Introduction
Bone modeling and remodeling are maintained by the
strictly coupled activities of bone-forming osteoblasts and
bone-resorbing osteoclasts (1,2).
Mechanical stimulation from weight-bearing activities and muscle
contraction plays an important role in bone modeling and
remodeling. Weight bearing and muscle contraction stimulate bone
formation and remodeling, whereas a lack of these stimuli leads to
bone mass loss, and can cause conditions such as disuse
osteoporosis (3–5). By contrast, exposure of the bone to
supraphysiological loads results in an increase in bone mass in
order to withstand the high stress (6–11). The
adaptation of bone to mechanical changes occurs due to osteoblasts
and osteoclasts, which sense the changes and regulate the function
of bone formation and resorption (12,13).
Numerous types of mechanical stimuli, such as
hydrostatic or hydrodynamic pressure, fluid shear stress and
hypergravity, have been applied to observe the effects on
bone-derived cells. Dynamic loading can stimulate human osteoblast
proliferation and increase extracellular matrix production
(14–16). In addition, mechanical stimulation of
osteoblasts has been to shown to increase the release of alkaline
phosphatase (ALP) (17), nitric
oxide (NO) (18) and prostaglandin
E2 (PGE2) (19), and regulate
Runt-related transcription factor 2 (Runx2) activation (20).
Osteoclasts are multinucleated cells that branch
from the monocyte or macrophage lineage early during the
differentiation process (21). The
osteoclast formation process includes several steps. Firstly,
precursor cells are genetically altered so that the proteins
expressed enable cell-cell recognition and attachment. The cells
then undergo differentiation to multinucleated pre-osteoclasts,
which do not resorb bone. These pre-osteoclasts are finally
activated into functional bone resorbing osteoclasts by a number of
hormones and cytokines, including parathyroid hormone,
1,25-dihydroxyvitamin D3, tumor necrosis factor (TNF) and
interleukin-1, which promote osteoclastogenesis. Receptor activator
of nuclear factor-κB (RANKL) and macrophage colony-stimulating
factor (M-CSF) are the key factors for osteoclast differentiation
(21,22). RANKL is expressed on the membrane
surface of osteoblasts and stromal cells and promotes
pre-osteoclast fusion into mature osteoclasts. The main role of
M-CSF is to induce the pre-osteoclast expression of RANK, which is
a receptor of RANKL (21). Mature
osteoclasts are tartrate-resistant acid phosphatase (TRAP)-positive
cells that cause bone resorption. Studies have reported that
mechanical stimulation affects the differentiation and bone
resorption function of osteoclasts (23,24).
Mature osteoclasts are terminally differentiated
cells and thus do not undergo mitosis. The lifespan of osteoclasts
is short, and the cells undergo spontaneous apoptosis. Numerous
drugs, such as bisphosphonates, can promote the apoptosis of
osteoclasts in order to have an anti-osteoporotic effect (25,26);
however, less is known about the effect of mechanical strain on
osteoclast apoptosis.
It is known that osteoclasts and osteoblasts are
sensitive to mechanical stimulation, which affects the
differentiation and bone resorption function of osteoclasts
(23,24); however, few studies have reported the
effect of cyclic tension stress on osteoclast apoptosis. In the
present study, the association between cyclic tension stress and
osteoclast apoptosis was investigated using the ElectroForce® 3200
mechanical testing instrument (EnduraTEC Systems Group, Bose Corp.,
Minnetonka, MN, USA) with a BioDynamic® bioreactor system (Bose
Corp.).
Materials and methods
Materials
RAW264.7 cells were purchased from the Institute of
Basic Medicine of Peking Union Medical College (Beijing, China).
High-glucose Dulbecco's Modified Eagle's Medium (DMEM) and fetal
bovine serum (FBS) were obtained from Invitrogen Life Technologies
(Carlsbad, CA, USA). RANKL was purchased from PeproTech Co. (Rocky
Hill, NJ, USA). The TRAP staining kit was purchased from
Sigma-Aldrich (St. Louis, MO, USA), and Silastic Sylgard 184 was
obtained from Dow Corning Corp. (Midland, MI, USA). The Annexin
V-fluorescein isothiocyanate (FITC) apoptosis detection kit, BD
FACSCalibur™ flow cytometer and flow cytometry and CellQuest
sub-analysis software were purchased from BD Biosciences (Franklin
Lakes, NJ, USA). The Endura ElectroForce 3200 mechanical testing
instrument and BioDynamic bioreactor system were obtained from Bose
Corp. For the reverse transcription-quantitative polymerase chain
reaction, All-in-One™ qPCR Mix and All-in-One qPCR Primer
(GeneCopoeia, Rockville, MD, USA) were used. Optical and inverted
phase contrast microscopes were obtained from Nikon Corp. (Tokyo
Japan). iQ™5 Real-Time PCR Detection Elution was purchased from
Bio-Rad (Hercules, CA, USA).
Cell culture and osteoclast
differentiation
RAW264.7 cells were grown in high-glucose DMEM
supplemented with 10% heat-inactivated FBS and 1%
penicillin-streptomycin at 37°C in a humidified atmosphere of 95%
air and 5% CO2. The differentiation of RAW264.7 cells
was induced using high-glucose DMEM containing RANKL (100 ng/ml),
as described in a previous study (27).
