Introduction
The annual percentage of recurrences following
coronary artery bypass graft (CABG) surgery that require further
revascularization therapy, is ∼8.6–10.4% (1). Patients with CABG have a tendency to
survive longer, leading to the issue of decreased long term patency
rates. The native coronary artery may also develop de novo
atherosclerosis, resulting in myocardial ischemia and angina. The
10-year patency rate of the internal mammary artery graft is
85–95%, whereas the 10-year patency rate of saphenous vein grafts
(SVG) is only ∼40% (2–5). Furthermore, 40% of patients with not
yet occluded SVG experience various extents of stenosis, the
treatment of which has become a common clinical problem (6–8). Graft
stenosis can be treated with secondary CABG or percutaneous
coronary intervention (PCI) in either the native vessel (NV) or the
graft. With significantly increased mortality, incidence of
myocardial infarction, and perioperative complications, the benefit
of secondary CABG is much lower, as compared with first-time CABG.
Therefore, PCI has become the preferential option for
revascularization following CABG treatment (9–10). The optimal
percutaneous revascularization strategy for patients with SVG
disease subsequent to CABG remains unclear and the results obtained
by previous retrospective studies are controversial (11–17);
thus, two questions remain unanswered. The first is the choice of
target vessel for either the graft or the native coronary artery.
The second is whether to use a drug eluting stent (DES) or bare
metal stent (BMS) for the PCI. The present study analyzed the
clinical and pathological manifestations of patients receiving
post-CABG PCI treatment. Furthermore, the risk factors for major
adverse cardiac events (MACEs) in patients subjected to PCI
post-CABG were investigated and the treatment strategy and
prognosis were discussed.
Materials and methods
Study design
Patients undergoing PCI for graft lesions post-CABG,
as demonstrated by ischemic symptoms or on angiography, were
investigated in the present study. The patients were treated at the
Tianjin Chest Hospital (Tianjin, China) between August 2005 and
March 2010 and included 211 males and 78 females with a mean age of
63.21±8.44 years. All demographic characteristics, cardiac risk
factors, clinical presentations, angiographic and procedural
results and in-hospital outcomes were prospectively recorded in
cardiovascular databases. Baseline patient demographic data are
shown in Table I. Patients with the
following characteristics were excluded: i) Liver or renal
dysfunction; ii) allergy or intolerance to aspirin or clopidogrel;
iii) PCI in both the NV and graft; iv) implanted with both DES and
BMS. The study protocol was approved by the Ethics Committee of
Tianjin Chest Hospital. Written informed consent was obtained from
all of the patients.
 | Table I.Baseline clinical characteristics of
the study population. |
Table I.
Baseline clinical characteristics of
the study population.
| Clinical
characteristic | Value |
|---|
| Age,
yearsa | 63.21±8.44 |
| Male, n (%) | 211 (73.01) |
| Hypertension, n
(%) | 186 (64.36) |
| Diabetes mellitus, n
(%) | 154 (53.29) |
| Hypercholesterolemia,
n (%) | 193 (66.78) |
| Smoking, n (%) | 154 (53. 29) |
| Previous MI, n
(%) | 115 (39.80) |
| Previous PCI, n
(%) | 61 (21.11) |
| Form of CHD |
| SA, n
(%) | 41 (14.19) |
| UA, n
(%) | 185 (64.01) |
| STEMI, n
(%) | 34 (11.76) |
| NSTEMI, n
(%) | 29 (10.03) |
| BMI,
kg/m2a | 25.99±3.27 |
| FBG,
mmol/la | 6.48±2.07 |
| FIB, g/la | 3.64±1.03 |
| CHO,
mmol/la | 4.80±1.16 |
| TG,
mmol/la | 2.01±1.51 |
| HDL-C,
mmol/la | 1.13±0.30 |
| LDL-C,
mmol/la | 2.72±0.74 |
| LVEF, %a | 56.80±7.03 |
| Graft age,
montdsa | 50.00±29.12 |
PCI procedure
Prior to the procedure, 300 mg/day aspirin and
300–600 mg clopidogrel were administered once. Quantitative
coronary angiographic analysis was performed using a validated,
edge-detection system (Medcon QCA software; Medcon Ltd., Tel Aviv,
Israel). All PCIs were carried out according to the practices and
preferences of the surgeon involved. This included the selection of
either a BMS or a DES and the anticoagulation therapy utilized
[heparin or bivalirudin, and the use of a glycoprotein IIb/IIIa
(GpIIb/IIIa) inhibitor]. Embolic protection devices, if technically
feasible, were used on a routine basis for SVG interventions. The
standard for a successful surgery was defined as a final residual
stenosis of <20% and thrombolysis in myocardial infarction (MI)
flow grade 3. Following the procedure, aspirin was administered
indefinitely. Clopidogrel (75 mg/day) was initially recommended for
≥6 months after DES implantation or for ≥3 months after BMS
implantation. Since December 2006, a minimum of 1 year of
clopidogrel has been recommended subsequent to DES placement
(18).
Clinical follow-up and study
end-points
Patients undergoing stent implantation at the
Tianjin Chest Hospital are routinely followed-up at 6 months, 1
year and annually thereafter by telephone interviews with the
patient or family and a review of the medical records. The primary
study end-point was all-cause mortality. The secondary study
end-point was a composite end-point of one of the following MACEs:
Cardiac mortality, non-fatal MI or target vessel revascularization
(TVR). MI was defined as the onset of chest pain in combination
with new, typical changes in the electrocardiogram and biochemical
evidence of myocardial necrosis. Since MIs recorded during the
follow-up period could have occurred in any region of the
myocardium it was not possible to establish whether the MI was
specific to the stented SVG segment. Target lesion
revascularization (TLR) was defined as the requirement for a
repeated revascularization procedure (either PCI or coronary bypass
surgery) due to re-stenosis in the stented segment. TVR was defined
as a new revascularization procedure in the target vessel, and also
included TLR. Any clinical events arising throughout the study were
adjudicated by an independent clinical events committee that was
blinded to the treatment assigned to the patient.
