog 3; NSE, neuron-specific enolase; NS,
normal saline; CON, control; SP, Streptococcus
pneumoniae. |
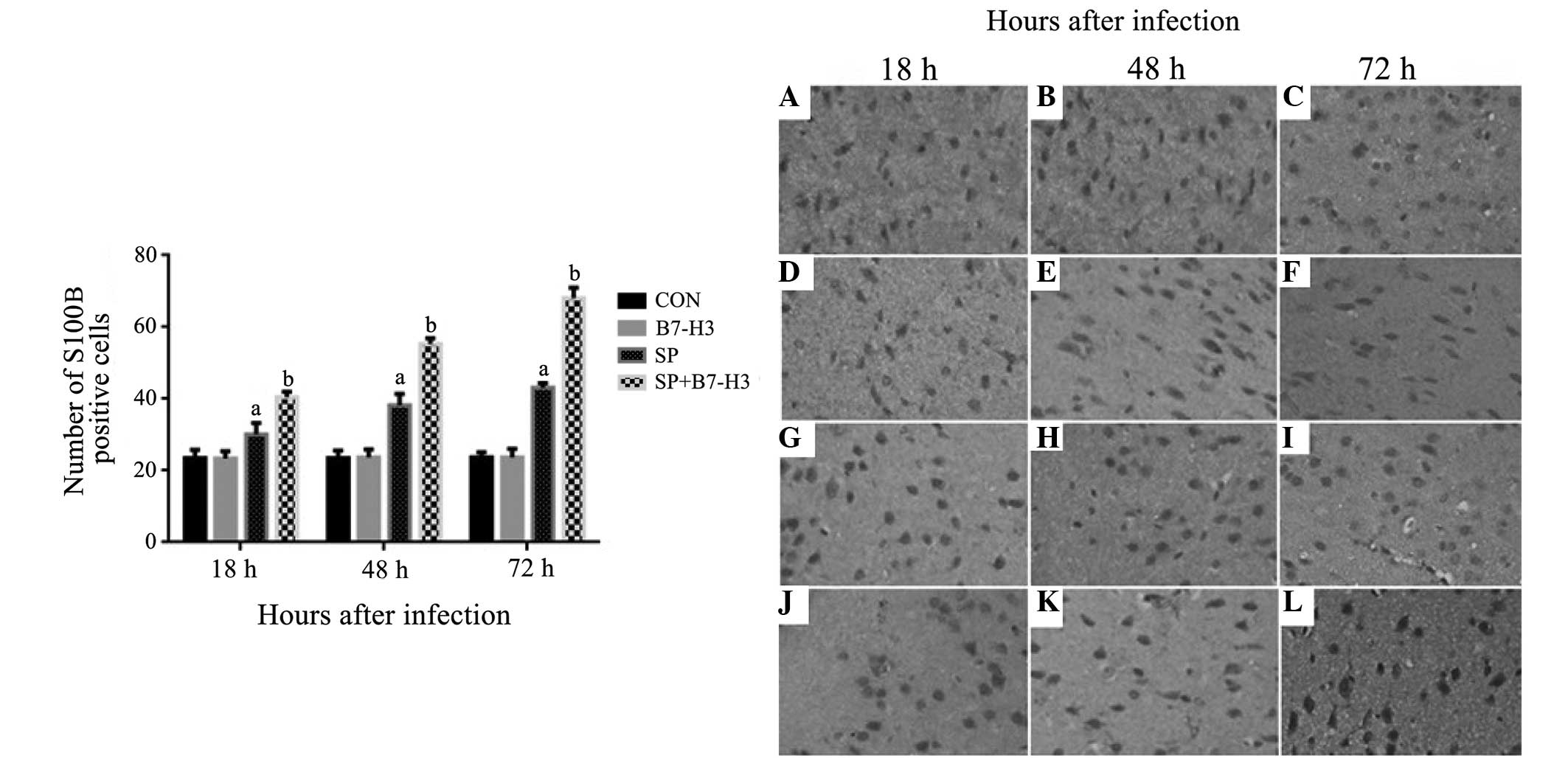 | Figure 6.B7-H3 upregulates S100B expression in
the cerebral tissues of Streptococcus pneumoniae-infected
mice. BALB/c mice were challenged with NS as the control, B7-H3,
live S. pneumoniae or live S. pneumoniae plus B7-H3
via intracerebroventricular injection. Brains were harvested from
(A–C) NS, (D–F) B7-H3, (G–I) S. pneumoniae and (J–L) S.