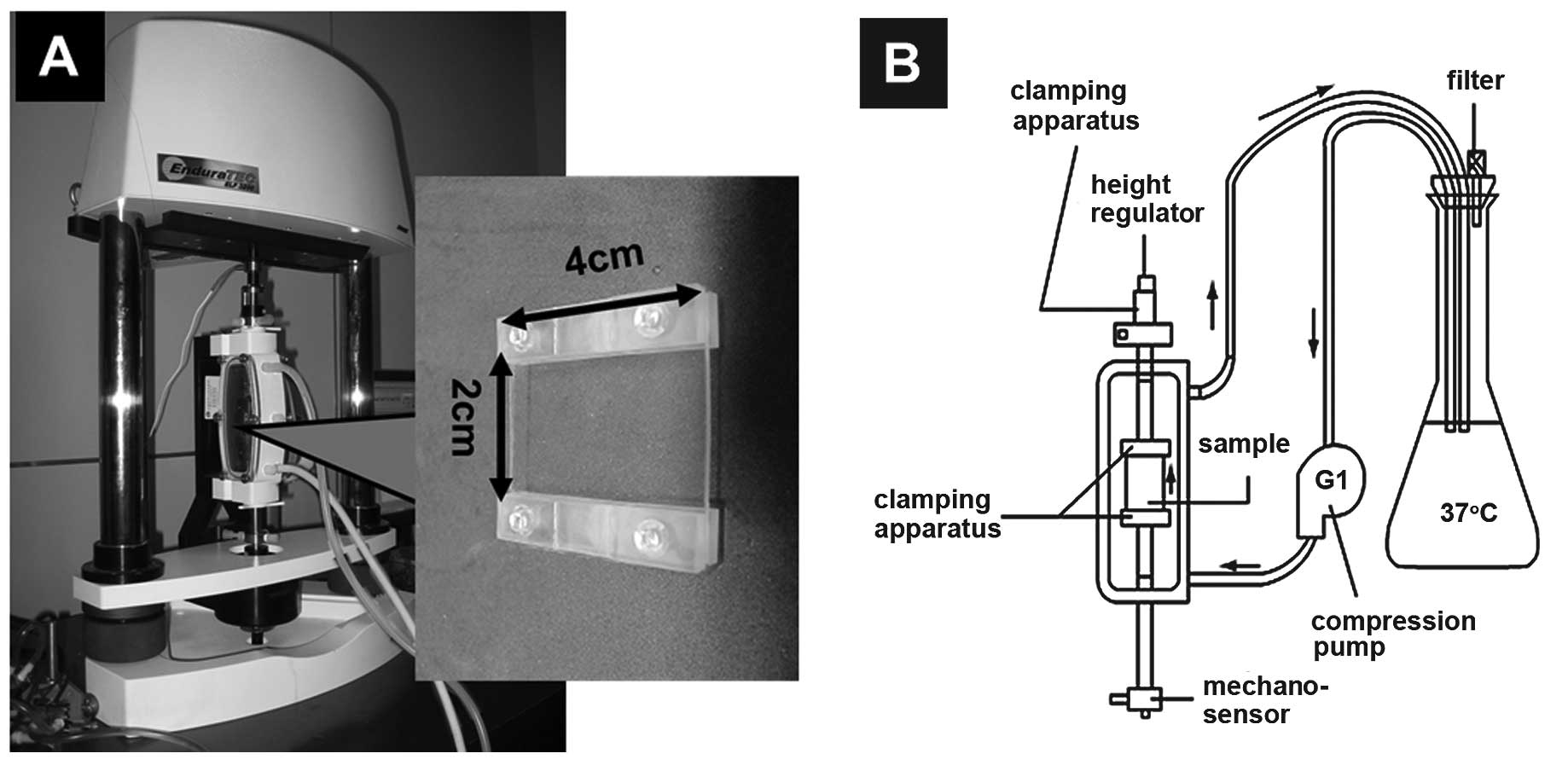
Mechanical loading system
Mechanical loading was performed using a rectangular
(4×2×0.1 cm) silicone rubber membrane developed by our research
group. The stress distribution on the surface of the silicone
rubber membrane and its biocompatibility were acceptable for cell
culture, and the material itself showed no cytotoxicity. The
silicone rubber membrane, which acted as a cell carrier, was
compatible with the Endura ElectroForce 3200 mechanical testing
instrument, and the mechanical parameters of the loading were
controlled via the Bose Peripheral Component Interconnect and
WinTest® system (Bose Corp.). The mechanical loading system was
able to produce precise deformations on the silicone rubber
membrane so that the osteoclasts plated on the membrane were
subjected to mechanical stimulation. The reservoir was settled
inside a CO2 incubator and the compression pump was
operated at a defined speed, so that the medium circulated
throughout the culture system via the piping system. This ensured
that the mechanical loading system temperature and CO2
concentration were stable (Fig.
1).
Following ethylene oxide sterilization, the silicone
rubber membrane was immersed in α-Minimum Essential Medium
(Shanghai and Shanghai Yu Biotechnology Co., Ltd., Shanghai, China)
with 5% FBS until required. The RAW264.7 cells were seeded at a
density of 1×105 cells in the silicone rubber membrane
with 100 ng/ml RANKL over the course of five days and were then
placed in the mechanical loading system and randomly divided into
four groups: A control group (cells were not subjected to any
mechanical tension stress) and three experimental groups (cells
were subjected to 5, 10 and 15% stretch microstrain, respectively,
at 0.25 Hz for 1 h a day). The mechanical loadings continued for
three days.
TRAP staining
Three days after the induction of the RAW264.7 cells
with 100 ng/ml RANKL, the osteoclasts were treated with 5, 10 and
15% stretch microstrain for three days (also with 100 ng/ml RANKL
induction), washed with phosphate-buffered saline (PBS) and then
fixed with 4% paraformaldehyde. Following fixation, the cells were
rinsed in distilled water and stained using the TRAP staining kit.
TRAP-positive cells with two or more nuclei were considered to be
osteoclast-like cells. The number of osteoclasts was counted in
each film (n=5/group) under a light microscope to observe the
effects of mechanical stimulation.