Statistical analysis
Continuous variables are expressed as the mean ±
standard deviation and were compared with the Student's t-test.
Categorical variables are expressed as frequencies and were
compared using the χ2 or Fisher's exact test. The
effects on survival were compared between the NV-PCI and graft-PCI,
and DES and BMS groups using the Kaplan-Meier survival curve and
log-rank test. Risk factors for MACEs post-PCI were analyzed with
multivariable Cox regression models. Odds ratios (ORs) and the 95%
confidence intervals (95% CIs) were used to express relative risk.
SPSS software (version 18.0; SPSS Inc., Chicago, IL, USA) was used
for the statistical analysis. All the tests were two-tailed, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Baseline clinical characteristics
Baseline patient demographic data are listed in
Table I.
Long-term follow-up outcomes
Among the 289 cases, only 24 were lost to follow-up
(8.3%), leaving a total of 265 patients who were followed. The mean
follow-up time was 37 months (range, 6–78 months). Eighty-eight
cases of MACEs occurred (33.2%), including 17 cardiac mortalities
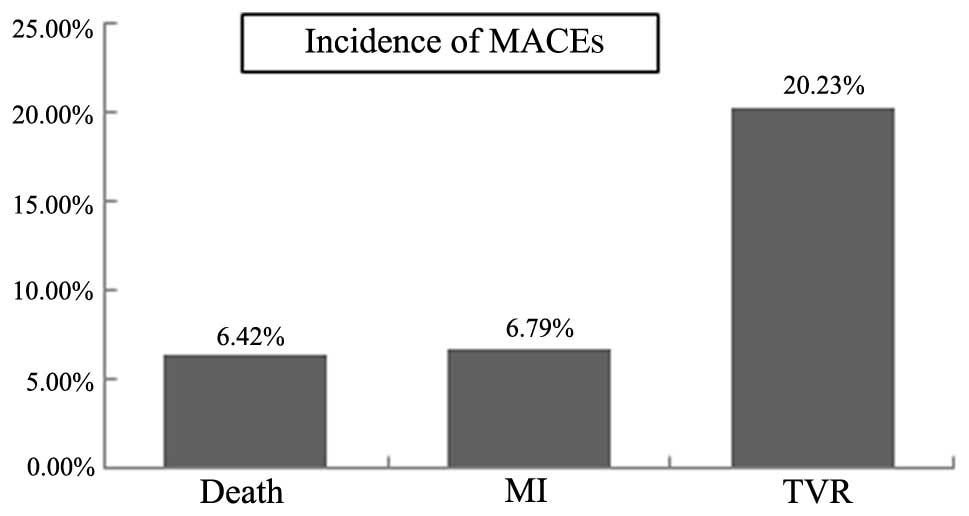
(6.4%), 18 MIs (6.8%) and 53 cases of TVR (20.0%) (Fig. 1).
Comparison of different PCI
strategies
NV-PCI was performed in 202 patients (69.9%) and
graft-PCI in 87 patients (30.1%). Compared with the NV-PCI group,
the graft-PCI group had more completely occluded NVs and fewer
completely occluded grafts, larger diameters of the smallest stents
and shorter stent lengths. Clinical baseline, angiographic and
procedural data of the two groups are shown in Table II. Two hundred sixty-five patients
were followed up for a mean time of 37 months, including 190
patients in the NV-PCI group and 75 patients in the graft-PCI
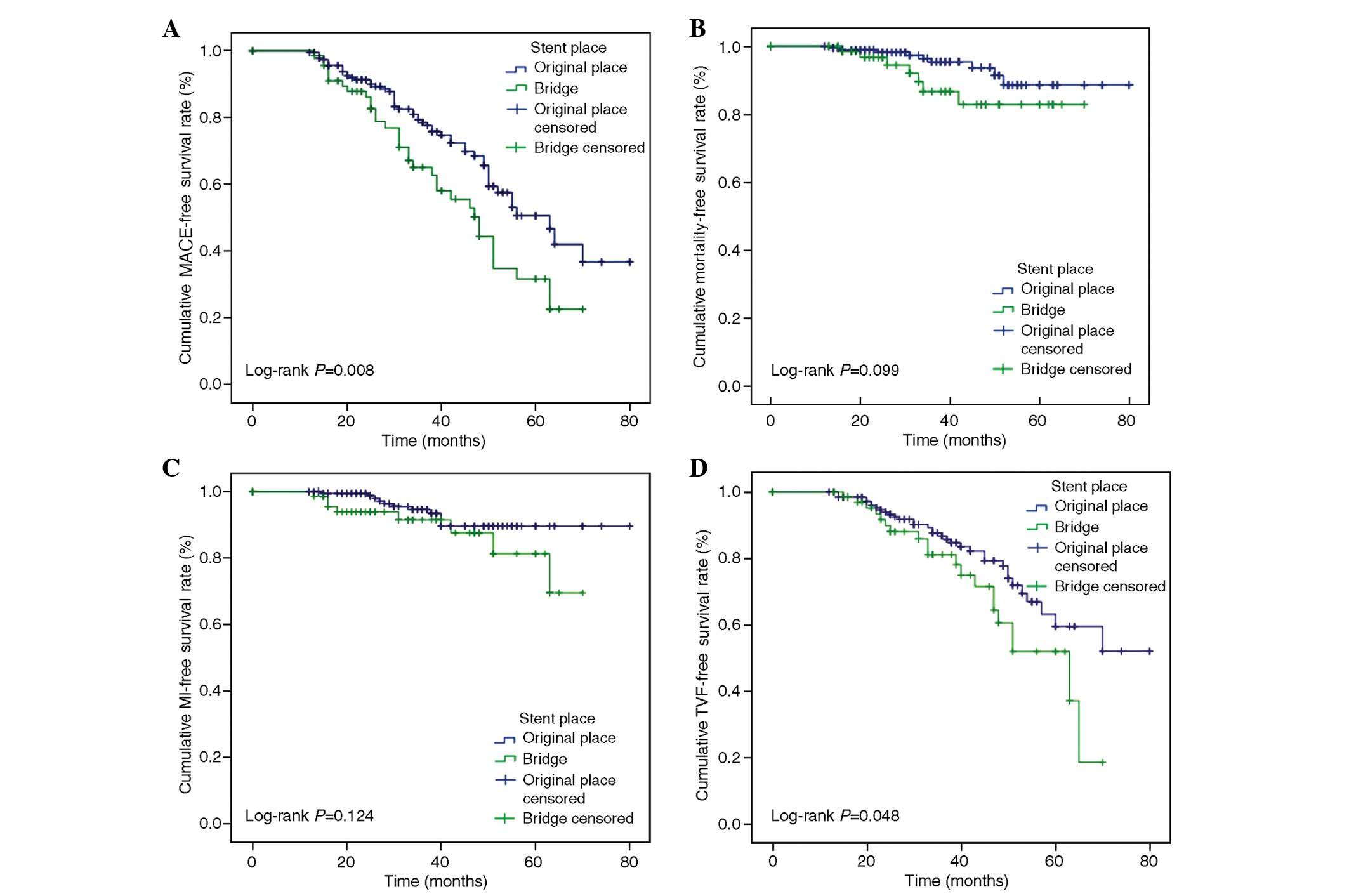
group. MACEs occurred in 54 patients in the NV-PCI group (28.4%)
and 34 patients in the graft-PCI group (45.3%). The NV-PCI group
had a higher MACE-free and revascularization-free survival compared
with the graft-PCI group (71.6 vs. 54.7%, log-rank P=0.008; 82.6
vs. 73.3%, log-rank P=0.048, respectively). No significant
difference was found in the overall survival and MI-free survival
between the groups (94.7 vs. 90.7%, log-rank P=0.099; 94.2 vs.
90.7%, log-rank P=0.124, respectively) (Fig. 2).
 | Table II.Comparison of baseline and procedural
