Introduction
A previous study demonstrated that immune-related
cytokines are involved in the pathology of cerebral ischemia and
subsequent neuronal death (1);
however, few cytokines, such as interleukin (IL)-l, nerve growth
factor, transforming growth factor-β and tumor necrosis factor
(TNF)-α, have been directly associated with cellular damage
(2,3)
following experimentally induced cerebral ischemia. IL-6 was
initially identified as B-cell stimulating factor (4), and is also synthesized by neurons and
glia. IL-6 mRNA expression in the brain is known to increase in
various central nervous system (CNS) disorders, including cerebral
ischemia (5,6). IL-6 has been demonstrated to be crucial
for neuron survival in culture (7,8), and
serves a key function in the regeneration of peripheral nerve cells
(9,10).
IL-6 functions via two subunits of its receptor: The
α-chain is the IL-6 binding protein gp80, and the β-chain is the
signal-transducing protein gp130 (11). Two pathways are activated by gp130.
The first pathway, the mitogen-activated protein kinase pathway, is
Ras-dependent and leads to the activation of a variety of
transcription factors, such as nuclear factor for IL-6, ETS
domain-containing protein Elk-1 and activator protein 1; the second
is the Janus kinase (JAK)-signal transducer and activator of
transcription (STAT) pathway, which involves the activation of JAK
and the STAT family members STAT1 and STAT3 (12,13). The
two pathways have been implicated in cell proliferation and
survival. Generally, the apoptosis-related B-cell lymphoma 2
(Bcl-2) protein family is believed to be a regulator of cell
survival (14) and Bcl-2, which is
highly expressed in malignant plasma cells, has been extensively
studied among the Bcl-2 family proteins. Furthermore, a prior study
has indicated that Bcl-2 protein is able to mediate cell cycle
function (15). By contrast, Bcl-xL
is thought to be a potential marker of chemoresistance regulating
cell apoptosis in myeloma (16). A
number of studies have indicated that induced myeloid leukemia cell
differentiation protein Mcl-1 is crucial for the survival of B
cells, particularly during the late stages of B-cell
differentiation (17,18). In addition, IL-6 is known to regulate
Mcl-1 and Bcl-xL proteins in myeloma cells (19,20). The
aim of the present study, therefore, is to investigate the
neuroprotective effects of the inflammatory cytokine IL-6 in a rat
model of cerebral ischemia, and to investigate the involvement of
the JAK/STAT pathway, i.e. the phosphorylation of STAT3 following
IL-6 treatment, in this process.
Materials and methods
Rat models
All experimental protocols were approved by the
Institutional Animal Care and Use Committee of Tongji Medical
College, Huazhong University of Science and Technology (Wuhan,
China). Adult male Sprague Dawley rats weighing 250–280 g were
obtained from Vital River Laboratory Animal Technology Co., Ltd.
(Beijing, China). Focal cerebral ischemia was induced via
intraluminal middle cerebral artery occlusion (MCAO), as described
by Longa et al (21), with
certain modifications. Briefly, the rats were intraperitoneally
(i.p.) anesthetized with chloral hydrate (350 mg/kg), and a
surgical nylon monofilament tip coated with 0.01% poly-L-lysine was
then introduced into the left internal carotid artery through the
external carotid stump. This filament was advanced 18–20 mm beyond
the carotid bifurcation until a slight resistance was detected. At
this point, the origin of the middle cerebral artery was obstructed
by the intraluminal filament, and all blood flow from the internal
carotid, anterior cerebral and posterior cerebral arteries was
occluded. The body temperature of the rats was maintained at
37±0.5°C throughout the procedure. The filament was left in
position for 2 h and then withdrawn. The rats were returned to
their cages and closely monitored until they were observed to have
recovered from the anesthesia. Any rats that exhibited an absence
of neurological deficits immediately following reperfusion
(neurological score, <3) were excluded from the study.
Sham-operated rats were treated identically, with the exception
that the MCAs were not occluded following the neck incision.
Drug preparation and treatment
schedule
Recombined IL-6 was purchased from PeproTech, Inc.
(Rocky Hill, NJ, USA) and dissolved in physiological saline. A
total of 52 rats were divided at random into sham, saline, IL-6 (50
ng, i.p.) and IL-6 (500 ng, i.p.) treatment groups. IL-6 solution
or a vehicle of physiological saline was administered 10 min after
the MCAO procedure.
Infarct volume determination
Each rat was sacrificed 24 h after reperfusion and
the brain was removed rapidly and frozen at −20°C for 5 min.
Coronal slices were collected at points 2 mm from the frontal tips
and immersed in 2% 2,3,5-tripenyltetrazolium chloride stain at 37°C
for 20 min. Following staining, color images of the slices were
captured using a Kodak 7230 digital camera (Kodak, Rochester, NY,
USA) and Adobe Photoshop software, version 7.0 (Adobe Systems,
Inc., San Jose, CA, USA). The infarct volume was calculated using
the Mias-2000 image analysis system (Institute of Graphics and
Images, Sichuan University, Chengdu, China).
Neurological deficit
determination
Symptoms of neurological deficit in the vehicle- and
drug-treated groups were assessed after 24 h of reperfusion
according to the method described by Longa et al (21). Neurological findings were scored on a
five-point scale, as follows: No neurological deficit, 0; failure
to extend right paw fully, 1; circling to the right, 2; falling to
the right, 3; and inability to walk spontaneously with depressed
levels of consciousness, 4.
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) staining
To detect neuronal apoptosis, in situ nick
end labeling was performed using a commercial kit (In Situ
Apoptosis Detection kit; Roche Diagnostics, Indianapolis, IN, USA).