Resorption pit assay
Three days after the induction of the RAW264.7 cells
with 100 ng/ml RANKL, the cells were treated with 5, 10 and 15%
stretch microstrain for three days (also with 100 ng/ml RANKL
induction). The osteoclasts were then seeded on bovine cortical
bone slices (0.5×0.5 cm) at a density of 20,000
cells/cm2 for three days. The cells were removed from
the bovine cortical bone slices by sonication in 0.1 N NaOH for 5
min, fixed with 2.5% glutaraldehyde for 30 min and then subjected
to ethanol gradient dehydration, drying and spraying. The cells
were observed using scanning electron microscopy. The surface of
each bovine cortical bone slice was examined using light microscopy
for evidence of lacunar resorption, and quantitative analysis of
the resorption area was performed with Image-Pro Plus software
version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA)
(n=5/group).
Analysis of osteoclast apoptosis
The effect of mechanical stimulation on osteoclast
apoptosis was quantified using the Annexin V-FITC apoptosis
detection kit. The osteoclasts were treated with 5, 10 and 15%
stretch microstrain for three days and then washed twice with PBS
and gently re-suspended in Annexin V binding buffer at a
concentration of 1×106 cells/ml. The osteoclasts
(1×105 cells, 100 µl) were added to a 5-ml flow tube,
prior to 5 µl Annexin V-FITC and 5 µl propidium iodide (PI) being
transferred. The cells were incubated for 15 min at room
temperature (25°C) in the dark and then analyzed by flow cytometry
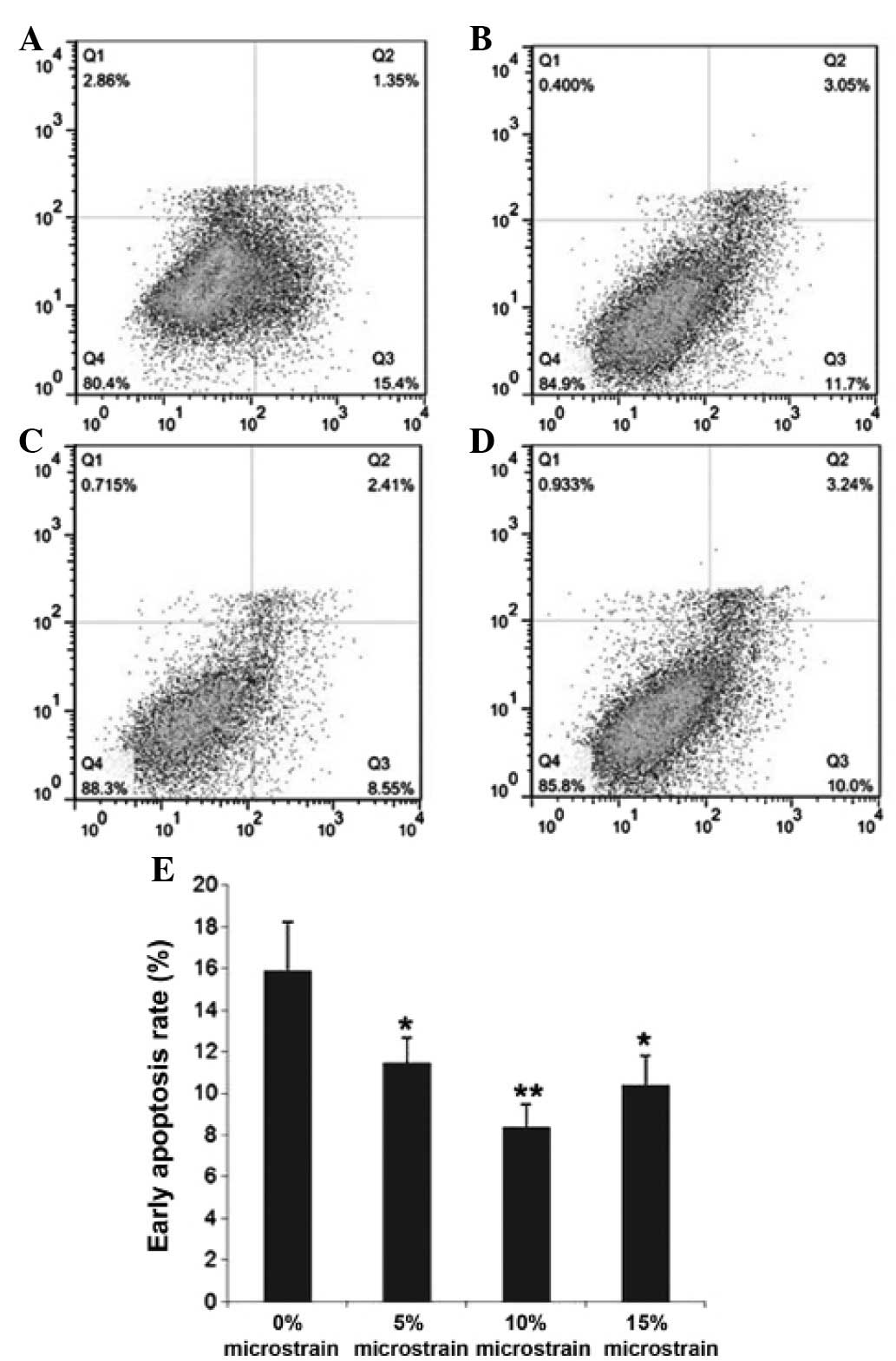
within 1 h. Q1 quadrant represented the mechanically damaged cells,
Q2 quadrant represented the post-apoptotic or necrotic cells, Q3
quadrant represented the early apoptotic cells and Q4 quadrant
represented the surviving cells.
RT-qPCR
Following treatment with the mechanical stimuli for
three days, the cells were washed twice with PBS and collected.
