Introduction
Spinal cord injury (SCI) predominantly affects
younger members of the population and is caused by traffic or
sports-related accidents. The condition can result in severe
neurological deficits, such as para- and quadriplegia (1). Since traumatic injury to the mammalian
spinal cord exhibits a highly dynamic nature, characterized by a
complex pattern of insidious, destructive biochemical and
pathophysiological events, the potential for functional recovery
from the condition is limited (2).
Following SCI, substantial secondary damage within the tissue is
caused by increased vascular permeability, infiltration of
inflammatory cells and subsequent focal edema, which may induce
apoptosis (3–5). Following SCI, cells at the site of
injury may undergo cell death through post-traumatic necrosis or
apoptosis, the latter of which can be demonstrated by nuclear DNA
fragmentation and caspase activation. Apoptosis, in particular, is
a prominent event in the spinal cord subsequent to SCI (6). Apoptosis has been shown to occur widely
in the white matter, concurrently with Wallerian degeneration, and
to affect neurons and oligodendrocytes. The apoptotic cell death of
both neurons and oligodendrocytes may therefore be a causative
factor contributing to the paralysis of patients with SCI (7,8).
Therapeutic interventions using neurotrophic factors have focused
on the prevention of such reactions to reduce cell death and
promote tissue regeneration (9).
Vascular endothelial growth factor (VEGF) has long
been known as a potent angiogenic factor that stimulates the
proliferation and migration of endothelial cells and the in
vivo formation of new blood vessels (10). The association of VEGF with the
central nervous system (CNS) has been predominantly studied in
models of ischemic stroke or brain tumor (11); however, VEGF has also been attracting
attention as a neuroprotective and neurotrophic factor involved in
nerve regeneration and the promotion of functional recovery
(12,13). It has been found that VEGF enhances
neurite outgrowth and neuroprotection, and reduces post-traumatic
apoptosis following CNS injury (14–16).
VEGF is crucial in a number of processes in the CNS, including
vascularization, neuronal proliferation and the growth of
coordinated vascular and neuronal networks (17). Accordingly, enhancing the expression
of VEGF may have therapeutic potential for the treatment of
SCI.
Batroxobin is a thrombin-like serine protease from
the venom of the snake Bothrops moojeni that can decrease
blood fibrinogen levels and promote blood flow (18). Batroxobin has been widely used
clinically in various ischemic disorders, such as stroke, deep-vein
thrombosis, myocardial infarction and peripheral arterial
thrombosis (19–21); however, it has not been fully
investigated whether batroxobin can exhibit protective effects by
promoting the expression of VEGF to reduce apoptosis in SCI. This
study was therefore designed to investigate whether batroxobin has
a beneficial effect on rats with SCI and to explore the possible
clinical application of batroxobin.
Materials and methods
This protocol was evaluated and approved by the
Governmental Animal Care Committee of the Medical College of Xiamen
University (Zhangzhou, China) and was performed according to the
National Institutes of Health guidelines on the ethical use of
animals. Every effort was made to minimize animal suffering and to
reduce the number of animals used.
Animals and surgical procedures
Ninety adult female Sprague Dawley rats
(Experimental Animal Center of Xiamen University) weighing 280–300
g were randomly assigned to the following three groups: Sham injury
(group I, n=30), SCI (group II, n=30) and batroxobin treatment
(group III, n=30). Any animals that died during the experiment were
not included. Batroxobin was obtained from Nuokang
Bio-Pharmaceutical, Inc. (Shenyang, China). Prior to surgery, the
animals were anesthetized by intraperitoneal injection of 400 mg/kg
chloral hydrate (Beyotime Institute of Biotechnology, Haimen,
China). During the surgery, the rats were placed in a prone
position on a warming pad to maintain a body temperature of
37.0±0.5°C. Upon completion of the surgery, the rats were housed in
individual cages with access to food and water ad libitum,
and administered an intramuscular injection of 200,000 U/day
penicillin (175th Hospital of the PLA, Zhangzhou, China) for 3
days.
All rats were injured at the thoracic level 12
(T12), using an established weight-drop model described in a
previous study (22). Briefly, the
skin and muscle overlying the spinal column were incised and a
laminectomy was performed at T12, leaving the dura intact. A
moderate-intensity weight-drop (10 g, 7.0 cm) was performed using
an impactor with a diameter of 2.5 mm (Xiamen University) onto the
exposed T12 cord. The rats in group I were treated in an identical
manner to the rats subjected to SCI with the omission of the
weight-drop step.
Following the surgery, the bladders of the rats were
manually pressed twice daily until spontaneous voiding occurred.
The dosage of batroxobin (DF-521; Beijing Tobishi Pharmaceutical
Co., Ltd., Beijing, China) was selected according to the
manufacturer's instructions, which recommended 10 batroxobin units
(BU) as the regular initial dose and 5 BU as the maintenance dose.
Considering the differences between humans and rats, the rats in
group III were injected with batroxobin at a dosage of 5 BU/kg/day
via the tail vein within 8 h of SCI until 3 days post-injury.
Instead of batroxobin, the rats in groups I and II were
administered saline through pumps as a control treatment.