Briefly, the tissue sections were washed in Tris-buffered saline
(TBS) and permeabilized using Proteinase K (20 µg/ml) for 10 min.
Following permeabilization, the sections were quenched for 5 min in
3% H2O2 in methanol at room temperature (RT).
The sections were then incubated in equilibration buffer for 20 min
prior to labeling for 100 min at 37°C. The reaction was terminated
by stop buffer. Subsequent to further washing in TBS, the sections
were incubated in peroxidase-streptavidin conjugate (In Situ
Apoptosis Detection Kit) for 45 min, and reacted with
3,3-diaminobenzidine tetrahydrochloride solution for 15 min at
RT.
Isolation of cortical neurons
Cerebral cortices were isolated and the meninges
removed, after which the tissue was minced and treated with 0.25%
trypsin in Earles balanced salt solution for 1 min. After
centrifugation, cortical neurons were isolated. Neurons were
isolated from each group, including the sham, saline, 50 ng IL-6
and 500 ng IL-6. Rats were treated with IL-6 (50 ng, i.p.) and IL-6
(500 ng, i.p.), IL-6 solution or a vehicle of physiological saline
was administered 10 min after the MCAO procedure.
Detection of annexin V staining and
caspase-3 expression
Cortical neurons, which were prepared and treated as
described above, were double-labeled with phycoerythrin
(PE)-conjugated caspase-3 monoclonal antibody and fluorescein
isothiocyanate (FITC)-conjugated annexin V (BD Pharmingen, San
Diego, CA, USA) for 1 h at RT. PE- and FITC-conjugated murine
immunoglobulin G were used as controls. Subsequent to staining, the
cells were assessed using flow cytometry. Cells were fixed and
permeabilized, then 5×105 cells were stained with 1
µg/ml antibodies against the active form of caspase-3 and annexin V
(BD-Pharmingen) for 60 min at room temperature. Cell were
subsequently washed with phosphate-buffered saline and analyzed in
a FACScan flow cytometer (FACSCalibur) and CellQuest software (BD
Biosciences, Franklin Lakes, NJ, USA).
Western blot analysis
Protein expression and phosphorylation were detected
by western blot analysis. After 12 h of culturing, the cells were
lysed in buffer containing 125 mM Tris-HCl (pH 6.8), 20% glycerol,
1% 2-mercaptoethanol and 2% sodium dodecyl sulfate (SDS). The total
protein from each sample was separated on a 12% SDS-polyacrylamide
gel and electroblotted onto a Hybond-C nitrocellulose membrane
(Amersham Pharmacia, Freiburg, Germany). The membrane was
subsequently blocked with 5% non-fat dry milk powder in TBS and
incubated for 1 h with rat monoclonal phospho-Stat1 (#8826), Stat3
(#9139) and phospho-Stat3 (#9145) and polyclonal Stat1 (#9172) and
Bcl2 (#2876) primary antibodies (1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA). In addition, a rat polyclonal
Mcl1 primary antibody (#A1832) from Selleck Chemicals was used
(1:500, Shanghai, China). Following incubation, the membrane was
washed four times with 0.05% Tween-20 in TBS and incubated with
peroxidase-conjugated anti-rabbit (#7074) and anti-mouse (#7076)
IgG secondary antibodies (Cell Signaling Technology, Inc.) for 1 h.
The membrane was then washed extensively and the bands in the
membrane were developed using enhanced chemiluminescence staining
(Amersham Pharmacia).
Measurement of myeloperoxidase (MPO)
activity
The enzymatic activity of MPO was measured as an
indicator of the accumulation of granulocytes in the ischemic brain
tissue (22). Briefly, the brains
were rapidly removed at different time-points after MCAO (STAT1/3
and p-STAT1/3, 15 min; Mcl-1 and Bcl-2, 4 h). Samples of ischemic
brain tissue weighing 100 mg were isolated, homogenized and
centrifuged for 15 min at 12,000 × g (4°C) for later biochemical
analysis. An MPO activity assay was conducted using a commercial
kit according to the manufacturers instructions (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) (23). Alterations in the absorbance at 460
nm were measured using a spectrophotometer (GE Healthcare
Bio-Sciences, Pittsburgh, PA, USA). One unit of MPO activity was
defined as the degradation of 1 ml H2O2 per
min at 37°C. The final results were expressed as units of MPO
activity per gram of wet brain tissue.
Cytokine content measurement in the
tissue
Cytokine levels of TNF-α, IL-1β and matrix
metalloproteinase (MMP)-9 in the rat brain were measured using
ELISA kits, according to the manufacturers instructions (R&D
Systems, Inc., Minneapolis, MN, USA).
Evans blue (EB) leakage
Blood-brain barrier (BBB) permeability was detected
by measuring the EB extravasation. EB leakage measurement was
performed as described previously (24). The quantity of EB in the supernatant
was measured spectrophotometrically at a wavelength of 610 nm and
compared with readings obtained from standard solutions.
Statistical analysis
Quantitative data are presented as the mean ±
standard deviation of at least three independent experiments.
Histological injury scoring data were analyzed by analysis of
variance (ANOVA) followed by the Kruskal-Wallis nonparametric test
for comparison, which is presented as a box-and-whisker plot. The
remaining data were analyzed by ANOVA and the Newman-Keuls test for
comparison. For comparisons among the groups, the unpaired Students
t-test was performed using GraphPad Prism software (GraphPad
Software, Inc., San Diego, CA, USA), in which P<0.05 was
considered to indicate a statistically significant difference.