Total RNA was extracted using TRIzol™ (Invitrogen Life
Technologies) according to the manufacturer's instructions. cDNA
was synthesized from the total RNA using All-in-One First-Strand
cDNA Synthesis kit and oligo(dt) primers (Wuhan Boster Biological
Engineering Co., Ltd., Wuhan, China). The primers used for the
genetic analysis of the osteoclasts were as follows: Bcl-2 forward,
5′-ACGGGGTGAACTGGGGGAGG-3′ and reverse, 5′-GCATGCTGGGGCCGTACAGT-3′;
Bax forward, 5′-GATGGACGGGTCCGGAGA-3′ and reverse,
5′-CTCAGCCCATCTTCTTCCAG-3′; caspase-3 forward,
5′-TTCAGAGGGGATCGTTGTAGAAGTC-3′ and reverse,
5′-CAAGCTTGTCGGCATACTGTTTCAG-3′; cytochrome c forward,
5′-TGGGCGGAAGACAGGTCA-3′ and reverse,
5′-TCCAGGGATGTACTTCTTGGGAT-3′; β-actin forward,
5′-GGGAAATCGTGCGTGACATT-3′ and reverse, 5′-GGAACCGCTCATTGCCAAT-3′.
The reaction conditions were set according to the kit
manufacturer's instructions. Subsequent to the completion of the
reaction, amplification and melting curve analyses were performed.
Gene expression values were analyzed for target gene expression
using the 2−ΔΔCt method.
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analysis was performed with a one-way analysis of
variance using SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
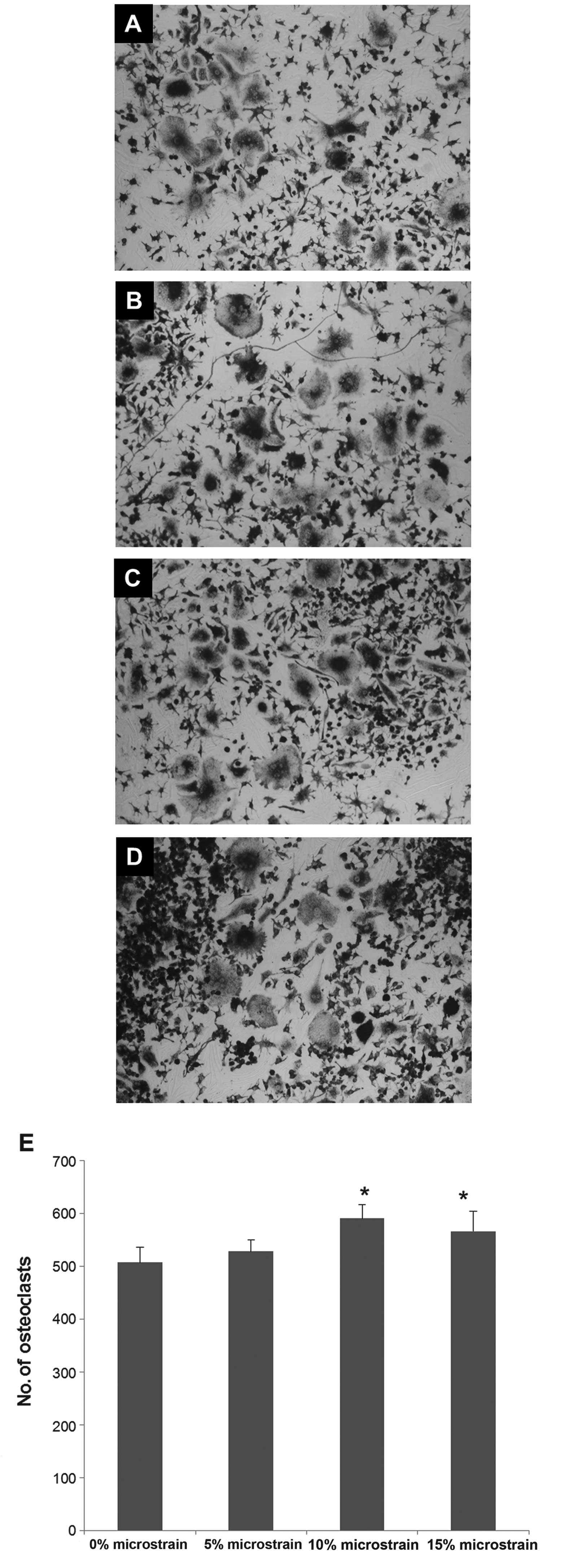
Effect of cyclic tension stress on the
number of TRAP-positive multinucleated osteoclasts
The effect of cyclic tension stress on osteoclast
differentiation was investigated by subjecting RAW264.7 cells to 5,
10 and 15% stretch microstrain and then counting the number of
multinucleated osteoclasts after three days of mechanical
stimulation. The number of mature osteoclasts was increased
following 10 and 15% mechanical stimulation (P<0.05), and the
strongest effect was noted following 10% microstrain (Fig. 2).
