Introduction
Penicillium marneffei is an opportunistic
pathogenic fungus and the only thermally dimorphic species in the
Penicillium genus (1). The
pathogen can lead to disseminated infection in immunocompromised
patients, particularly those infected with human immunodeficiency
virus (HIV). Penicilliosis marneffei has become one of the most
common diseases in patients with acquired immune deficiency
syndrome (AIDS) in Southeast Asian nations, such as Southern China,
Thailand, Malaysia, the Philippines, Vietnam and Singapore. If
penicilliosis marneffei is not diagnosed early and treated
appropriately, its mortality rates can reach 91.3% (2).
Histoplasma capsulatum can lead to
histoplasmosis, a systemic mycosis affecting humans and animals
that is considered to be a leading indicator of HIV infection.
Histoplasmosis was previously a rare disease; however, since the
1980s it has developed into a major opportunistic infection in
patients with AIDS. Although the majority of patients with
histoplasmosis only present with mild symptoms, ∼5% are likely to
develop a severe and life-threatening disease if early diagnosis is
not conducted (3,4).
Mucor, a serious opportunistic pathogenic
fungus, can cause mucormycosis. Patients with immunosuppression or
diabetes are more sensitive to the acquisition of mucormycosis
(5). Mucormycosis is a dangerous
illness with a high mortality rate (6).
Leishmania donovani causes visceral
leishmaniasis, a zoonosis and one of most important emerging
diseases worldwide. Its wide distribution and global prevalence
lead to serious consequences; at present the condition is prevalent
in 88 countries, affecting ≤1.2 billion individuals (7).
The described diseases are seriously harmful to
human health and have a significant impact on the life conditions
of the affected individuals. Pathogen culture is the most reliable
method of identifying the pathogen, but this takes a long time. The
rapid identification of the four pathogens and the early diagnosis
of the four diseases are therefore of great importance; however,
each of the pathogens have a similar size and shape in the
Wright-Giemsa staining of bone marrow smears (BMSs). Furthermore,
the clinical manifestations of the four diseases are similar, to a
certain extent, since the four pathogens always invade the
mononuclear phagocyte system and can be found in the macrophages.
The aim of the present study was therefore to explore a novel
method combining Periodic Acid Schiff (PAS) and Wright-Giemsa
staining for BMSs in order to rapidly identify and distinguish the
four pathogens.
Materials and methods
Specimens
The BMSs (n=55) were obtained from bone marrow
invaded by P. marneffei (25 BMSs), H. capsulatum (10
BMSs), Mucor (3 BMSs) and L. donovani (17 BMSs). The
diagnosis of the four diseases (penicilliosis marneffei,
histoplasmosis, mucormycosis and visceral leishmaniasis) was
confirmed by pathogen culture. The cases of penicilliosis marneffei
and visceral leishmaniasis were from the First Affiliated Hospital
of Guangxi Medical University (Nanning, China); the cases of
mucormycosis were from the People's Hospital of Guangxi Zhuang
Autonomous Region (Nanning, China); and the cases of histoplasmosis
were referred by experts from other hospitals and identified and
confirmed by the Shanghai Huashan Hospital (Shanghai, China) and
Nanjing Institute of Skin Disease (Nanjing, China). Informed
consent was obtained from all patients prior to participation in
the study and ethical approval was obtained from the First
Affiliated Hospital of Guangxi Medical University Ethical Review
Committee (Nanning, China).
PAS staining methods
Each BMS was fixed by 95% alcohol for 10 min, prior
to being washed, dried, stained by 1% periodic acid for 20 min and
washed and dried once more. Each BMS was then stained by a Schiff
reagent for 60 min, washed and dried, and subsequently stained by
hematoxylin for 5 min, washed and dried. The BMSs were observed
using light microscopy. Wright-Giemsa staining was also performed,
following standard protocols.