Results
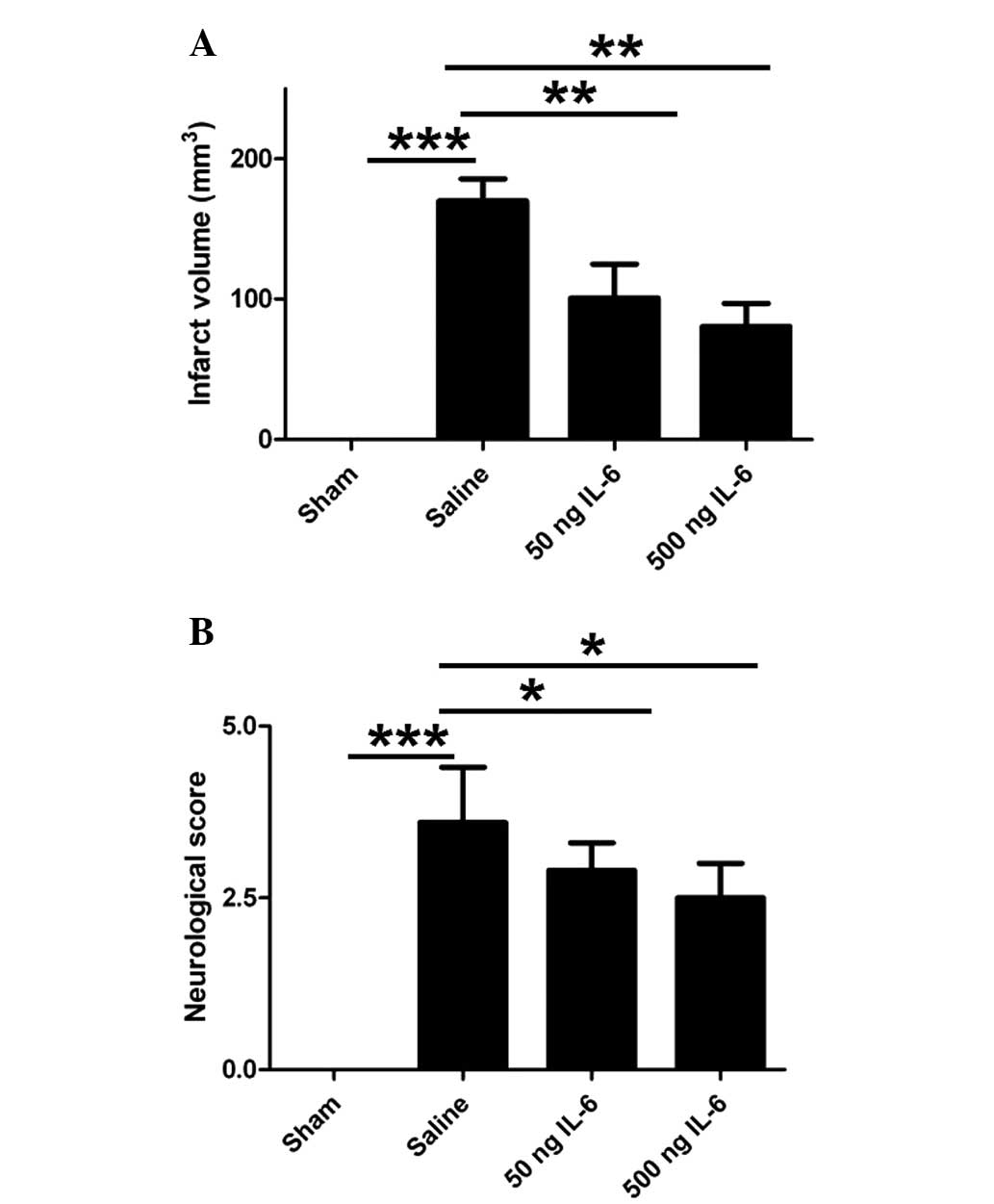
IL-6 treatment reduces infarct volume
and neurological score
To evaluate the efficacy of IL-6 in the rat model of
cerebral ischemia/reperfusion, the infarct volume and neurological
score were measured 24 h after the MCAO procedure. In the IL-6
treated rats, the infarct volume (Fig.
1A) and neurological score (Fig.
1B) were reduced significantly in a dose-dependent manner
compared with those in the vehicle-treated rats. These results
indicate that IL-6 is able to mitigate the damage associated with
ischemia/reperfusion-induced brain injury.