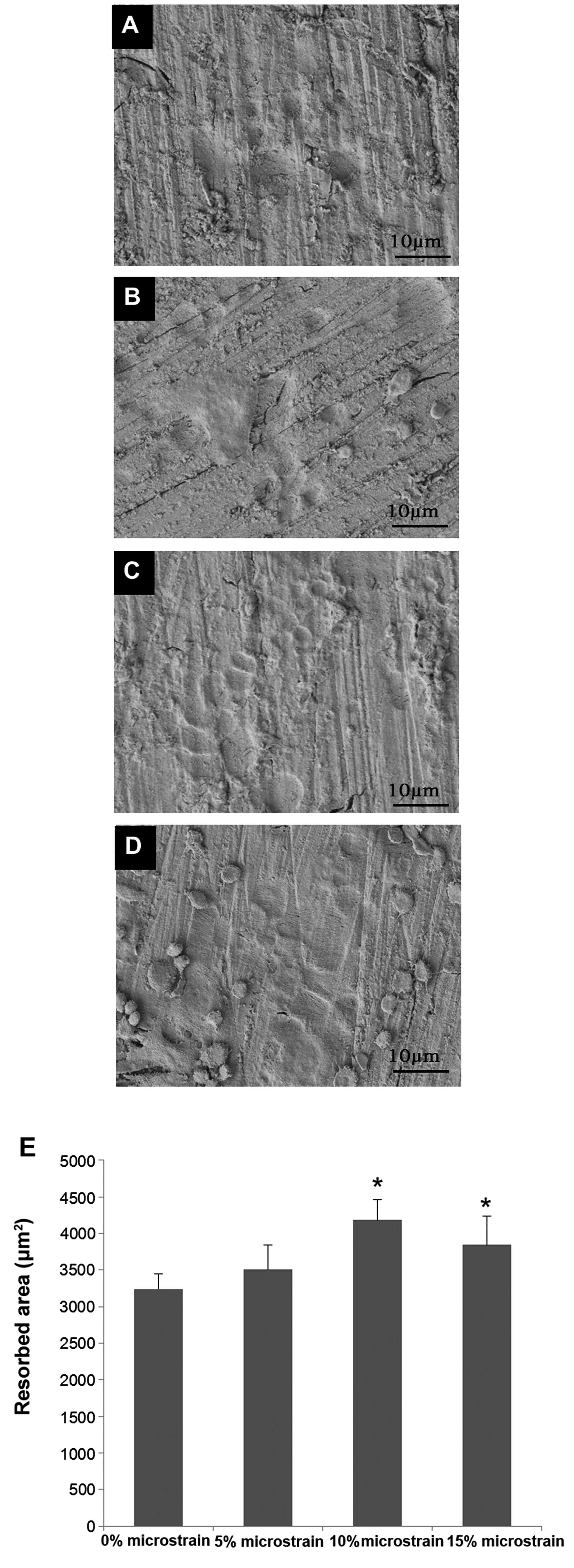
Resorption pit assay
As shown in Fig. 3,
treatment with mechanical stimulation significantly increased the
ability of the experimental group cells to resorb bovine cortical
bone slices compared with the control group. No significant
difference was observed between the cells subjected to 5%
microstrain and the control cells (P>0.05).
Cyclic tension stress inhibits
osteoclast apoptosis
To determine the effect of cyclic tension stress on
osteoclast apoptosis, the Annexin-V/PI binding assay was performed
(Fig. 4). Compared with the control
group cells (15.9±2.36%), the cells subjected to 5, 10 and 15%
stretch microstrain for three days showed significantly decreased
early apoptosis (Q3 quadrant) rates (11.47±1.21, 8.39±1.08 and
10.41±1.4%, respectively) (P<0.05). These results indicate that
cyclic tension stress inhibits osteoclast apoptosis.
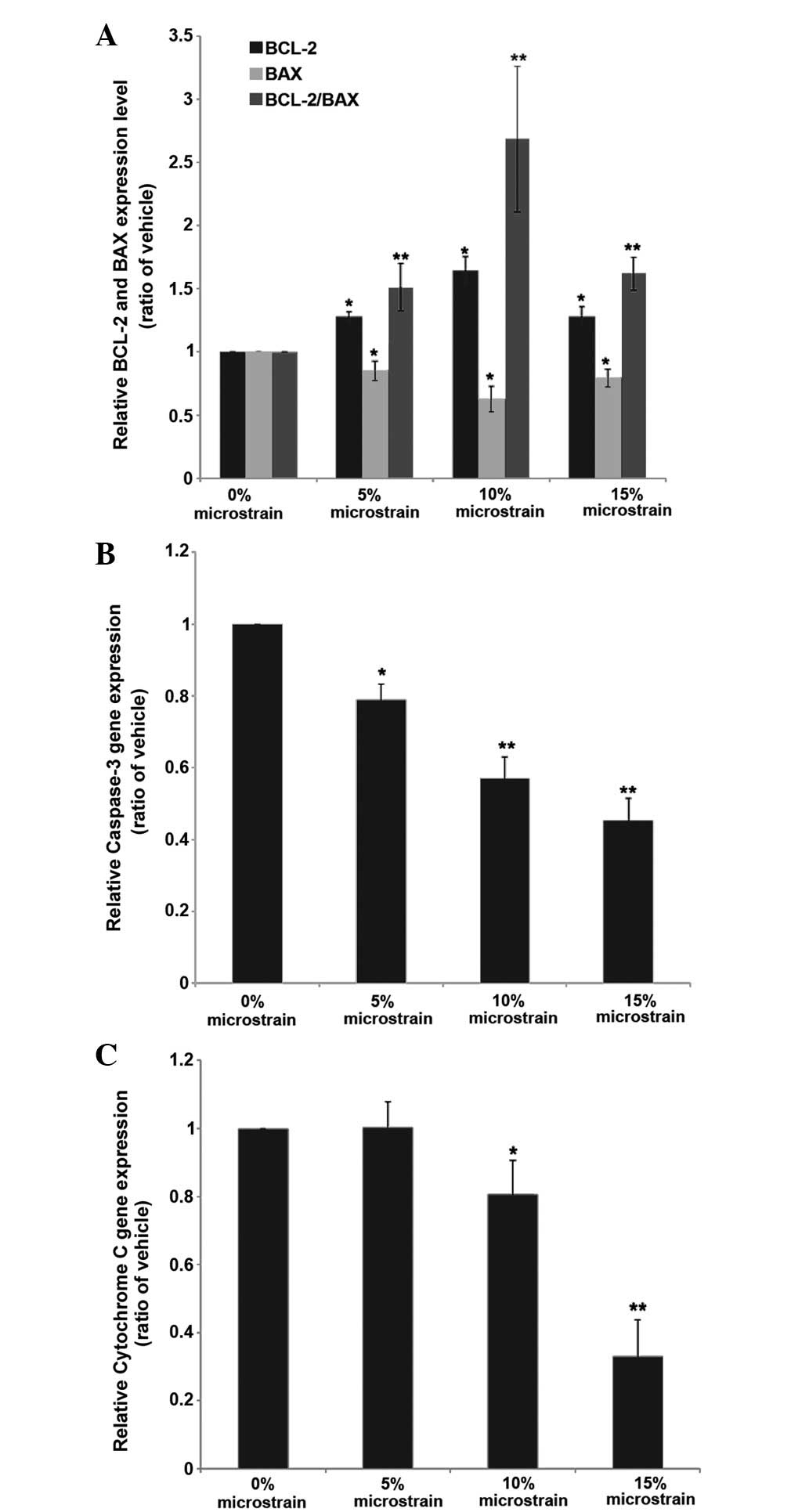
Effect of cyclic tension stress on the
expression of osteoclast apoptosis genes
In order to confirm the effect of three days of
cyclic tension stress on osteoclast apoptosis, RT-qPCR was
performed (Fig. 5). Compared with
the control group, the mRNA expression of caspase-3 and cytochrome
c was decreased in the mechanical loading group (5, 10 and
15% stretch microstrain) in a load-dependent manner. The mRNA
expression of Bcl-2 showed upregulation and that of Bax showed
downregulation. The Bcl-2/Bax ratio of the mechanical stress groups
was increased compared with that of the control group, and the
increase was greatest in the 10% stretch microstrain group. These
results suggest that cyclic tension stress has an important effect
on osteoclast apoptosis.