characteristics according to PCI strategy. |
Table II.
Comparison of baseline and procedural
characteristics according to PCI strategy.
| Clinical
characteristic | NV-PCI | Graft-PCI | P-value |
|---|
| Age,
yearsa | 63.78±8.58 | 61.90±8.01 | 0.082 |
| Male, n (%) | 152 (75.25) | 59 (67.82) | 0.192 |
| Hypertension, n
(%) | 131 (64.85) | 55 (63.22) | 0.790 |
| Diabetes mellitus,
n (%) | 109 (53.96) | 45 (51.72) | 0.727 |
|
Hypercholesterolemia, n (%) | 139 (68.81) | 54 (62.07) | 0.264 |
| Smoking, n (%) | 103 (51.00) | 51 (58.62) | 0.283 |
| Previous MI, n
(%) | 80 (39.60) | 35 (40.23) | 0.921 |
| Previous PCI, n
(%) | 43 (21.30) | 18 (20.69) | 0.909 |
| Form of CHD |
|
|
|
| SA, n
(%) | 32 (15.84) | 9 (10.34) | 0.219 |
| UA, n
(%) | 130 (64.36) | 55 (63.22) | 0.853 |
| STEMI,
n (%) | 19 (9.41) | 15 (17.24) | 0.058 |
| NSTEMI,
n (%) | 21 (10.40) | 8 (9.20) | 0.755 |
| BMI,
kg/m2a | 26.06±3.27 | 25.83±3.26 | 0.573 |
| FBG,
mmol/la | 6.36±1.96 | 6.77±2.27 | 0.116 |
| FIB,
g/la | 3.64±1.01 | 3.65±1.07 | 0.933 |
| CHO,
mmol/la | 4.80±1.23 | 4.82±0.97 | 0.904 |
| TG,
mmol/la | 1.82±1.20 | 2.47±2.03 | 0.001 |
| HDL-C,
mmol/la | 1.13±0.31 | 1.12±0.30 | 0.757 |
| LDL-C,
mmol/la | 2.73±0.75 | 2.68±0.69 | 0.537 |
| LVEF,
%a | 56.74±6.23 | 56.94±8.64 | 0.825 |
| Graft age,
montdsa | 48.50±31.40 | 53.45±22.75 | 0.185 |
| Number of occluded
NVs, n (%) |
|
|
|
| 0 | 110 (54.46) | 28 (32.18) | 0.001 |
| 1 | 40 (19.80) | 19 (21.84) | 0.694 |
| 2 | 32 (15.84) | 21 (24.14) | 0.095 |
| ≥3 | 20 (9.90) | 19 (21.84) | 0.006 |
| Number of occluded
grafts, n (%) |
|
|
|
| 0 | 64 (31.68) | 35 (40.23) | 0.160 |
| 1 | 66 (32.67) | 38 (43.68) | 0.074 |
| 2 | 36 (17.82) | 7 (8.05) | 0.032 |
| ≥3 | 36 (17.82) | 7 (8.05) | 0.032 |
| Number of
stentsa | 2.24±1.12 | 2.15±1.14 | 0.544 |
| Minimal stent
diameter, mma | 2.95±0.69 | 3.17±0.58 | 0.008 |
| Total stent lengtd,
mma | 45.35±22.14 | 39.29±19.92 | 0.029 |
| GpIIb/IIIa
inhibitor, n (%) | 54 (26.73) | 26 (29.89) | 0.583 |
| Embolic protection
device, n (%) | - | 31 (35.63) | NA |
| Complete
revascularization, n (%) | 53 (26.24) | 18 (20.60) | 0.315 |
Comparison of the stent types for
PCI
DESs were used in 239 patients (82.7%) and BMSs in
50 patients (17.3%). Patients in the BMS group were older compared
with those in the DMS group. The groups did not differ
significantly in the number of occluded NVs or grafts (P>0.05).