Results
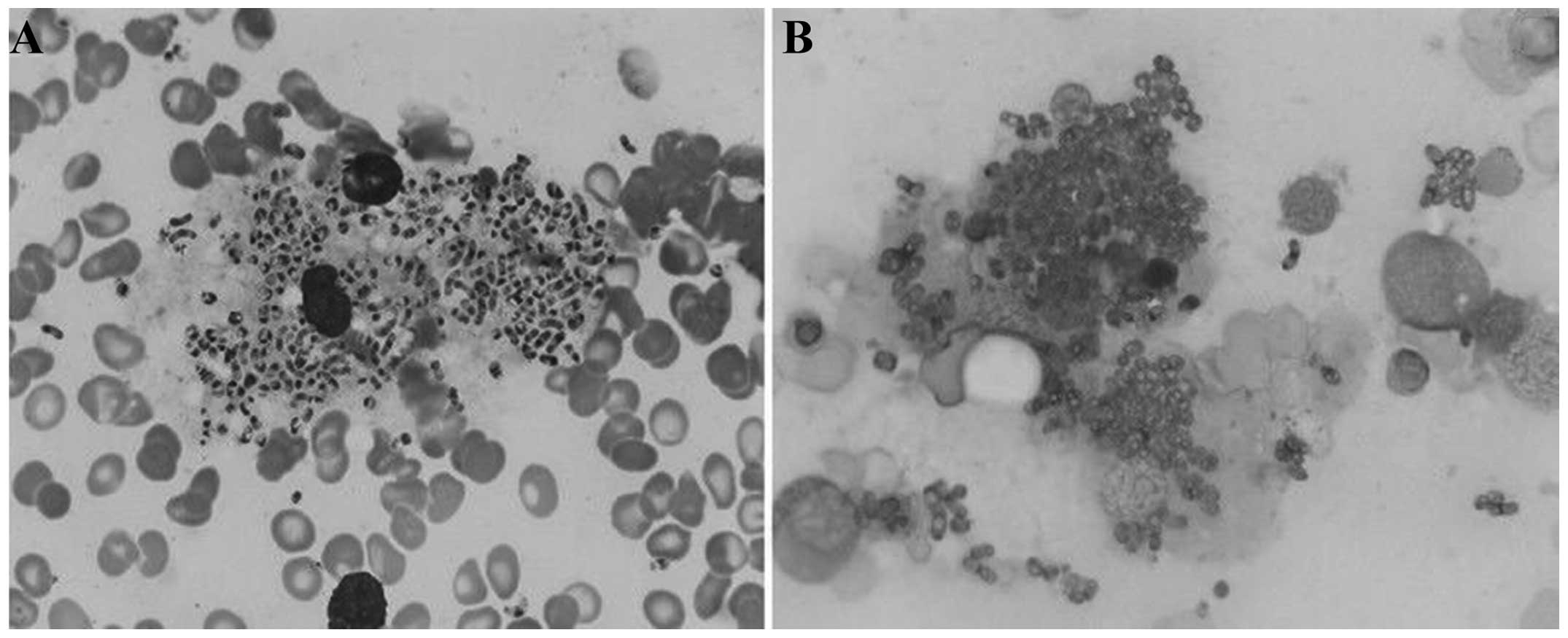
P. marneffei
The pathogens were mostly located in the cytoplasm
of macrophages; a few pathogens were freely scattered
extracellularly. In the Wright-Giemsa staining, the yeast forms of
P. marneffei were round, ellipsoidal or sausage-like with
one or two purplish-red, small nuclei and a light blue cytoplasm.
The multiplying P. marneffei had two nuclei, which were on
both sides of the fungus. The P. marneffei pathogens were
not of a uniform size and measured 2–8 µm. The cell wall was not
clearly visible (Fig. 1A). In the
PAS staining, the cell walls of the P. marneffei were red,
distinct and continuous. A number of sausage-like cells with
cross-walls could be observed and the intracellular contents did
not stain easily (Fig. 1B).