Discussion
The present study utilized a novel tension stress
loading system designed by our research group. The apoptotic
activity of osteoclasts was detected when the cells were subjected
to cyclic tension stress during cultivation. The results showed
that cyclic tension stress increased the number of TRAP-positive
cells and inhibited osteoclast apoptosis. The mRNA expression
levels of caspase-3, cytochrome c and Bax were significantly
decreased under mechanical stimulation, and the expression of Bcl-2
was increased, suggesting that the apoptotic activity of the
osteoclasts was inhibited by the mechanical stimulation.
The advantage of the mechanical loading system used
in the present study was that all cells on the silicone rubber
membrane were subjected to the same mechanical stimulation, meaning
that an effective ‘uniaxial’ loading was applied to the
osteoclasts. In addition, the WinTest software system of the
mechanical loading system could control the duration, tensile
elongation and tensile frequency of the loading regime, which was
important for the cellular responses. The silicone rubber membrane
had the three basic characteristics of a cell carrier: i) Good
transparency, so the microscopic observation of the cells was not
affected; ii) good elasticity (the silicone rubber membrane could
stretch up to ∼20% under the precise control of the operating
system); iii) good cytocompatibility.
There were two main methods that could have been
used to obtain mature osteoclasts in the present study. Although
mature osteoclasts can be isolated from the long bones, the purity
is poor and the short life of the cells is not suitable for the
needs of the experiment (28–30).
Numerous methods have therefore been developed to acquire
sufficient osteoclast-like cells in vitro (31–33). In
the present study, TRAP-positive, mature osteoclasts with
resorptive capabilities were obtained by inducing RAW264.7 cells
with 100 ng/ml RANKL.
Mechanical stimulation plays an important role in
the regulation of bone cells. Numerous studies have investigated
the effect of mechanical stress on bone tissue in vivo and
in vitro (34,35), and it has been reported that the
osteoblasts and osteoclasts sense and respond to mechanical
stimuli. Studies have confirmed that mechanical stimulation
increases the osteoblastic release of ALP (17), NO (18) and PGE2 (19) and regulates Runx2 activation
(20). Additionally, studies have
reported that mechanical stress can inhibit osteoclast
differentiation (23) and increase
the activity of bone resorption (24); however, the effect of mechanical
stimuli on osteoclast apoptosis as been less well studied. The cell
apoptosis pathway is activated by a variety of physical, chemical
and biological factors. Apoptosis may occur through activation of
the death receptor pathway (Fas/TNF) or the mitochondrial apoptosis
pathway (36). The latter is
achieved by regulating the expression of the anti-apoptotic protein
B-cell lymphoma 2 (Bcl-2) and the pro-apoptotic protein
Bcl2-associated X protein (Bax) (37). The anti-apoptotic factor Bcl-2 and
pro-apoptotic factors (such as Bax) regulated the mitochondrial
membrane permeability to decide whether cytochrome c and other
pro-apoptotic factors are release into the cytoplasm.
Apoptosis-related genes of the Bcl-2 family are the
key regulators of apoptosis, which exert their effects via the
following mechanisms: i) Inhibition of oxygen free radicals; ii)
control of intracellular Ca2+ influx, iii) inhibition of
the release of cytochrome c, iv) inhibition of p53- and
c-myc-induced apoptosis. Bax and Bcl-2 are embedded in the
mitochondrial membrane. Bax dimers enhance mitochondrial membrane
permeability, while Bcl-2 and Bax can combine to form a
heterodimeric body, which prevents the formation of pro-apoptotic
Bax dimers (37). Bcl-2 dimers can
additionally inhibit mitochondrial depolarization (38–40).
When cytochrome c is released into the cytoplasm, it can
combine with apoptosis protease activating factor-1 (APAF-1) and
enhance the combined capacity of APAF-1 and adenosine triphosphate.