The BMS group had larger stent diameters but fewer stents (both
P<0.05). Baseline clinical, angiographic and procedural data of
the two groups are listed in Table
III. Of the 265 patients who completed the long-term follow-up,
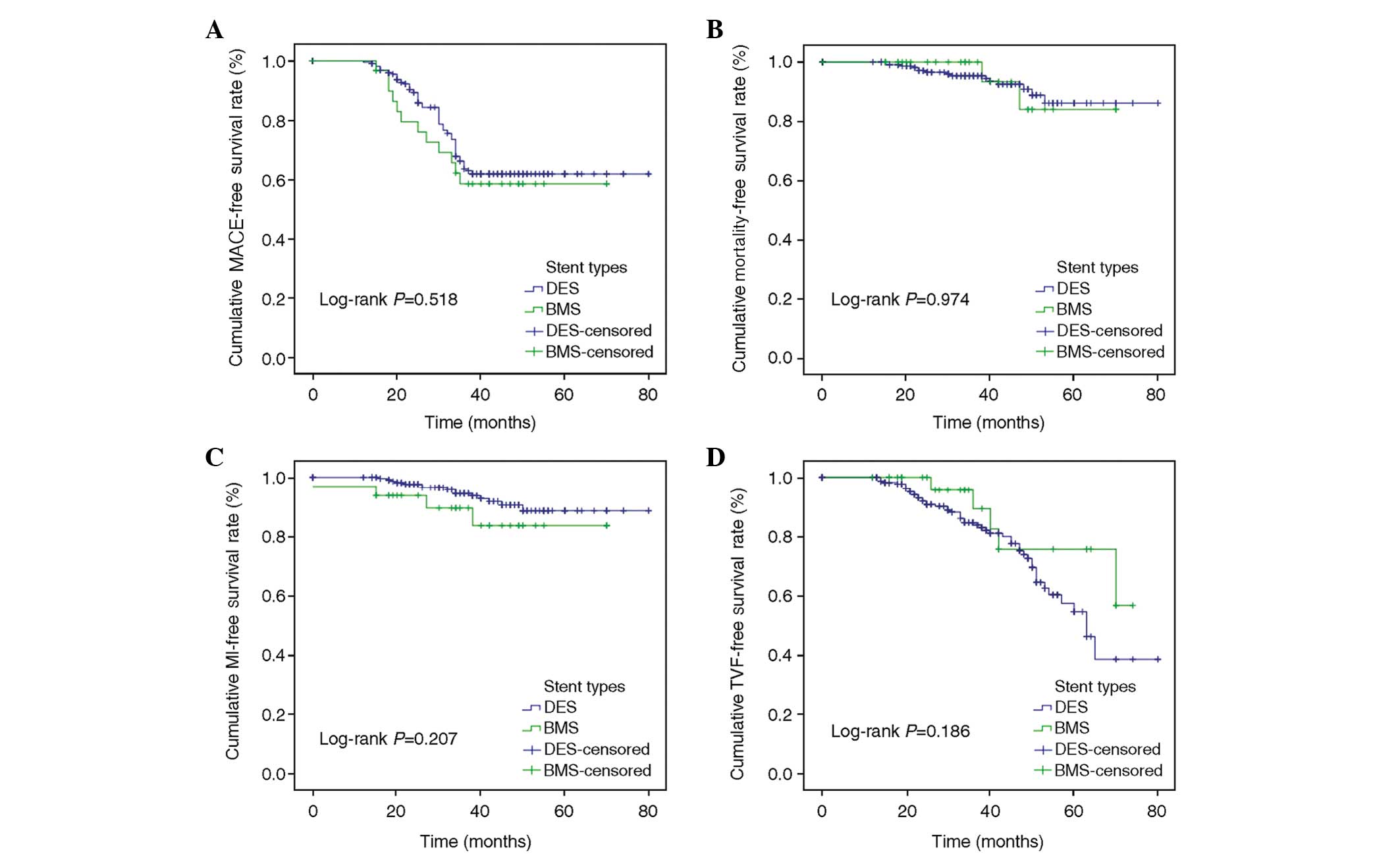
217 were in the DES group and 48 in the BMS group. There were 63
occurrences (29.0%) of MACEs in the DES group and 25 (52.1%) in the
BMS group. The DES group had a higher MACE-free and MI-free
survival compared with the BMS group (71.0 vs. 47.9%, log-rank
P=0.013; 94.9 vs. 85.4%, log-rank P=0.028, respectively). No
significant difference was found in the overall and
revascularization-free survival (95.9 vs. 89.6%, log-rank P=0.356;
81.6 vs. 72.9%, log-rank P=0.386, respectively) (Fig. 3).
 | Table III.Comparison of baseline and procedural
characteristics according to type of stent implanted. |
Table III.