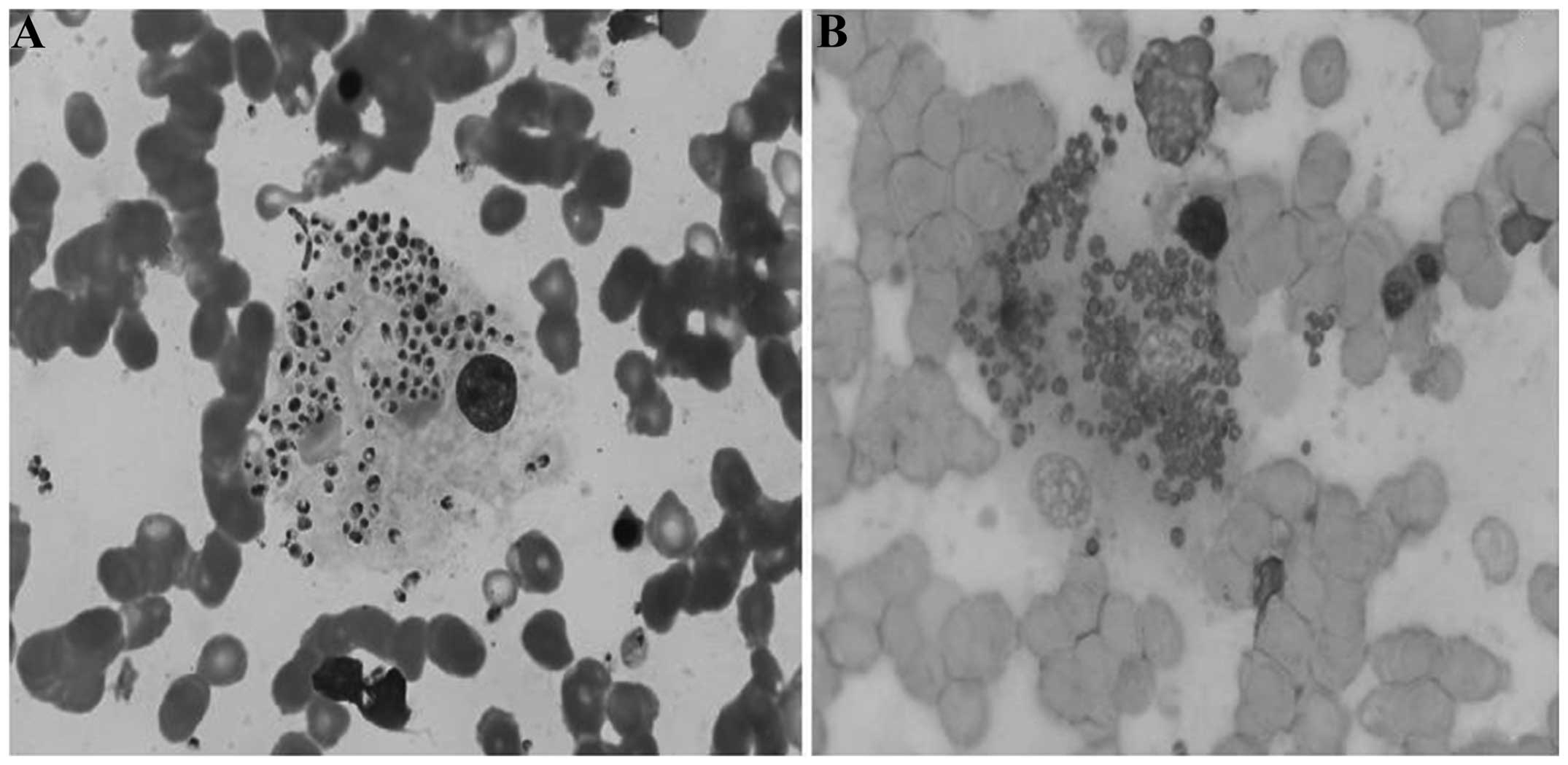
H. capsulatum
In the Wright-Giemsa staining, the majority of the
histoplasmosis-causing pathogens were located in the cytoplasm of
macrophages; only a few pathogens were observed to be outside of
the macrophages. The H. capsulatum exhibited round or
ellipsoidal shapes and measured 2–5 µm. The cell membrane of the
pathogens was not clearly visible. A purplish nucleus, which
occupied between one-third and one-half of the spore, and a light
blue cytoplasm could be observed in the pathogens. Peripheral
spores formed a halo-like circle (Fig.