Recruitment of the caspase-9 precursor molecule to the APAF-1
complex results in caspase-9 activation. The effectors caspase-3,
−6 and −7 are then gradually activated, furthering the cells along
the apoptotic pathway (41–43). The caspase family of cysteine
aspartic proteases plays an important role at the start and finish
of cell apoptosis and acts as the executors of cell apoptosis. Two
pathways can result in caspase activation. In the first pathway,
caspase activation is mediated by death receptors, such as Fas or
the TNF receptor. Fas ligand and Fas receptor combine, and then
Fas-Associated protein with Death Domain binds to the receptor,
leading to pro-caspase-8 combination and automatic activation.
Caspase-3 and other downstream caspases are then activated, which
in turn lead to a caspase cascade amplification reaction, cracking
protein substrates in the cell (44). The second pathway leading to caspase
activation begins with pro-apoptotic signals promoting cytochrome
c release from the mitochondria into the cytosol, where it
enters the cytoplasm, combines with APAF-1 and then binds to and
activates pro-caspase-9. The composite activated caspase-3 leads to
the caspase cascade amplification. Caspase-3 acts as the converging
point of a variety of apoptosis-stimulating signals and the
ultimate enforcer of cell apoptosis. Activation of caspase-3 is
representative of the cell reaching the irreversible phase of
apoptosis (45).
In the present study, it was demonstrated that
cyclic tension stress could upregulate the mRNA expression of Bcl-2
and downregulate that of Bax, thus increasing the Bcl-2/Bax ratio.
This reduced the permeability of the mitochondrial membrane and
suppressed the release of cytochrome c and other
pro-apoptotic factors into the cytoplasm. The final caspase cascade
mediating the apoptosis of osteoclasts was inhibited. Changes in
the deformation variables affected each gene differently; for
example, 10% stretch microstrain elicited the strongest effect on
the expression of Bcl-2 and Bax and the Bcl-2/Bax ratio, while the
expression levels of caspase-3 and cytochrome c were
inhibited most strongly by 15% stretch microstrain. The results
suggest that mild mechanical stimulation (5% stretch microstrain)
has only a slight effect on osteoclast apoptosis and that moderate
mechanical stimulation (10% stretch microstrain) inhibits
osteoclast apoptosis mainly by regulating the ratio of Bcl-2/Bax
but also by inhibiting the expression of cytochrome c and
caspase-3. By contrast, high levels of mechanical stimulation (15%
stretch microstrain) regulate osteoclast apoptosis mainly by
inhibiting the expression of cytochrome c and caspase-3, and
secondly by regulating the ratio of Bcl-2/Bax.
In conclusion, cyclic tension stress can inhibit
osteoclast apoptosis through the mitochondria-mediated apoptosis
pathway. The mechanism by which stretch microstrain stimuli affect
osteoclast apoptosis varies depending on the size of the stimulus.
Since osteoblasts and osteoclasts are closely associated in
vivo, the experimental study of the mechanical regulation of
osteocytes requires further development.
Acknowledgements
This study was funded by the Public Health Bureau of
Science and Technology of Tianjin, China (no. 2011KZ57) and the
Traditional Chinese Medicine Administration of Tianjin, China (no.
13123).
Glossary
Abbreviations
Abbreviations:
|
RANKL
|
receptor activator of nuclear
factor-κB ligand
|
|
TRAP
|
tartrate-resistant acid
phosphatase
|
|
NO
|
nitric oxide
|
|
PGE2
|
prostaglandin E2
|
|
M-CSF
|
macrophage colony-stimulating
factor
|
|
APAF-1
|
apoptosis protease activating
factor-1
|
References
|
1
|
Nakahama K: Cellular communications in
bone homeostasis and repair. Cell Mol Life Sci. 67:4001–4009. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu H and Li B: p53 control of bone
remodeling. J Cell Biochem. 111:529–534. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ehrlich PJ and Lanyon LE: Mechanical
strain and bone cell function: A review. Osteoporos Int.
13:688–700. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chow JW, Jagger CJ and Chambers TJ:
Reduction in dynamic indices of cancellous bone formation in rat
tail vertebrae after caudal neurectomy. Calcif Tissue Int.
59:117–120. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huiskes R, Ruimerman R, van Lenthe GH and
Janssen JD: Effects of mechanical forces on maintenance and
adaptation of form in trabecular bone. Nature. 405:704–706. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rubin CT and Lanyon LE: Kappa Delta Award
paper. Osteoregulatory nature of mechanical stimuli: Function as a
determinant for adaptive remodeling in bone. J Orthop Res.
5:300–310. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jee WS, Li XJ and Schaffler MB: Adaptation
of diaphyseal structure with aging and increased mechanical usage
in the adult rat: A histomorphometrical and biomechanical study.
Anat Rec. 230:332–338. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Turner CH, Akhter MP, Raab DM, Kimmel DB
and Recker RR: A noninvasive, in vivo model for studying strain
adaptive bone modeling. Bone. 12:73–79. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chambers TJ, Evans M, Gardner TN,
Turner-Smith A and Chow JW: Induction of bone formation in rat tail
vertebrae by mechanical loading. Bone Miner. 20:167–178. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Forwood MR and Turner CH: Skeletal
adaptations to mechanical usage: Results from tibial loading
studies in rats. Bone. 17(4 Suppl): 197S–205S. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mosley JR, March BM, Lynch J and Lanyon
LE: Strain magnitude related changes in whole bone architecture in
growing rats. Bone. 20:191–198. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cowin SC, Moss-Salentijn L and Moss ML:
Candidates for the mechanosensory system in bone. J Biomech Eng.
113:191–197. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weinbaum S, Cowin SC and Zeng Y: A model
for the excitation of osteocytes by mechanical loading-induced bone
fluid shear stresses. J Biomech. 27:339–360. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaspar D, Seidl W, Neidlinger-Wilke C, et
al: Proliferation of human-derived osteoblast-like cells depends on
the cycle number and frequency of uniaxial strain. J Biomech.