pneumoniae plus B7-H3 challenged mice and the expression of
S100B in the brain was assessed by immunohistochemistry as
described in materials and methods. Representative
immunohistochemical analyses are shown (magnification, x400). The
number of S100B-positive cells differed significantly between the
S. pneumoniae plus B7-H3 group at (J) 18, (K) 48 (L) and 72
h, and the S. pneumoniae group at these time-points (G–I,
respectively); there was no significant difference in the number of
S100B-positive cells between the control group at (A) 18, (B) 48
and (C) 72 h and the B7-H3 group at these time-points (D–F,
respectively) 72 h. The data in the bar chart are expressed as the
mean ± standard deviation of 6 mice per time-point and represent 3
separate experiments. aP<0.05 vs. mice that received
NS, bP<0.05 vs. mice that received S.
pneumoniae alone. B7-H3, B7 homolog 3; NS, normal saline; CON,
control; SP, Streptococcus pneumoniae. |
Discussion
SP infection resulted in significantly decreased
expression of NSE at 18, 48 and 72 h post SP inoculation, and
combined SP and B7-H3 challenge further reduced NSE expression
levels when compared with that induced by SP infection alone. The
expression of S100B was substantially increased in SP-infected
mice, and was further elevated in the mice treated with SP and
B7-H3. Furthermore, similar expression levels of NSE and S100B were
observed in the B7-H3 group and the control group. These results
indicate that SP infection results in neurological damage, and that
the administration of B7-H3 does not cause neurological damage
directly but aggravates SP infection-induced brain injury.
NSE (γ-enolase) is a stable cell-specific isoenzyme
of the glycolytic enzyme enolase, a dimer protein consisting of α,
β and γ subunits. In the brain, NSE is concentrated exclusively in
the cytoplasm of neurons (10) and
has been suggested to be a specific serum marker for neuronal
damage. In the results of the present study, the reduction of NSE
expression in the brain tissues of the mice treated with SP plus
B7-H3 may reflect the following: i) NSE is primarily localized in
the cytoplasm of neurons (10) and
is not secreted; thus, an increase in its levels in the CSF or
blood indicates structural damage of neuronal cells (8); ii) The number of existing functional
neurons in the brain tissues that are available to be stained as
NSE-positive by immunohistochemical analysis is markedly decreased
due to apoptosis or necrosis caused by multiple factors exerting
injurious effects, including exacerbated inflammation in response
to bacteria stimuli (5), oxidative
stress, lipid peroxidation, mitochondrial damage and BBB breakdown
(1). Further reductions in NSE
expression in brain tissues in response to combined SP and B7-H3
challenge indicate a greater degree of neuronal loss and structural
neuronal damage, which is consistent with our previous study where
it was identified that B7-H3 exacerbated the SP infection-induced
proinflammatory response and BBB disruption (6).
S100B belongs to the multi-genic family of
Ca2+-binding proteins and is abundant in astrocytes,
where it is found diffusely in the cytoplasm and is associated with
membranes and cytoskeleton constituents (12). Extensive evidence indicates that
S100B in high levels (micromolar) may contribute to the severity of
and neurological damage during bacterial meningitis (13,14). As
a biomarker that indicates damage or dysfunction of the CNS
(15), S100B levels could be used as
an additional monitoring parameter in CNS infection (16). In the present study, the
significantly higher level of expression of S100B in brain tissues
in the B7-H3 plus SP group compared with that in the SP group
indicates that B7-H3 aggravates SP infection-induced neurological
damage, since elevated S100B expression reflects an ongoing
cellular injury (specifically of astrocytes) (17,18).
Furthermore, persistent increases in S100B may have a neurotoxic
effect by inducing apoptosis, causing the release of
proinflammatory cytokines as well as nitric oxide from astroglial
cells, and exhibiting detrimental effects on neurons (13,19).
Consistent with the dynamic changes of S100B expression, a greater
amount of neuronal apoptosis and/or loss in the brain tissues, and
a worse clinical status were observed from 18 to 72 h after SP
infection. Administration of B7-H3 exacerbated SP infection-induced
apoptosis or loss of neurons and clinical status, demonstrating
that B7-H3 plays an important role in the pathological process of
SP-induced brain injury.