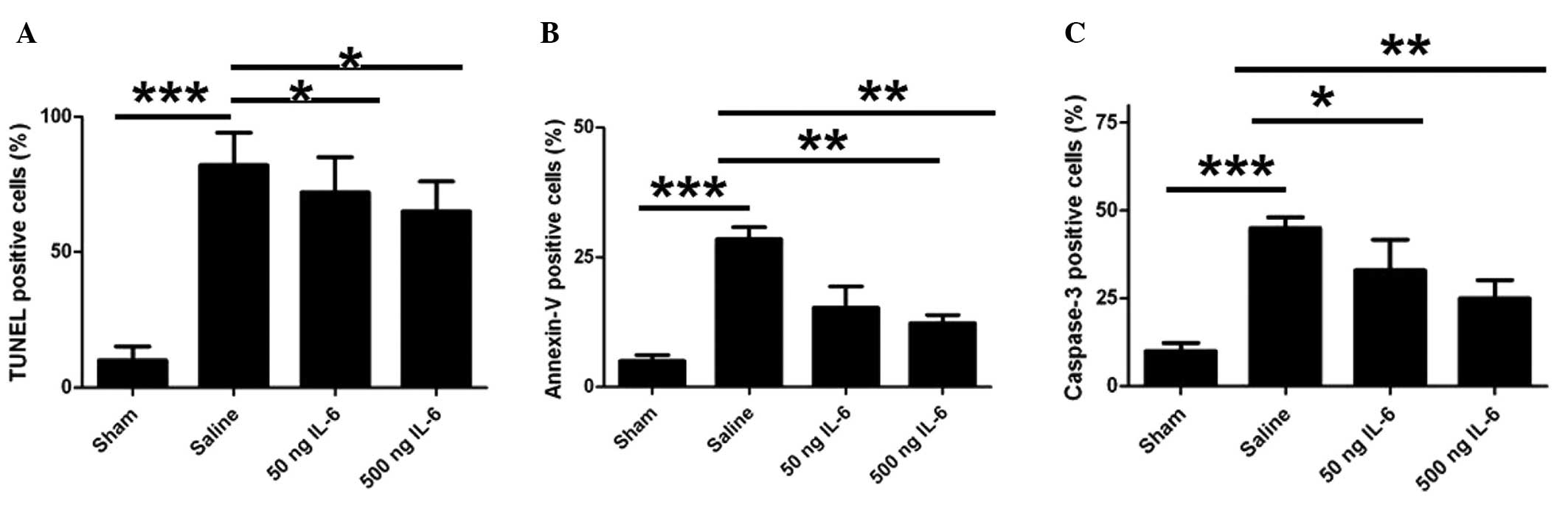
IL-6 treatment effectively inhibits
ischemia-induced apoptosis
To elucidate the mechanism underlying the
neuroprotective effect of IL-6 on ischemia/reperfusion, neuronal
apoptosis was determined using TUNEL staining. After 24 h of
reperfusion, MCAO induced considerable DNA fragmentation and a
large number of TUNEL-positive cells in the vehicle group compared
with the sham group; however, the TUNEL-positive cell count was
significantly reduced by the IL-6 treatment (Fig. 2A). To further confirm the effect of
IL-6 on neuronal apoptosis, pure cortical neurons were isolated and
the in vivo regulation of apoptosis by IL-6 injection was
assessed. Consistently, 50 and 500 ng doses of IL-6 effectively
inhibited the ischemia-induced apoptosis, as indicated by annexin V
binding, compared with the vehicle control (Fig. 2B). In addition, the number of
activated caspase-3-positive neurons also increased markedly in the
vehicle group compared with the sham group (Fig. 2C), while IL-6 treatment attenuated
this increase (Fig. 2C). These
results indicate that IL-6 mitigates ischemia-induced neuronal
apoptosis.
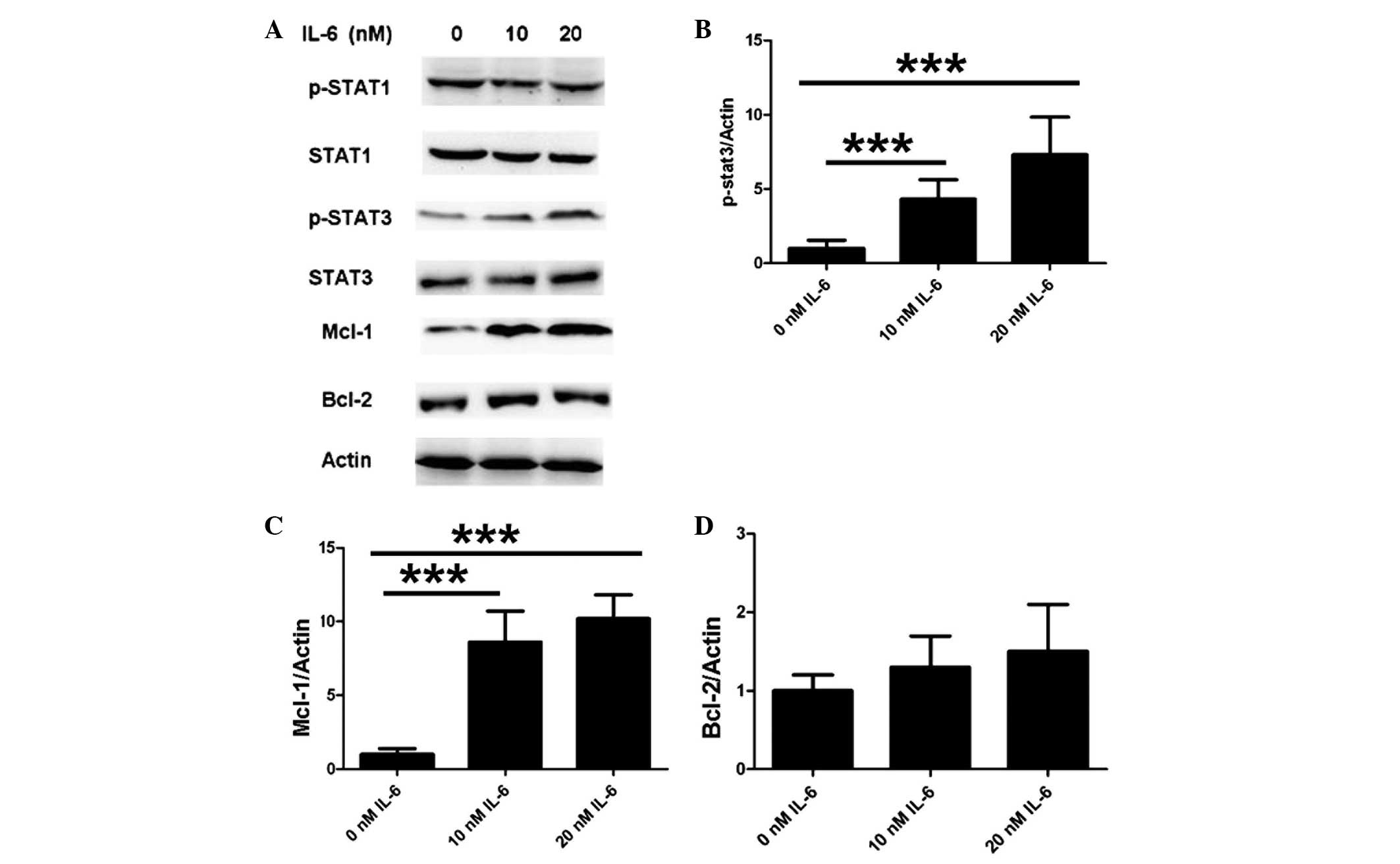
IL-6 modulates neuronal anti-apoptotic
proteins by activating STAT3 in the JAK/STAT pathway
To further elucidate the mechanism underlying the
inhibitory function of IL-6 on apoptosis, apoptotic proteins were
examined using western blot analysis. To specifically investigate
the JAK/STAT pathway, the cells were treated with various
concentrations of IL-6 in vitro. As shown in Fig. 3A and B, IL-6 induced the
phosphorylation of STAT3 while exerting no effect on total STAT3
expression. No difference was observed in the phosphorylation of
STAT1 and total STAT1 expression following IL-6 treatment under
identical experimental conditions (Fig.