35:873–880. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaspar D, Seidl W, Neidlinger-Wilke C, et
al: Dynamic cell stretching increases human osteoblast
proliferation and CICP synthesis but decreases osteocalcin
synthesis and alkaline phosphatase activity. J Biomech. 33:45–51.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Neidlinger-Wilke C, Wilke HJ and Claes L:
Cyclic stretching of human osteoblasts affects proliferation and
metabolism: A new experimental method and its application. J Orthop
Res. 12:70–78. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen YJ, Huang CH, Lee IC, et al: Effects
of cyclic mechanical stretching on the mRNA expression of
tendon/ligament-related and osteoblast-specific genes in human
mesenchymal stem cells. Connect Tissue Res. 49:7–14. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hara F, Fukuda K, Ueno M, et al: Pertussis
toxin-sensitive G proteins as mediators of stretch-induced decrease
in nitric-oxide release of osteoblast-like cells. J Orthop Res.
17:593–597. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Searby ND, Steele CR and Globus RK:
Influence of increased mechanical loading by hypergravity on the
microtubule cytoskeleton and prostaglandin E2 release in primary
osteoblasts. Am J Physiol Cell Physiol. 289:C148–C158. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kanno T, Takahashi T, Tsujisawa T, et al:
Mechanical stress-mediated Runx2 activation is dependent on
Ras/ERK1/2 MAPK signaling in osteoblasts. J Cell Biochem.
101:1266–1277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Quinn JM and Gillespie MT: Modulation of
osteoclast formation. Biochem Biophys Res Commun. 328:739–745.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suzuki N, Yoshimura Y, Deyama Y, et al:
Mechanical stress directly suppresses osteoclast differentiation in
RAW264. 7 cells. Int J Mol Med. 21:291–296. 2008.
|
|
24
|
Kurata K, Uemura T, Nemoto A, et al:
Mechanical strain effect on bone-resorbing activity and messenger
RNA expressions of marker enzymes in isolated osteoclast culture. J
Bone Miner Res. 16:722–730. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weinstein RS and Manolagas SC: Apoptosis
and osteoporosis. Am J Med. 108:153–164. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abe K, Yoshimura Y, Deyama Y, et al:
Effects of bisphosphonates on osteoclastogenesis in RAW264. 7
cells. Int J Mol Med. 29:1007–1015. 2012.
|
|
27
|
Mdel M Arriero, Ramis JM, Perelló J and
Monjo M: Inositol hexakisphosphate inhibits osteoclastogenesis on
RAW 264.7 cells and human primary osteoclasts. PLoS One.
7:e431872012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Osdoby P, Martini MC and Caplan AI:
Isolated osteoclasts and their presumed progenitor cells, the
monocyte, in culture. J Exp Zool. 224:331–344. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zallone A Zambonin, Teti A and Primavera
MV: Isolated osteoclasts in primary culture: First observations on
structure and survival in culture media. Anat Embryol (Berl).
165:405–413. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tezuka K, Tezuka Y, Maejima A, et al:
Molecular cloning of a possible cysteine proteinase predominantly
expressed in osteoclasts. J Biol Chem. 269:1106–1109.
1994.PubMed/NCBI
|
|
31
|
Udagawa N, Takahashi N, Akatsu T, et al:
The bone marrow-derived stromal cell lines MC3T3-G2/PA6 and ST2
support osteoclast-like cell differentiation in cocultures with
mouse spleen cells. Endocrinology. 125:1805–1813. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Collin-Osdoby P, Oursler MJ, Webber D and
Osdoby P: Osteoclast-specific monoclonal antibodies coupled to
magnetic beads provide a rapid and efficient method of purifying
avian osteoclasts. J Bone Miner Res. 6:1353–1365. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Quinn JM, Neale S, Fujikawa Y, et al:
Human osteoclast formation from blood monocytes, peritoneal
macrophages, and bone marrow cells. Calcif Tissue Int. 62:527–531.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Duncan RL and Turner CH:
Mechanotransduction and the functional response of bone to
mechanical strain. Calcif Tissue Int. 57:344–358. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Skerry TM and Suva LJ: Investigation of
the regulation of bone mass by mechanical loading: From
quantitative cytochemistry to gene array. Cell Biochem Funct.
21:223–229. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kroemer G, Galluzzi L and Brenner C:
Mitochondrial membrane permeabilization in cell death. Physiol Rev.
87:99–163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Adams JM and Cory S: Bcl-2-regulated
apoptosis: Mechanism and therapeutic potential. Curr Opin Immunol.
19:488–496. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yin XM: Bid, a BH3-only multi-functional
molecule, is at the cross road of life and death. Gene. 369:7–19.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Certo M, Del Gaizo Moore V, Nishino M, et
al: Mitochondria primed by death signals determine cellular
addiction to antiapoptotic BCL-2 family members. Cancer Cell.
9:351–365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kuwana T and Newmeyer DD: Bcl-2-family
proteins and the role of mitochondria in apoptosis. Curr Opin Cell
Biol. 15:691–699. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hellebrand EE and Varbiro G: Development
of mitochondrial permeability transition inhibitory agents: A novel
drug target. Drug Discov Ther. 4:54–61. 2010.PubMed/NCBI
|
|
42
|
Jilka RL, Noble B and Weinstein RS:
Osteocyte apoptosis. Bone. 54:264–271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Timmer JC and Salvesen GS: Caspase
substrates. Cell Death Differ. 14:66–72. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dai C, Zhang B, Liu X, et al:
Pyrimethamine sensitizes pituitary adenomas cells to temozolomide
through cathepsin B-dependent and caspase-dependent apoptotic
pathways. Int J Cancer. 133:1982–1993. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cryns V and Yuan J: Proteases to die for.
Genes Dev. 12:1551–1570. 1998. View Article : Google Scholar : PubMed/NCBI
|