Comparison of baseline and procedural
characteristics according to type of stent implanted.
| Clinical
characteristic | DES | BMS | P-value |
|---|
| Age,
yearsa | 62.01±8.25 | 72.72±4.11 | 0.000 |
| Male, n (%) | 188 (74.60) | 23 (62.16) | 0.111 |
| Hypertension, n
(%) | 160 (63.49) | 26 (70.27) | 0.421 |
| Diabetes mellitus,
n (%) | 128 (50.79) | 26 (70.27) | 0.027 |
|
Hypercholesterolemia, n (%) | 164 (65.08) | 29 (78.38) | 0.109 |
| Smoking, n (%) | 136 (53.97) | 18 (48.65) | 0.545 |
| Previous MI, n
(%) | 96 (38.10) | 16 (43.24) | 0.124 |
| Previous PCI, n
(%) | 50 (19.84) | 11 (29.73) | 0.169 |
| Form of CHD |
|
|
|
| SA, n
(%) | 35 (13.89) | 6 (16.22) | 0.705 |
| UA, n
(%) | 162 (64.29) | 23 (62.16) | 0.802 |
| STEMI,
n (%) | 30 (11.90) | 4 (10.81) | 1.000 |
| NSTEMI,
n (%) | 25 (9.92) | 4 (10.81) | 0.775 |
| BMI,
kg/m2a | 25.97±3.19 | 26.14±3.81 | 0.767 |
| FBG,
mmol/la | 6.52±2.08 | 6.15±2.01 | 0.263 |
| FIB,
g/la | 3.67±1.00 | 3.54±1.13 | 0.832 |
| CHO,
mmol/la | 4.69±1.03 | 5.56±1.61 | 0.000 |
| TG,
mmol/la | 2.02±1.54 | 1.94±1.28 | 0.716 |
| HDL-C,
mmol/la | 1.12±0.31 | 1.15±0.27 | 0.632 |
| LDL-C,
mmol/la | 2.70±0.73 | 2.82±0.75 | 0.350 |
| LVEF,
%a | 56.91±7.14 | 56.05±6.25 | 0.489 |
| Graft age,
montds | 49.67±28.03 | 52.14±36.01 | 0.631 |
| Number of occluded
NVs, n (%) |
|
|
|
| 0 | 120 (47.62) | 18 (48.65) | 0.907 |
| 1 | 54 (21.43) | 5 (13.51) | 0.265 |
| 2 | 41 (16.27) | 12 (32.43) | 0.018 |
| ≥3 | 37 (14.68) | 2 (5.41) | 0.194 |
| Number of occluded
grafts, n (%) |
|
|
|
| 0 | 88 (34.92) | 11 (29.73) | 0.534 |
| 1 | 88 (34.92) | 16 (43.24) | 0.325 |
| 2 | 37 (14.68) | 6 (16.22) | 0.807 |
| ≥3 | 39 (15.48) | 4 (10.81) | 0.457 |
| Number of
stentsa | 2.26±1.15 | 1.86±0.82 | 0.045 |
| Minimal stent
diameter, mma | 2.98±0.65 | 3.28±0.71 | 0.010 |
| Total stent lengtd,
mma | 44.26±22.34 | 38.51±15.40 | 0.132 |
| GpIIb/IIIa
inhibitor, n (%) | 71 (28.17) | 9 (24.32) | 0.625 |
| Embolic protection
device, n (%) | 24 (10.04) | 7 (14.00) | 0.411 |
| Complete
revascularization, n (%) | 60 (25.10) | 11 (22.00) | 0.643 |
Stent performance according to
intervention strategy
In the 190 patients undergoing NV-PCI, DESs were
implanted in 161 patients (84.7%) and BMSs in 29 (15.3%). The
incidence rates of MACEs and TVR in patients with DESs were
significantly lower than those in patients with BMSs (24.2 vs.
51.7%, P=0.003; 14.9 vs. 31.0%, P=0.035, respectively), while the
incidence of mortality and MI (4.4 vs. 10.3%, P=0.182; 5.0 vs.
10.3%, P=0.254, respectively) did not differ significantly between
the two groups. In the 75 patients undergoing graft-PCI, DESs were
implanted in 56 patients (74.7%) and BMSs in 19 patients (25.3%).
The DES group showed a tendency for lower incidence rates of MACEs
(42.9 vs. 52.6%, P=0.460), cardiac mortality (8.9 vs. 10.5%,
P=0.836), MI (7.1 vs. 15.8%, P=0.360) and TVR (25.0 vs. 31.6%,
P=0.575).
Risk factors for MACEs post-PCI
The following factors were considered to be
independent variables in the multivariable Cox regression model
analysis of risk factors for MACEs subsequent to PCI: Site of the
PCI (NV or graft), age of >70 years, gender, diabetes, graft age
of >5 years, use of a GpIIb/IIIa inhibitor, embolic protection
device, number of completely occluded NVs or grafts, stent type,
mean minimal stent diameter and mean total stent length. Diabetes,
age >70 years and graft-PCI were independent risk factors for
the development of MACEs (Table
IV).
 | Table IV.Analysis of risk factors for major
adverse cardiac events. |
Table IV.
Analysis of risk factors for major
adverse cardiac events.
| Variables | β | SE | Wald | OR | 95% CI | P-value |
|---|
| Diabetes | 0.193 | 0.242 | 0.636 | 1.213 | 1.056–1.950 | 0.045 |
| Age >70
years | 0.325 | 0.291 | 1.249 | 1.384 | 1.123–2.448 | 0.037 |
| Graft age >5
years | 0.092 | 0.243 | 0.144 | 1.096 | 0.681–1.764 | 0.704 |
| Occluded graft
≥2 | 0.016 | 0.282 | 0.003 | 1.016 | 0.585–1.765 | 0.954 |
| Occluded NV ≥2 | 0.051 | 0.265 | 0.037 | 1.052 | 0.626–1.767 | 0.847 |
| Graft-PCI | 0.796 | 0.284 | 7.851 | 2.218 | 1.270–3.871 | 0.005 |
| DES | 0.028 | 0.418 | 0.004 | 0.973 | 0.429–2.205 | 0.947 |
| GpIIb/IIIa | 0.561 | 0.124 | 0.678 | 1.122 | 0.672–2.342 | 0.543 |
| Embolic protection
device | 0.432 | 0.098 | 0.754 | 0.876 | 0.544–1.434 | 0.786 |
| Stent diameter | 0.319 | 0.171 | 3.497 | 0.876 | 0.817–1.092 | 0.061 |
| Stent lengtd | 0.006 | 0.012 | 0.288 | 1.006 | 0.984–1.029 | 0.592 |
Discussion
The 10-year patency rate of the internal mammary
artery graft has been previously reported to be 85–95% (5) compared with only 40% for SVGs (6). Approximately 40% of unoccluded SVGs may
develop stenosis (6). Patients with
diseased graft vessels are older and the primary coronary lesion
prior to CABG is often severe (10).