2A). Differentiation between the H. capsulatum and P.
marneffei was difficult under Wright-Giemsa staining; however,
significant differences between H. capsulatum and P.
marneffei were observed with PAS staining. The cell walls of
the H. capsulatum were red, distinct and continuous, and the
intracellular contents were not easily stained. No sausage-like
cells or cross-walls were visible but, occasionally, narrow-necked,
single spores could be observed (Fig.
2B). It was not difficult, therefore, to identify the two
pathogens when using Wright-Giemsa staining combined with PAS
staining.
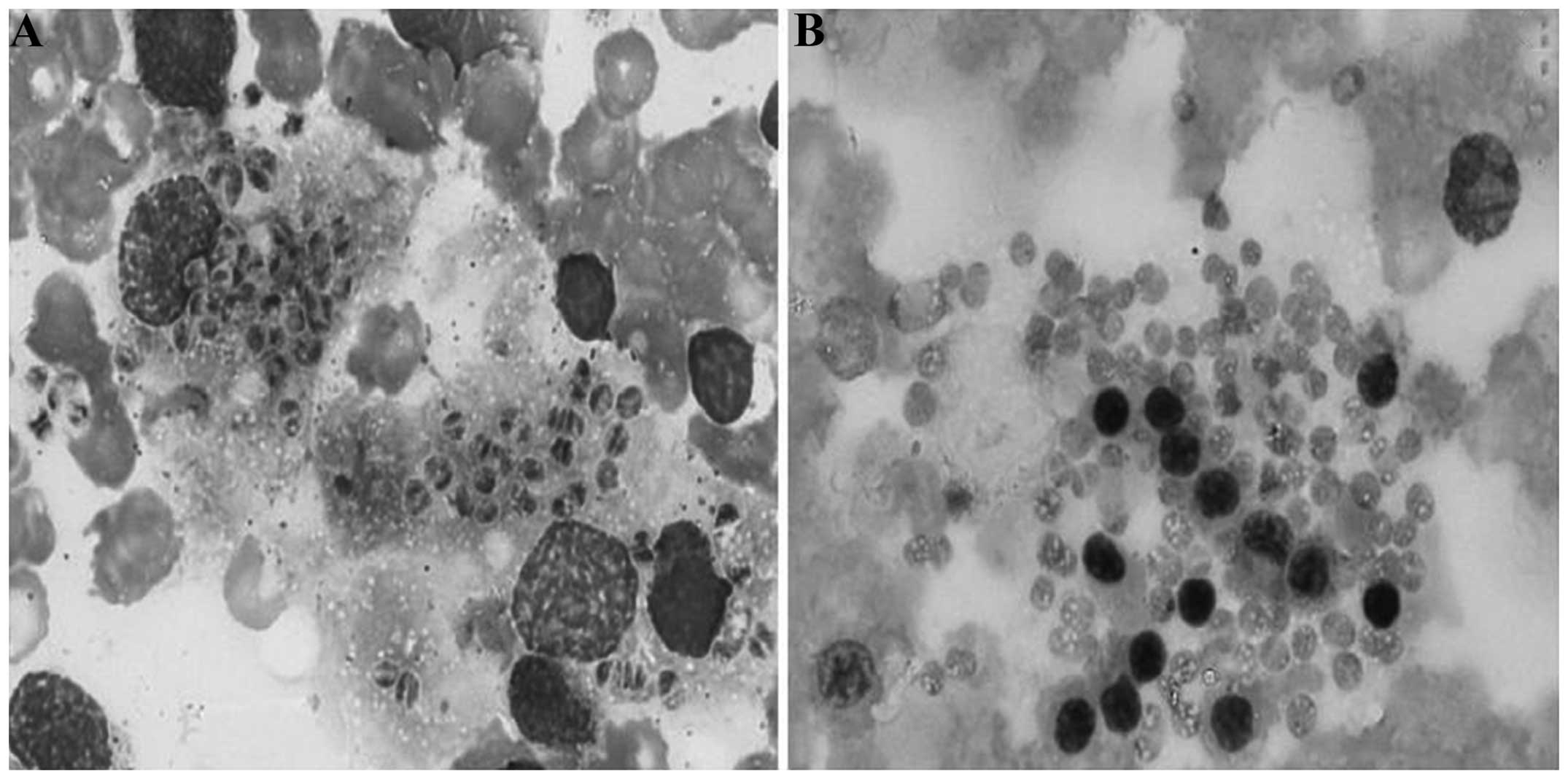
Mucor
In the Wright-Giemsa staining, the location of the
Mucor pathogens was similar to that of the P.