A number of studies have demonstrated that neurons
in the CNS undergo apoptotic cell death during SP-induced bacterial
meningitis (20–23). Consistent with this, the
B7-H3-amplified cell apoptosis and neuronal loss as demonstrated by
TUNEL and Nissl staining in the present study are also associated
with deteriorated clinical disease status. Challenge with B7-H3
caused a further increase in apoptotic cell death and increased the
severity of disease with significantly impaired spontaneous motor
activity and increased body weight loss in SP-infected mice. Thus,
these in vivo data indicate that B7-H3 not only amplifies
the SP infection-initiated expression of neuronal (NSE) and glial
(S100B) markers, but also results in aggravated cell apoptosis in
the CNS, suggesting that B7-H3 plays a detrimental role in the
development of SP meningitis and its intracranial
complications.
SP is recognized by antigen-presenting cells binding
to Toll-like receptor 2 (TLR2), one of the main pattern recognition
receptors that lead to the activation of NF-κB (6,24)
contributing to the transcription of numerous genes involved in the
pathogenesis of SP meningitis, such as tumor necrosis factor-α,
interleukin-1β (6), inducible nitric
oxide synthase, and intercellular adhesion molecules (25,26). Our
previous study (6) demonstrated that
B7-H3 participates in the development of SP infection-induced
bacterial meningitis by amplifying the inflammatory response and
exacerbating BBB disruption through a TLR2-dependent mechanism.
Consistent with the above findings, the present study demonstrates
that the administration of B7-H3 leads to increased cell apoptosis,
upregulated S100B expression and downregulated NSE expression in
brain tissues, which are probably associated with the ongoing loss
of neurons.
In summary, the present study documents a critical
role of B7-H3 in the pathogenesis of neuronal injury in a model of
SP meningitis. As part of a series of studies on the effects of
B7-H3 on the development of SP meningitis, these results may
contribute to further understanding of the factors involved in the
pathogenesis of brain in bacterial meningitis, and may have further
clinical implications. Other adjunctive approaches targeting B7-H3
may be useful for altering the outcome of SP-induced
meningitis.
Acknowledgements
The authors would like to thank Xueming Zhu, Yunzhen
Tao and Bin Zhou for the excellent technical support and Yumin Hu
for mice breeding and care. This study was supported by grants from
the National Natural Science Foundation of China (no. 81273242,
81272143 and 81301494), Natural Science Foundation of Jiangsu
Province (no. BK2012605), Jiangsu Province Program of Innovative
and Entrepreneurial Talents (2011–2014) and Priority Academic
Program Development of Jiangsu Higher Education Institutions.
References
|
1
|
Barichello T, Generoso JS, Collodel A,
Moreira AP and Almeida SM: Pathophysiology of acute meningitis
caused by Streptococcus pneumoniae and adjunctive therapy
approaches. Arq Neuropsiquiatr. 70:366–372. 2012.PubMed/NCBI
|
|
2
|
Shapiro MA, Donovan KD and Gage JW:
Comparative therapeutic efficacy of clinafloxacin in a pneumococcal
meningitis mouse model. J Antimicrob Chemother. 45:489–492. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Streitbürger DP, Arelin K, Kratzsch J,
Thiery J, Steiner J, Villringer A, Mueller K and Schroeter ML:
Validating serum S100B and neuron-specific enolase as biomarkers
for the human brain - A combined serum, gene expression and MRI
study. PLoS One. 7:e432842012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao X, Li DC, Zhu XG, et al: B7-H3
overexpression in pancreatic cancer promotes tumor progression. Int
J Mol Med. 31:283–91. 2013.PubMed/NCBI
|
|
5
|
Chen X, Zhang G, Li Y, Feng X, Wan F,
Zhang L, Wang J and Zhang X: Circulating B7-H3(CD276) elevations in
cerebrospinal fluid and plasma of children with bacterial
meningitis. J Mol Neurosci. 37:86–94. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen X, Quinn EM, Ni H, Wang J, Blankson
S, Redmond HP, Wang JH and Feng X: B7-H3 participates in the
development of experimental pneumococcal meningitis by augmentation
of the inflammatory response via a TLR2-dependent mechanism. J
Immunol. 189:347–355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Leib SL, Clements JM, Lindberg RL, et al:
Inhibition of matrix metalloproteinases and tumour necrosis factor
alpha converting enzyme as adjuvant therapy in pneumococcal