3A). Furthermore, neuronal Mcl-1 expression was upregulated
following the IL-6 treatment (Fig.
3C), but the treatment did not result in any difference in
Bcl-2 expression (Fig. 3D). These
results suggest that IL-6 modulates neuronal anti-apoptotic
proteins by activating STAT3 in the JAK/STAT pathway.
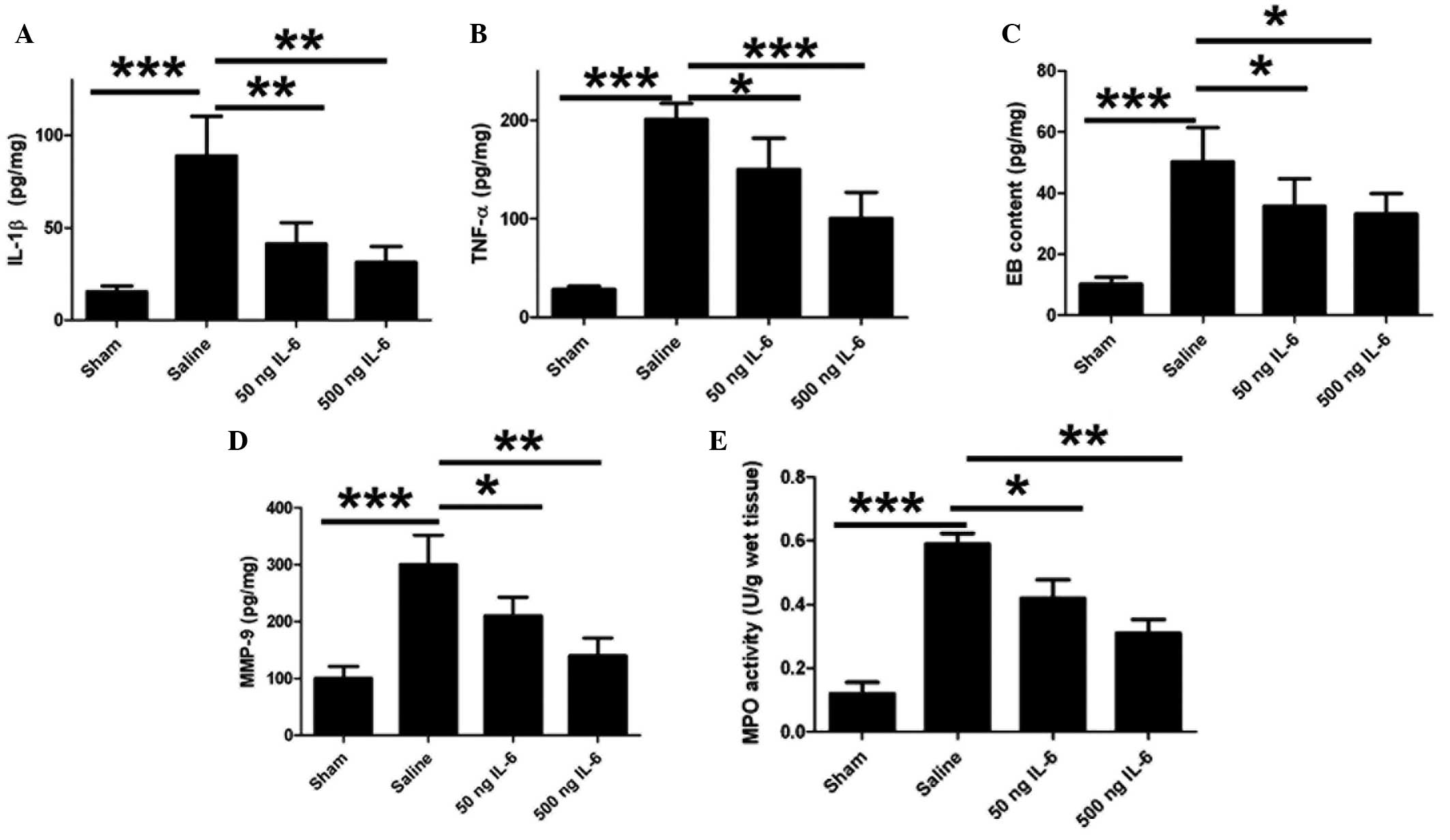
IL-6 treatment reduces levels of IL-1β
and TNF-α and ameliorates EB leakage
The levels of a number of inflammatory cytokines
were quantified in order to determine whether IL-6 influences the
regulation of cytokine secretion and BBB integrity in cerebral
ischemia As shown in Fig. 4A and B,
IL-6 induced a significant reduction in the levels of IL-1β and
TNF-α in the rat brain, suggesting that IL-6 mediates the immune
response following the inhibition of neuronal apoptosis. As
inflammatory cytokines are responsible for the BBB integrity, the
effect of IL-6 on the BBB permeability following
ischemia/reperfusion was subsequently investigated. EB
extravasation was detected in the ischemic region; however, IL-6
injection notably reduced this EB leakage in vivo (Fig. 4C). High levels of MMP-9 expression
were observed in the ischemic brain tissue, and IL-6 reduced these
levels (Fig. 4D). Furthermore, IL-6
reduced MPO activity in a dose-dependent manner (Fig. 4E).