Grafts, and particularly SVGs, usually deteriorate
within 3 years, resulting in ischemia and refractory heart failure
with a poor prognosis (19). Graft
lesions following CABG have remained an important clinical
challenge. Graft revascularization can be achieved with a second
CABG or PCI; however, a second CABG can be difficult, with an
increased incidence of complications and mortality, and inferior
results with regard to symptom relief, graft patency and event-free
survival (20). Older age,
systematic atherosclerosis, vital organ dysfunction and malignancy
are also contraindications for a second CABG. In addition,
potential donor sites for a new graft are sparse following two or
more attempts at CABG. PCI has therefore become the preferred mode
of treatment for graft lesions, the majority of which are SVGs
(10). PCI has also become the
first-line treatment for post-CABG myocardial ischemia due to its
excellent safety and efficacy (21–22).
NV-PCI and graft-PCI are the two options for graft
revascularization following CABG. Graft-PCI shows superior outcomes
to repeated CABG; however, graft-PCI is complex due to the anatomy
of the saphenous vein and results in low success rates (23). Graft-PCI is easily complicated by
distal thrombosis during the procedure, post-procedural re-stenosis
and unconfirmed long-term efficacy; therefore, current guidelines
do not recommend PCI for the treatment of completely occluded
grafts (22,24,25). PCI
for graft stenosis is optional when the NV is totally occluded, has
diffuse lesions, failed opening or is unlikely to open, as judged
by the surgeon. With sufficient training and a good surgical
technique, NV-PCI does not require highly specialized
instrumentation and, with sufficient training, it is a
straightforward surgical procedure. Compared with graft-PCI, NV-PCI
has a higher success rate in complicated coronary disease (26). The reopened native coronary artery is
preferred due to its long-term durability compared with the
degenerated SVG; however, the complexity of NV lesions affects the
success rate of PCI (11–13).
Comparison studies of different PCI strategies for
post-CABG graft lesions show conflicting findings (27–29). In
a study with 1,000 patients with a mean follow-up time of 29
months, SVG-PCI was shown to have a 2.1-fold mortality risk and
1.6-fold MACE occurrence compared with NV-PCI (27). In a prospective study including 190
patients with post-CABG NV-PCI and 88 patients with graft-PCI, the
graft-PCI group had significantly higher incidence rates of MACEs,
mortality and TVR than the NV-PCI group (43.2 vs. 19.6%, log-rank
P<0.001; 19.3 vs. 6.9%, log-rank P=0.008; 23.9 vs. 12.7%,
log-rank P=0.02, respectively), and graft-PCI was shown to be an
independent risk factor for MACEs [hazard ratio (HR), 2.84; 95% CI,
1.45–5.57; P=0.002] (21). By
contrast, in a retrospective study of 618 patients subjected to PCI
post-CABG with a mean follow-up time of 27 months, the NV-PCI and
SVG-PCI groups did not show significant differences in the
incidence rates of mortality (10.0 vs. 8.0%, P=0.22), MI (9.0 vs.
6.0%, P=0.20) or TVR (26.0 vs. 25.0%, P=0.80) (29).
In the present study, 265 patients completed the
follow-up, with a significantly higher proportion of NV-PCI cases
than graft-PCI cases (190 NV-PCI and 75 graft-PCI). Seventy-five
patients with graft-PCI had completely occluded NVs, failed opening
due to diffuse lesions or unlikely opening, as determined by the
surgeon. The mean follow-up time was 37 months, during which the
incidence of MACEs was 33.2% (mortality, 6.4%; MI, 6.8%; TVR,
20.2%). The NV-PCI group had an improved prognosis and higher
MACE-free and revascularization-free survival compared with the
graft-PCI group. We thus recommend that NV-PCI be used as the
first-line treatment for post-CABG graft disease. In the case of
failed NV-PCI, graft-PCI can be considered.
The two major types of stents available for PCI
following CABG are BMSs and DESs. Re-stenosis can significantly
affect the efficacy of SVG-PCI (30). A meta-analysis revealed DESs to be
superior to BMSs in SVG-PCI (31).
Hakeem et al (31) analyzed
29 studies with a total of 7,994 patients (4,187 with DESs and
3,807 with BMSs) and a mean follow-up time of 6–48 months. In their
meta-analysis, DESs were found to be superior to BMSs with regard
to the incidence rates of MACEs (19 vs. 28%, P<0.00001),
mortality (7.8 vs. 9%, P=0.02), MI (5.7 vs. 7.6%, P=0.007) and TVR
(12 vs. 17%, P=0.0002), demonstrating a higher safety and efficacy.
In addition, compared with BMSs, DESs had significantly lower
incidences of mortality (OR, 0.68; 95% CI, 0.53–0.88; P=0.004),
MACEs (OR, 0.64; 95% CI, 0.51–0.82; P<0.001), TLR (OR, 0.6; 95%
CI, 0.43–0.83; P=0.002) and target vessel failure (OR, 0.57; 95%
CI, 0.41–0.80; P=0.001) (31). By
contrast, other studies did not find DESs to be superior to BMSs in
the long-term follow-up subsequent to SVG-PCI (17,32,33). The
SOS study (17) found that the
overall mortality rate did not differ significantly between the DES
and BMS groups at the end of 1.5 years of follow-up (5 vs. 12%; HR,
1.56; 95% CI, 0.72–4.11; P=0.27). In a study of 284 patients with
DESs and 95 patients with BMSs, the incidence of MACEs at 3 years
did not differ significantly between the groups, despite a
significantly higher inpatient mortality rate in the BMS group.