marneffei and H. capsulatum pathogens. With the
exception of a few pathogens outside the macrophages, others were
located in the macrophage cytoplasm. The majority of the pathogens
were round and a few were ellipsoidal; no sausage-like pathogens
were observed. The size of the pathogens varied between 2 and 5 µm.
The cell membrane of the pathogens could not be clearly observed. A
purplish-red, small nucleus and light blue cytoplasm were evident
in the pathogens. The boundary of the pathogens was indistinct. A
long, lightly stained area could be noted in the majority of
pathogens, which made the cell body appear to have two separate,
half-moon-shaped areas. This was easily confused with the dinuclear
P. marneffei (Fig. 3A). In
the PAS staining, the cell walls of the pathogens were not stained,
lightly stained or granulated and discontinuous, which made it
easier to differentiate Mucor from P. marneffei and
H. capsulatum (Fig. 3B).
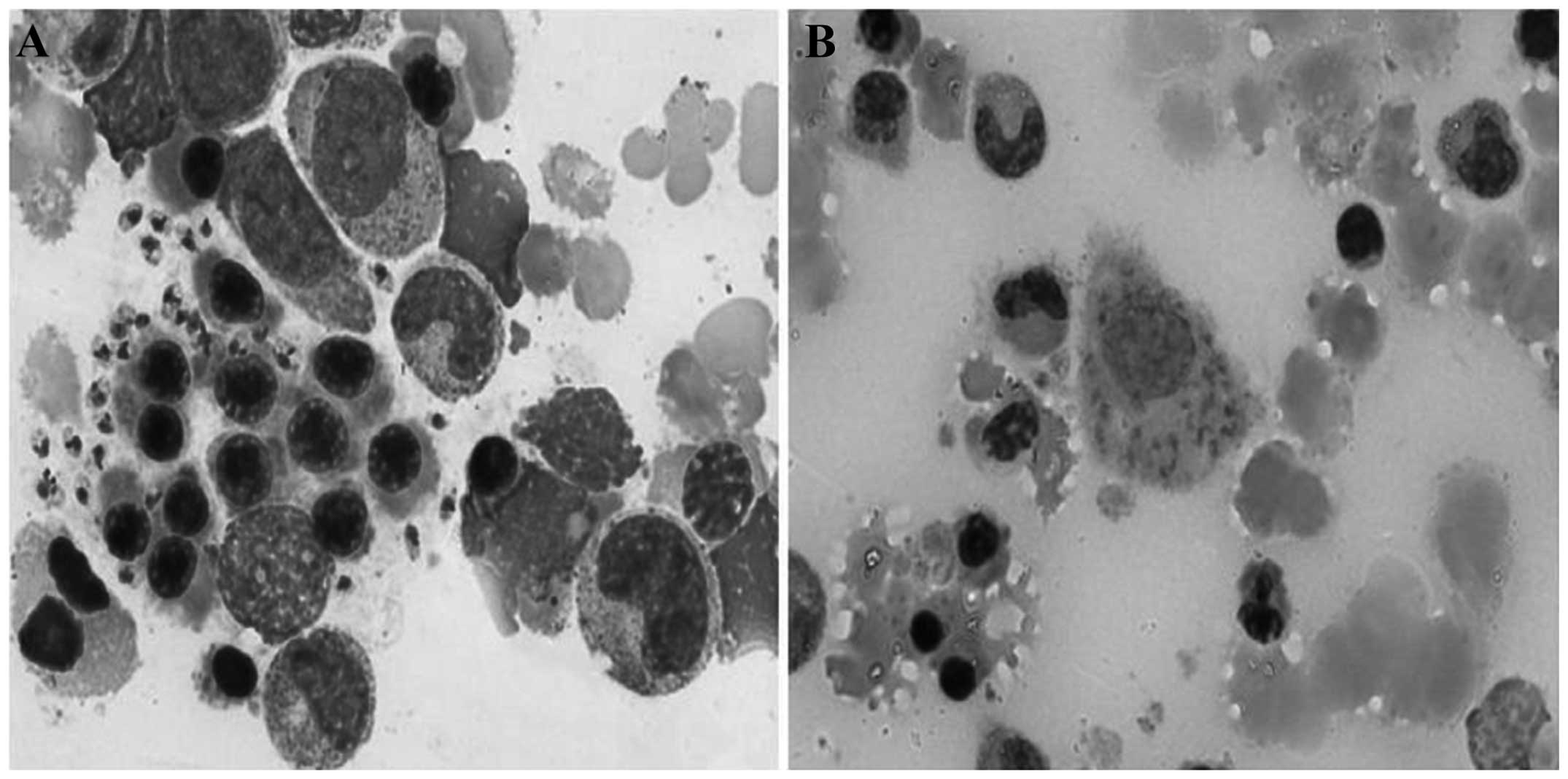
L. donovani
In the Wright-Giemsa staining, the majority of the
L. donovani pathogens were located in the cytoplasm of the
macrophages, and only a few pathogens were located extracellularly.
The majority of the pathogens exhibited round or ellipsoidal
shapes; only a few were sausage-like. Their sizes varied between 2
and 5 µm. The boundary of the pathogens could not be clearly
observed. A purplish-red, small nucleus and light blue cytoplasm
were evident in the pathogens. Furthermore, the L. donovani
exhibited a thin, rhabditiform or granulated, purplish kinetoplast
near the nucleus; this should not be confused with the dual-core
P. marneffei (Fig. 4A). In
the PAS staining, the cell walls and intracellular contents of the
pathogens were lightly stained or not stained. It was therefore
relatively simple to identify L. donovani when combining PAS
and Wright-Giemsa staining (Fig.
4B).
Discussion