Discussion
Apoptosis is a typical cell function with various
characteristic morphological features, including DNA fragmentation,
nuclear chromatin condensation and cell shrinkage. Apoptosis is
widely implicated in neuronal disease; for example, apoptosis can
lead to neuronal death in Alzheimers disease, and amyloid β-protein
may be involved in this process (25,26). It
is, however, unknown which specific factors regulate the apoptosis
of neurons following cerebral ischemia. To confirm the
anti-apoptosis effect of IL-6, the rate of neuronal apoptosis was
measured using a number of independent methods in the present
study. A TUNEL assay demonstrated that cerebral ischemia induced
DNA fragmentation. Consistently, IL-6 also reduced annexin V
binding and caspase-3 expression in freshly isolated cortical
neurons compared with the cells from the saline-treated group.
These results therefore demonstrate that IL-6 protects neurons
against apoptosis. To elucidate the possible associated mechanism,
the signal transduction was investigated in neurons from cerebral
ischemia mice in vitro.
In the present study, high expression levels of
Mcl-1 were observed to be associated with reduced levels of
apoptosis in the IL-6-treated injured neurons. Mcl-1 is implicated
in myeloid pathways upon exposure to
12-O-tetradecanoylphorbol-13-acetate (27). Although Mcl-1 has a key function in
B-cell differentiation and survival, the exact of role of Mcl-1 has
not been defined (28,29). The results of the present study
additionally reveal that IL-6 induces STAT3 phosphorylation in
primary neuronal cells. We therefore hypothesized that the
phosphorylation of STAT3 in the JAK/STAT pathway stimulates Mcl-1
expression. Consequently, these results suggest that STAT3 is
involved in the IL-6-mediated anti-apoptosis activity, and the
JAK/STAT pathway (30,31) may serve a key function in mediating
Mcl-1 and the apoptotic processes.
The present study indicated that IL-6 is able to
alleviate the cerebral ischemic/reperfusion damage in a rat model.
Furthermore, IL-6 exerted a neuroprotective effect by inhibiting
neuronal apoptosis and inflammatory mediators in the brain.
Increased MPO activity was observed following the ischemic injury;
however, IL-6 reduced the MPO activity, suggesting that IL-6 is
able to inhibit the inflammatory responses in the brain. This
observation was confirmed by the downregulation of inflammatory
cytokines, including TNF-α and IL-1β. These results indicate that
IL-6 ameliorates the symptoms of ischemic brain injury by
preventing the secretion of inflammatory cytokines and the immune
response in the brain. The breakdown of the BBB is involved in the
pathogenesis of cerebral ischemia, and the present data regarding
the reduced MMP-9 expression following IL-6 treatment are
consistent with the reduction in EB content in the rat brain
(32,33). This therefore suggests that IL-6
protects the BBB and exerts a neuroprotective effect in
MCAO-induced cerebral ischemia. To the best of our knowledge, the
present study is the first to demonstrate the efficacy of an
inflammatory cytokine in a rat model of cerebral ischemia in rats
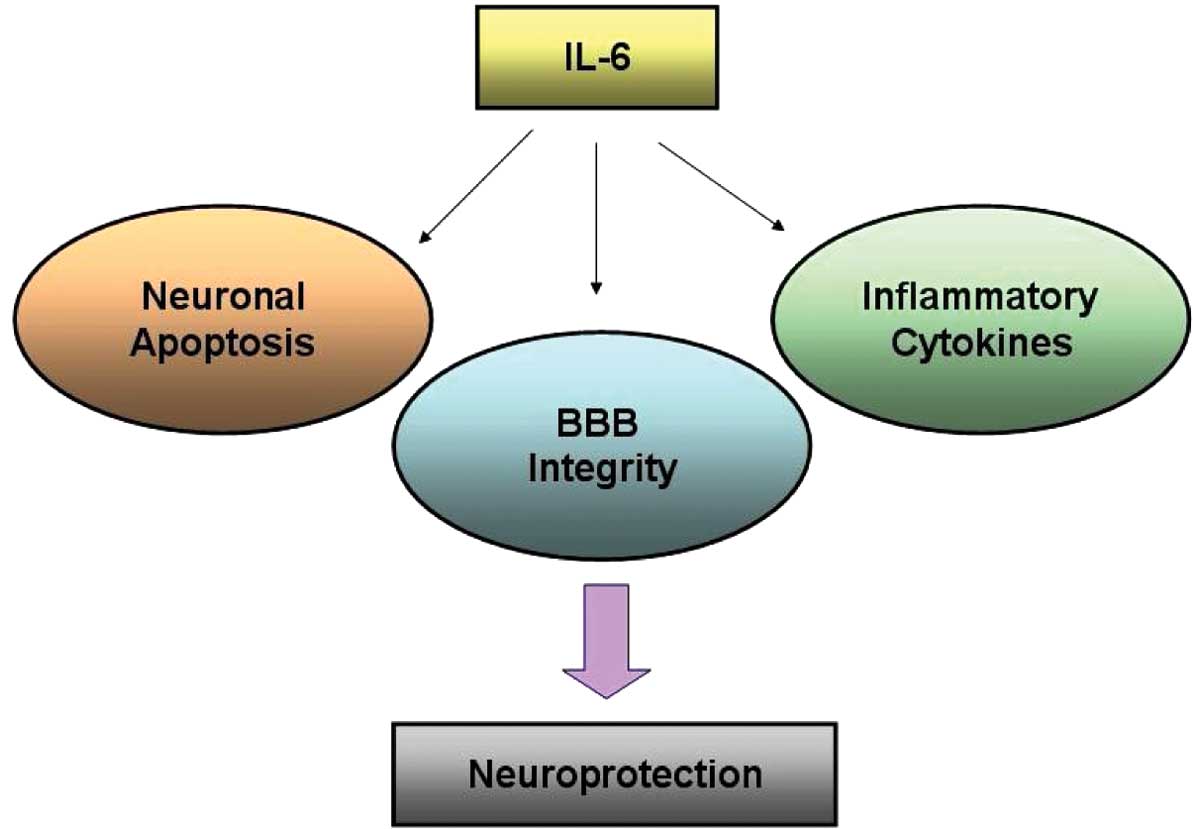
via anti-inflammation and anti-apoptosis pathways (Fig. 5).
In conclusion, the results of the present study
suggest that IL-6 plays a comprehensive role in cerebral ischemia
by mediating neuronal apoptosis, inflammatory cytokines and BBB