This suggested a good safety profile (but non-superiority) of DESs
in long-term implantation (33).
In the present study, DESs were employed in 239
patients (82.7%) and BMSs in 50 patients (17.3%), who were followed
up for a mean period of 37 months. There was a trend towards an
improved outcome in the DES group compared with the BMS group. In
patients undergoing NV-PCI, DESs were superior to BMSs with regard
to the incidence rates of MACEs and TVR, but no significant
differences in mortality and MI were found between the groups. In
patients undergoing graft-PCI, DESs were implanted in 56 patients
and BMSs in 19 patients. The DES group showed a tendency for lower
incidence rates of MACEs, cardiac mortality, MI and TVR.
It should be acknowledged that there were several
limitations to the present study. Firstly, the design of the study
was retrospective and non-randomized, and the course of treatment
was determined by the individual surgeon. Secondly, antiplatelet
treatment was administered for a variable duration and there was a
lack of routine angiographic follow-up. Additionally, the length of
the time-frame for inclusion in this study (5 years) may have
introduced confounding effects as a result of developments in
techniques and equipment. Finally, the use of BMSs in the graft-PCI
procedures was relatively low. A prospective, randomized study with
angiographic follow-up is therefore warranted to control for
confounding factors. Despite these limitations, however, the
present study has collated the information for a large patient
population and reports the clinical presentation and outcomes of
SVG disease treatment in routine, daily practice.
In conclusion, NV-PCI has an improved long-term
prognosis compared with graft-PCI in the treatment of post-CABG
graft disease. NV-PCI should be considered as the first-line
treatment for graft lesions, but graft-PCI remains a viable option.
There is insufficient data on the long-term efficacy and safety of
DESs and BMSs in SVG-PCI; however, compared with BMSs, DESs are
currently the preferred stents for SVG-PCI.
Acknowledgements
The authors would like to thank the Department of
Catheter Laboratory and Cardiac Surgery, Tianjin Chest Hospital,
for their helpful suggestions in the preparation of this study.
References
|
1
|
Lee MS, Park SJ, Kandzari DE, et al:
Saphenous vein graft intervention. JACC Cardiovasc Interv.
8:831–843. 2011. View Article : Google Scholar
|
|
2
|
Campeau L, Enjalbert M, Lespérance J,
Vaislic C, Grondin CM and Bourassa MG: Atherosclerosis and late
closure of aortocoronary saphenous vein grafts: Sequential
angiographic studies at 2 weeks, 1 year, 5 to 7 years, and 10 to 12
years after surgery. Circulation. 68:111–117. 1983.
|
|
3
|
Fitzgibbon GM, Leach AJ, Kafka HP and Keon
WJ: Coronary bypass graft fate: longerm angiographic study. J Am
Coll Cardiol. 17:1075–1080. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weintraub WS, Jones EL, Craver JM and
Guyton RA: Frequency of repeat coronary bypass or coronary
angioplasty after coronary artery bypass surgery using saphenous
venous grafts. Am J Cardiol. 73:103–112. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goldman S, Zadina K, Moritz T, Ovitt T,
Sethi G, Copeland JG, et al: VA Cooperative Study Group
#207/297/364: Long-term patency of saphenous vein and left internal
mammary artery grafts after coronary bypass surgery: results from a
Department of Veterans Affairs Cooperative Study. J Am Coll
Cardiol. 44:2149–2156. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fitzgibbon GM, Kafka HP, Leach AJ, Keon
WJ, Hooper GD and Burton JR: Coronary bypass graft fate and patient
outcome: angiographie follow-up of 5,065 grafts related to survival
and reoperation in 1,388 patients during 25 years. J Am Coll
Cardiol. 28:616–626. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eagle KA, Guyton RA, Davidoff R, et al:
American College of Cardiology; American Heart Association: ACC/AHA
2004 guideline update for coronary artery bypass graft surgery: A
report of the American College of Cardiology/American Heart
Association Task Force on Practice Guidelines (Committee to Update
the 1999 Guidelines for Coronary Artery Bypass Graft Surgery).
Circulation. 110:e340–e437. 2004.PubMed/NCBI
|
|
8
|
Baim DS: Percutaneous treatment of
saphenous vein graft disease: The ongoing challenge. J Am Coll
Cardiol. 42:1370–1372. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baldwin DE, Abbott JD, Trost JC, et al:
Comparison of drug-eluting and bare metal stents for saphenous vein
graft lesions (from the National Heart, Lung, and Blood Institute
Dynamic Registry). Am J Cardiol. 106:946–951. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gyenes G, Norris CM and Graham MM:
APPROACH Investigators: Percutaneous revascularization improves
outcomes in patients with prior coronary artery bypass surgery.
Catheter Cardiovasc Interv. 82:E148–E154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hiscock M, Oqueli E and Dick R:
Percutaneous saphenous vein graft intervention - a review. Heart
Lung Circ. 16:S51–S55. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sabik JF III, Blackstone E, Gillinoy M,
Smedira NG and Lytle BW: Occurrence and risk factors for
reintervention after coronary artery bypass grafting. Circulation.
114:1454–1460. 2006. View Article : Google Scholar
|
|
13
|
Pucelikova T, Mehran R, Kirtane A, et al:
Short- and long-term outcomes after stent-assisted percutaneous
treatment of saphenous vein grafts in the drug-eluting stent era.