Penicilliosis marneffei, histoplasmosis,
mucormycosis and visceral leishmaniasis are commonly found in
immunocompromised patients (1). In
the present study, a total of 55 patients who suffered from the
four described diseases were enrolled. It was found that all the
patients were HIV-positive, with the exception of one patient with
mucormycosis, who suffered from disseminated intravascular
coagulation caused by multiple organ damage; this patient succumbed
having only been in hospital for two days, meaning that the HIV
test had not yet been performed. The clinical manifestations,
including fever, hepatosplenomegaly, anemia and emaciation, were
evident in each of the 55 patients. The size and shape of the four
pathogens on the BMSs were similar, making it relatively difficult
to discriminate between them based solely on Wright-Giemsa
staining.
In the present study, a novel method of combining
Wright-Giemsa staining with PAS staining was utilized to help to
solve this problem. To the best of our knowledge, this is the first
study to provide a new method to identify the four easily confused
pathogens with Wright-Giemsa staining. P. marneffei and
H. capsulatum can cause serious systemic infections.
Following staining with PAS, the cell walls of these pathogens were
red and their outlines were clear and distinct. It was therefore
possible to observe and distinguish the two pathogens under a
low-power lens. Furthermore, as P. marneffei depends on
fissiparity, cells with cross-walls could be observed in the
mitotic phase. Cross-walls are the cell walls of two unseparated
cells, and they therefore appear thick and deep red in PAS
staining. The characteristic septate forms and the absence of
budding enable the differentiation between P. marneffei and
H. capsulatum. We propose that fission is a reliable
indicator for the diagnosis of penicilliosis marneffei (8,9).