integrity in the CNS (Fig. 5). The
present study has thus elucidated a possible mechanism underlying
the actions of IL-6 in this disease and has indicated the
possibility of the application of IL-6 as a therapeutic agent for
cerebral ischemia.
Acknowledgements
The study was supported by a grant from the Natural
Science Foundation of Hubei Province, China (no. 070413066).
References
|
1
|
Amantea D, Nappi G, Bernardi G, Bagetta G
and Corasaniti MT: Post-ischemic brain damage: Pathophysiology and
role of inflammatory mediators. FEBS J. 276:13–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Y, Cao M, Liu A, Di W, Zhao F, Tian Y
and Jia J: Changes of inflammatory cytokines and neurotrophins
emphasized their roles in hypoxic-ischemic brain damage. Int J
Neurosci. 123:191–195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pradillo JM, Denes A, Greenhalgh AD,
Boutin H, Drake C, McColl BW, Barton E, Proctor SD, Russell JC,
Rothwell NJ and Allan SM: Delayed administration of interleukin-1
receptor antagonist reduces ischemic brain damage and inflammation
in comorbid rats. J Cereb Blood Flow Metab. 32:1810–1819. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kishimoto T: The biology of interleukin-6.
Blood. 74:1–10. 1989.PubMed/NCBI
|
|
5
|
Wakabayashi K, Nagai A, Sheikh AM, Shiota
Y, Narantuya D, Watanabe T, Masuda J, Kobayashi S, Kim SU and
Yamaguchi S: Transplantation of human mesenchymal stem cells
promotes functional improvement and increased expression of
neurotrophic factors in a rat focal cerebral ischemia model. J
Neurosci Res. 88:1017–1025. 2010.PubMed/NCBI
|
|
6
|
Nakamachi T, Tsuchida M, Kagami N, Yofu S,
Wada Y, Hori M, Tsuchikawa D, Yoshikawa A, Imai N, Nakamura K, et
al: IL-6 and PACAP receptor expression and localization after
global brain ischemia in mice. J Mol Neurosci. 48:518–525. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hama T, Kushima Y, Miyamoto M, Kubota M,
Takei N and Hatanaka H: Interleukin-6 improves the survival of
mesencephalic catecholaminergic and septal cholinergic neurons from
postnatal, two-week-old rats in cultures. Neuroscience. 40:445–452.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dooley D, Vidal P and Hendrix S:
Immunopharmacological intervention for successful neural stem cell
therapy: New perspectives in CNS neurogenesis and repair. Pharmacol
Ther. 141:21–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brázda V, Klusáková I, Hradilová Svíženská
I and Dubový P: Dynamic response to peripheral nerve injury
detected by in situ hybridization of IL-6 and its receptor mRNAs in
the dorsal root ganglia is not strictly correlated with signs of
neuropathic pain. Mol Pain. 9:422013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Allodi I, Udina E and Navarro X:
Specificity of peripheral nerve regeneration: Interactions at the
axon level. Prog Neurobiol. 98:16–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hibi M, Nakajima K and Hirano T: IL-6
cytokine family and signal transduction: A model of the cytokine
system. J Mol Med Berl. 74:1–12. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heim MH: The Jak-STAT pathway: Cytokine
signalling from the receptor to the nucleus. J Recept Signal
Transduct Res. 19:75–120. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heinrich PC, Behrmann I, Müller-Newen G,
Schaper F and Graeve L: Interleukin-6-type cytokine signalling
through the gp130/Jak/STAT pathway. Biochem J. 334:297–314.