Am J Cardiol. 101:63–68. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meliga E, García-García H, Kukreja N, et
al: Chronic total occlusion treatment in post-CABG patients:
Saphenous vein graft versus native vessel recanalization-long-term
follow-up in the drug-eluting stent era. Catheter Cardiovasc
Interv. 70:21–25. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hoffmann R, Hamm C, Nienaber CA, et al:
German Cypher Registry: Implantation of sirolimus-eluting stents in
saphenous vein grafts is associated with high clinical follow-up
event rates compared with treatment of native vessels. Coron Artery
Dis. 18:559–564. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vermeesch P, Agostoni P, Verheye S, et al:
DELAYED RRISC (Death and Events at Long-term follow-up AnalYsis:
Extended Duration of the Reduction of Restenosis In Saphenous vein
grafts with Cypher stent) Investigators: Increased late mortality
after sirolimus-eluting stents versus bare-metal stents in diseased
saphenous vein grafts: Results from the randomized DELAYED RRISC
Trial. J Am Coll Cardiol. 50:261–267. 2007.PubMed/NCBI
|
|
17
|
Brilakis ES, Lichtenwalter C, de Lemos JA,
Roesle M, Obel O, Haagen D, et al: A randomized controlled trial of
a paclitaxel-eluting stent versus a similar bare-metal stent in
saphenous vein graft lesions the SOS (Stenting of Saphenous Vein
Grafts) trial. J Am Coll Cardiol. 53:919–928. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mauri L, Kereiakes DJ, Yeh RW, et al: DAPT
Study Investigators: Twelve or 30 months of dual antiplatelet
therapy after drug-eluting stents. N Engl J Med. 371:2155–2166.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morrison DA, Sethi G, Sacks J, et al:
Investigators of the Department of Veterans Affairs Cooperative
Study #385, angina With Extremely Serious Operative Mortality
Evaluation (AWESOME): Percutaneous coronary interventions versus
coronary bypass graft surgery for patients with medically
refractory myocardial ischemia and risk factors for adverse
outcomes with bypass. The VA AWESOME Multicenter Registry:
Comparison With the Randomized Clinical Trial. J Am Coll Cardiol.
39:266–273. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yap CH, Sposato L, Akowuah E, et al:
Contemporary results show repeat coronary artery bypass grafting
remains a risk factor for operative mortality. Ann Thorac Surg.
87:1386–1391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Runyan D, Gorges R, Feldman D, McCullough
PA, David S and Saba S: Long-term follow-up of lesion-specific
outcomes comparing drug-eluting stents and bare metal stents in
diseased saphenous vein grafts. Rev Cardiovasc Med. 14:1–6.
2013.PubMed/NCBI
|
|
22
|
Gupta S and Cigarroa JE: The quest for
optimal interventional strategy in saphenous vein graft
interventions - are we there yet? Catheter Cardiovasc Interv.
80:1118–1119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brilakis ES, Rao SV, Banerjee S, et al:
Percutaneous coronaryintervention in native arteries versus bypass
grafts in prior coronary artery bypass grafting patients: a report
from the National Cardiovascular Data Registry. JACC Cardiovasc
Interv. 4:844–850. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ko DT, Guo H, Wijeysundera HC, Zia MI,
Džavík V, Chu MW, et al: Long-term safety and effectiveness of
drug-eluting stents for the treatment of saphenous vein grafts
disease: a population-based study. JACC Cardiovasc Interv.
4:965–973. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hindnavis V, Cho SH and Goldberg S:
Saphenous vein graft intervention: a review. J Invasive Cardiol.
24:64–71. 2012.PubMed/NCBI
|
|
26
|
Ho PC, Lee AC and Fortuna R: Drug-eluting
stenting of saphenous vein graft versus native coronary artery
supplying the same myocardial perfusion territory: a pilot
retrospective 3-year follow-up. J Invasive Cardiol. 24:516–520.
2012.PubMed/NCBI
|
|
27
|
de Feyter PJ, van Suylen RJ, de Jaegere
PP, Topol EJ and Serruys PW: Balloon angioplasty for the treatment
of lesions in saphenous vein bypass grafts. J Am Coll Cardiol.
21:1539–1549. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xanthopoulou I, Davlouros P, Tsigkas G,
Panagiotou A, Hahalis G and Alexopoulos D: Long-term clinical
outcome after percutaneous coronary intervention in grafts vs
native vessels in patients with previous coronary artery bypass
grafting. Can J Cardiol. 27:716–724. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Leal S, Campante Teles R, Calé R, Sousa
PJ, Brito J, Raposo L, et al: ACROSS Registry Investigators:
Percutaneous revascularization strategies in saphenous vein graft
lesions: long-term results. Rev Port Cardiol. 31:11–18.
2012.PubMed/NCBI
|
|
30
|
Vermeersch P, Agostoni P, et al:
Randomized double-blind comparison of sirolimus-eluting stent
versus bare-metal stent implantation in diseased saphenous vein
grafts: six-month angiographic, intravascular ultrasound and
clinical follow-up of the RRISC Trial. J Am Coll Cardiol.
48:2423–2431. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hakeem A, Helmy T, Munsif S, Bhatti S,
Mazraeshahi R, Cilingiroglu M, et al: Safety and efficacy of drug
eluting stents compared with bare metal stents for saphenous vein
graft interventions: a comprehensive meta-analysis of randomized
trials and observational studies comprising 7,994 patients.
Catheter Cardiovasc Interv. 77:343–355. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lozano I, García-Camarero T, Carrillo P,
Baz JA, de la Torre JM, López-Palop R, et al: Comparison of
drug-eluting and bare metal stents in saphenous vein grafts.
Immediate and long-term results. Rev Esp Cardiol. 62:39–47.
2009.[(In Spanish)]. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Goswami NJ, Gaffigan M, Berrio G, Plessa
AL, Pfeiffer AM, Markwell SJ and Mishkel GJ: Long-term outcomes of
drug-eluting stents versus bare-metal stents in saphenous vein
graft disease: results from the Prairie ‘Real World’ Stent
Registry. Catheter Cardiovasc Interv. 75:93–100. 2010.PubMed/NCBI
|