H. capsulatum and Mucor depend on
spore reproduction. Neither sausage-like cells nor cross-walls
appeared in the two pathogens, but narrow-necked, single spores
could be observed in microscopy examination of H.
capsulatum. A long, lightly colored area was evident in the
majority of the Mucor pathogens, which made the cell body
appear in the form of two separate, half-moon-shaped areas. In the
PAS staining, the cell walls of the pathogens were not stained,
lightly stained or granulated and discontinuous, which made it
easier to distinguish Mucor from P. marneffei and
H. capsulatum.
L. donovani can cause visceral leishmaniasis
in humans, dogs and wild animals. In the Wright-Giemsa staining,
the pathogens exhibited a purplish-red, small nucleus and a thin,
rhabditiform or granulated, purplish kinetoplast. In the PAS
staining, the cell walls and intracellular contents of the
pathogens were lightly stained or not stained. It was therefore
relatively simple to distinguish L. donovani from the
dual-core P. marneffei when combining PAS and Wright-Giemsa
staining.
P. marneffei, H. capsulatum and Mucor
are opportunistic pathogens. While microbiological culture remains
the ‘gold standard’ for the diagnosis of opportunistic infection,
the examination takes 3–10 days. Examinations of biopsied tissue
samples provide an early diagnosis, as the fungus can be identified
by the representative morphological characteristics. The
examination of BMSs is always conducted for morphological
examination in patients with AIDS, and the cases in the present
study were also patients with HIV infection. The examination of
BMSs lays the foundation for the further identification and early
diagnosis of the four pathogens and their corresponding
diseases.
In conclusion, the four diseases of the present
study can lead to similar clinical manifestations, which include
the first symptoms of headache, cough or fever. Furthermore, these
pathogens are found in patients with low immunity and exhibit
similarities in microscopic morphology and size. They are
additionally all located in mononuclear macrophages, meaning that
the four pathogens are easily confused. The differentiation of the
four pathogens is therefore relatively problematic when relying
solely on Wright-Giemsa staining. In the present study, it was
shown that a method combining PAS and Wright-Giemsa staining could
effectively enhance the identification of the four pathogens. This
method is simple and can enable the rapid diagnosis of
penicilliosis marneffei, histoplasmosis, mucormycosis and visceral
leishmaniasis. Furthermore, the method may have an important role
in diagnosing the four diseases in cases in which it is not
possible to conduct pathogen culture and serology tests.
References
|
1
|
Cooper CR Jr and McGinnis MR: Pathology of
Penicillium marneffei. An emerging acquired immunodeficiency
syndrome-related pathogen. Arch Pathol Lab Med. 121:798–804.
1997.PubMed/NCBI
|
|
2
|
Bo L and Ping F: Research progress of
penicilliosis marneffei. J Dermatol Venereol. 32:26–28. 2010.[(In
Chinese)].
|
|
3
|
Freifeld AG, Iwen PC, Lesiak BL, Gilroy
RK, Stevens RB and Kalil AC: Histoplasmosis in solid organ
transplant recipients at a large Midwestern university transplant
center. Transpl Infect Dis. 7:109–115. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kauffman CA: Histoplasmosis: a clinical
and laboratory update. Clin Microbiol Rev. 20:115–132. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kauffman CA and Malani AN: Zygomycosis: an
emerging fungal infection with new options for management. Curr
Infect Dis Rep. 9:435–440. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roden MM, Zaoutis TE, Buchanan WL, et al:
Epidemiology and outcome of zygomycosis: a review of 929 reported
cases. Clin Infect Dis. 41:634–653. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singh S and Sivakumar R: Challenges and
new discoveries in the treatment of leishmaniasis. J Infect
Chemother. 10:307–315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mo W, Deng Z and Li S: Clinical blood
routine and bone marrow smear manifestations of disseminated
penicilliosis marneffei. Chin Med J (Engl). 115:1892–1894.
2002.PubMed/NCBI
|
|
9
|
Deng Z and Liu X: Disseminated
Penicilliosis marneffei in a patient with acquired immunodeficiency
syndrome: a first case report from China. Chin Med J (Engl).
113:1049–1050. 2000.PubMed/NCBI
|