1998.PubMed/NCBI
|
|
14
|
Adams JM and Cory S: The Bcl-2 protein
family: Arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Puthier D, Pellat-Deceunynck C, Barillé S,
Robillard N, Rapp MJ, Juge-Morineau N, Harousseau JL, Bataille R
and Amiot M: Differential expression of Bcl-2 in human plasma cell
disorders according to proliferation status and malignancy.
Leukemia. 13:289–294. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tu Y, Renner S, Xu F, Fleishman A, Taylor
J, Weisz J, Vescio R, Rettig M, Berenson J, Krajewski S, et al:
BCL-X expression in multiple myeloma: Possible indicator of
chemoresistance. Cancer Res. 58:256–262. 1998.PubMed/NCBI
|
|
17
|
Altmeyer A, Simmons RC, Krajewski S, Reed
JC, Bornkamm GW and Chen-Kiang S: Reversal of EBV immortalization
precedes apoptosis in IL-6-induced human B cell terminal
differentiation. Immunity. 7:667–677. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lømo J, Smeland EB, Krajewski S, Reed JC
and Blomhoff HK: Expression of the Bcl-2 homologue Mcl-1 correlates
with survival of peripheral blood B lymphocytes. Cancer Res.
56:40–43. 1996.PubMed/NCBI
|
|
19
|
Mérino D, Khaw SL, Glaser SP, Anderson DJ,
Belmont LD, Wong C, Yue P, Robati M, Phipson B, Fairlie WD, et al:
Bcl-2, Bcl-x(L), and Bcl-w are not equivalent targets of ABT-737
and navitoclax (ABT-263) in lymphoid and leukemic cells. Blood.
119:5807–5816. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dimberg LY, Dimberg A, Ivarsson K, Fryknäs
M, Rickardson L, Tobin G, Ekman S, Larsson R, Gullberg U, Nilsson
K, et al: Stat1 activation attenuates IL-6 induced Stat3 activity
but does not alter apoptosis sensitivity in multiple myeloma. BMC
Cancer. 12:3182012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsuo Y, Onodera H, Shiga Y, Nakamura M,
Ninomiya M, Kihara T and Kogure K: Correlation between
myeloperoxidase-quantified neutrophil accumulation and ischemic
brain injury in the rat. Effects of neutrophil depletion. Stroke.
25:1469–1475. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lou HY, Zhang XM, Wei XB, Wang RX and Sun
X: Anti-inflammatory effect of hydroxyethylpuerarin on focal brain
ischemia/reperfusion injury in rats. Chin J Physiol. 47:197–201.
2004.PubMed/NCBI
|
|
24
|
Gürsoy-Ozdemir Y, Bolay H, Saribaş O and
Dalkara T: Role of endothelial nitric oxide generation and
peroxynitrite formation in reperfusion injury after focal cerebral
ischemia. Stroke. 31:1974–1981. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
LaFerla FM, Tinkle BT, Bieberich CJ,
Haudenschild CC and Jay G: The Alzheimers A beta peptide induces
neurodegeneration and apoptotic cell death in transgenic mice. Nat
Genet. 9:21–30. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miravalle L, Tokuda T, Chiarle R, Giaccone
G, Bugiani O, Tagliavini F, Frangione B and Ghiso J: Substitutions
at codon 22 of Alzheimers abeta peptide induce diverse
conformational changes and apoptotic effects in human cerebral
endothelial cells. J Biol Chem. 275:27110–27116. 2000.PubMed/NCBI
|
|
27
|
Yang T, Buchan HL, Townsend KJ and Craig
RW: MCL-1, a member of the BLC-2 family, is induced rapidly in
response to signals for cell differentiation or death, but not to
signals for cell proliferation. J Cell Physiol. 166:523–536. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou P, Qian L, Bieszczad CK, Noelle R,
Binder M, Levy NB and Craig RW: Mcl-1 in transgenic mice promotes
survival in a spectrum of hematopoietic cell types and
immortalization in the myeloid lineage. Blood. 92:3226–3239.
1998.PubMed/NCBI
|
|
29
|
Reynolds JE, Yang T, Qian L, Jenkinson JD,
Zhou P, Eastman A and Craig RW: Mcl-1, a member of the Bcl-2
family, delays apoptosis induced by c-Myc overexpression in Chinese
hamster ovary cells. Cancer Res. 54:6348–6352. 1994.PubMed/NCBI
|
|
30
|
Jin S, Mutvei AP, Chivukula IV, Andersson
ER, Ramsköld D, Sandberg R, Lee KL, Kronqvist P, Mamaeva V, Ostling
P, et al: Non-canonical Notch signaling activates IL-6/JAK/STAT
signaling in breast tumor cells and is controlled by p53 and
IKKα/IKKβ. Oncogene. 32:4892–4902. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fahmi A, Smart N, Punn A, Jabr R, Marber M
and Heads R: p42/p44-MAPK and PI3K are sufficient for IL-6 family
cytokines/gp130 to signal to hypertrophy and survival in
cardiomyocytes in the absence of JAK/STAT activation. Cell Signal.
25:898–909. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang X, Zhang X, Wang C, Li Y, Dong L,
Cui L, Wang L, Liu Z, Qiao H, Zhu C, et al: Neuroprotection of
early and short-time applying berberine in the acute phase of
cerebral ischemia: Up-regulated pAkt, pGSK and pCREB,
down-regulated NF-κB expression, ameliorated BBB permeability.
Brain Res. 1459:61–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nagaraja TN, Keenan KA, Brown SL,
Fenstermacher JD and Knight RA: Relative distribution of plasma
flow markers and red blood cells across BBB openings in acute
cerebral ischemia. Neurol Res. 29:78–80. 2007. View Article : Google Scholar : PubMed/NCBI
|