meningitis. Brain. 124:1734–1742. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Q, Wang C, Shu Z, et al: Valeriana
amurensis improves Amyloid-beta 1–42 induced cognitive deficit by
enhancing cerebral holinergic function and protecting the brain
neurons from apoptosis in mice. J Ethnopharmacol. 153:318–325.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rohlwink UK and Figaji AA: Biomarkers of
brain injury in cerebral infections. Clin Chem. 60:823–834. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marangos PJ and Schmechel DE: Neuron
specific enolase, a clinically useful marker for neurons and
neuroendocrine cells. Annu Rev Neurosci. 10:269–295. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
El-Naga RN, Ahmed HI2 and Abd Al Haleem
EN: Effects of indole-3-carbinol on clonidine-induced neurotoxicity
in rats: Impact on oxidative stress, inflammation, apoptosis and
monoamine levels. Neurotoxicology. 44:48–57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Adami C, Sorci G, Blasi E, Agneletti AL,
Bistoni F and Donato R: S100B expression in and effects on
microglia. Glia. 33:131–142. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hamed SA, Hamed EA and Zakary MM:
Oxidative stress and S-100B protein in children with bacterial
meningitis. BMC Neurol. 9:512009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park JW, Suh GI and Shin HE: Association
between cerebrospinal fluid S100B protein and neuronal damage in
patients with central nervous system infections. Yonsei Med J.
54:567–71. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang XY, Lin J, Lu XY and Zhao XY:
Expression of S100B protein levels in serum and cerebrospinal fluid
with different forms of neuropsychiatric systemic lupus
erythematosus. Clin Rheumatol. 27:353–357. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lins H, Wallesch CW and Wunderlich MT:
Sequential analyses of neurobiochemical markers of cerebral damage
in cerebrospinal fluid and serum in CNS infections. Acta Neurol
Scand. 112:303–308. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Raabe A and Seifert V: Protein S-100B as a
serum marker of brain damage in severe head injury: Preliminary
results. Neurosurg Rev. 23:136–138. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Raabe A and Seifert V: Fatal secondary
increase in serum S-100B protein after severe head injury. Report
of three cases. J Neurosurg. 91:875–877. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Steiner J, Bernstein HG, Bielau H, Berndt
A, Brisch R, Mawrin C, et al: Evidence for a wide extraastrocytic
distribution of S100B in human brain. BMC Neurosci. 8:22007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leib SL, Kim YS, Chow LL, Sheldon RA and
Tӓuber MG: Reactive oxygen intermediates contribute to necrotic and
apoptotic neuronal injury in an infant rat model of bacterial
meningitis due to group B streptococci. J Clin Invest.
98:2632–2639. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tauber SC, Harms K, Falkenburger B, Weis
J, Sellhaus B, Nau R, Schulz JB and Reich A: Modulation of
hippocampal neuroplasticity by Fas/CD95 regulatory protein 2
(Faim2) in the course of bacterial meningitis. J Neuropathol Exp
Neurol. 73:2–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zysset-Burri DC, Bellac CL, Leib SL and
Wittwer M: Vitamin B6 reduces hippocampal apoptosis in experimental
pneumococcal meningitis. BMC Infect Dis. 13:3932013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Braun JS, Sublett JE, Freyer D, Mitchell
TJ, Cleveland JL, Tuomanen EI and Weber JR: Pneumococcal
pneumolysin and H2O2 mediate brain cell apoptosis during
meningitis. J Clin Invest. 109:19–27. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Opitz B, Eitel J, Meixenberger K and
Suttorp N: Role of Toll-like receptors, NOD-like receptors and
RIG-I-like receptors in endothelial cells and systemic infections.
Thromb Haemost. 102:1103–1109. 2009.PubMed/NCBI
|
|
25
|
Koedel U, Bayerlei I, Paul R, Sporer B and
Pfister HW: Pharmacologic interference with NF-kappaB activation
attenuates central nervous system complications in experimental
pneumococcal meningitis. J Infect Dis. 182:1437–1445. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kastenbauer S, Koedel U, Weih F,
Ziegler-Heitbrock L and Pfister HW: Protective role of NF-kappaB1
(p50) in experimental pneumococcal meningitis. Eur J Pharmacol.
498:315–318. 2004. View Article : Google Scholar : PubMed/NCBI
|