Basso-Beattie-Bresnahan (BBB)
evaluation of locomotion
The rats were tested for locomotor deficits at 1 day
before and 1, 4 and 7 days after SCI with a standard open-field
locomotor test, developed by Basso et al (23). This BBB
locomotor rating scale evaluates the following criteria: Extent of
joint movement, weight support and stepping/walking behavior of the
hindlimbs. The rating scale ranges from 0 (no observable hindlimb
movement) to 21 (normal locomotion), and scores were assigned for
both hind limbs by two independent observers blinded to the
experiments. The main functional outcome was calculated by the mean
value.
Hematoxylin and eosin (HE) staining
for the detection of pathological changes
For the histological staining, 5-µm transverse
sections of injured spinal cord tissue from each group at 1, 3, 5,
7, 14 and 28 days post-injury were deparaffinized and placed into
fresh xylene for 15 min twice. The sections were re-hydrated in
100% alcohol for 5 min twice, and then 95 and 70% alcohol once for
3 min, respectively. The sections were subsequently washed briefly
in double-distilled (dd)H2O and stained in Harris
hematoxylin (Beyotime Institute of Biotechnology) solution for 5
min. Following staining, the sections were washed in running tap
water for 8 min, subjected to differentiation with 1% acid alcohol
for 30 sec and blued in 0.2% ammonia water for 30 sec. The sections
were then washed in running tap water for a further 5 min and
rinsed in 95% alcohol for ∼15 dips. The sections were stained in
Eosin-Phloxine solution (Beyotime Institute of Biotechnology) for 1
min, prior to undergoing 95 and 100% alcohol dehydration (5 min
each) and clearing in two changes of xylene (5 min each). Finally,
the sections were mounted with mounting medium (Beyotime Institute
of Biotechnology). The images were captured using an FV300 confocal
microscope (Olympus Corp., Tokyo, Japan).
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) test for apoptosis
For the detection of apoptosis, TUNEL staining was
performed using a TUNEL detection kit according to the
manufacturer's instructions (ApopTag® horseradish peroxidase kit;
DBA, Milan, Italy). Briefly, sections of SCI tissue at 1, 3, 5, 7,
14 and 28 days post-injury were immersed in xylene for 5 min twice
at room temperature, and in 100, 90, 80 and 70% ethanol for 5 min
twice. The sections were then incubated in 15 µg/ml Proteinase K
solution for 20 min at room temperature and washed with
phosphate-buffered saline (PBS). Hydrogen peroxide (3%), applied
for 5 min at room temperature, was utilized to terminate any
endogenous peroxidase activity, prior to the sections being washed
with PBS. The sections were then immersed in terminal
deoxynucleotidyl transferase (TdT) buffer containing TdT and
biotinylated dUTP, incubated in a humid atmosphere at 37°C for 90
min, and washed with PBS. Subsequent to being washed, the sections
were incubated at room temperature for a further 30 min with
anti-horseradish peroxidase-conjugated antibody (GeneTex, San
Antonio, Texas, USA), and 3,3′-diaminobenzidine was used to
visualize the signals. The sections were then washed in
ddH2O and mounted. Images were captured using an FV300
confocal microscope (Olympus Corp.).
Immunohistochemistry of VEGF
For immunohistochemical staining, each specimen was
embedded in paraffin and a microtome was used to cut serial
sections. VEGF immunohistochemical staining was performed using an
avidin-biotin peroxidase complex technique and a Histostain® SP kit
(Maixin-Bio, Inc., Fuzhou, China) in accordance with the
manufacturer's instructions. Mouse monoclonal antibody against VEGF
(1:200; #sc-30343; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) and rabbit polyclonal antibody against VEGF (1:100; #sc-33547;
Santa Cruz Biotechnology, Inc.) were used for this study. Two
pathologists who were unaware of the experimental data were
responsible for counting the number of VEGF-positive cells in 10
high-power fields (magnification, ×400) in each specimen. The
average number of VEGF-positive cells per specimen was then
calculated. Images were captured using an FV300 confocal microscope
(Olympus Corp.).
Statistical analysis
Statistical analysis was performed using SPSS
version 13.0 for Windows (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard deviation. The Mann-Whitney U-test
and Spearman's rank correlation were used for the statistical
analyses. P<0.05 was considered to indicate a statistically
significant difference.
Results
During surgery, the rectal temperature of the rats
was maintained at 37±0.5°C. The mean body weight of the rats in the
sham surgery group was 371.8±9.7 g (range, 362–385 g), while the
mean body weights of the control and batroxobin group rats were
375.9±7.1 g (range, 367–388 g) and 369.0±11.3 g (range, 354–389 g),
respectively. No significant differences in these physiological
parameters existed between the groups.
Behavioral test
To evaluate the extent of motor function recovery,
the BBB locomotor rating scale was used. The BBB scores were
assessed for the three groups at different time-points following
SCI. Table I shows the mean BBB
scores of the rats in the three groups over the time-course of the
experiment. Prior to surgery, the rats were all healthy (BBB score,
21±0.00; data not shown). In the group I rats, no significant
difference was observed in the hind limb movement scores measured
prior to and following SCI, and the rats exhibited normal movement
throughout the observation period (BBB score, 21 points). In groups
II and III, the rats showed improvements in motor function at day 5
post-SCI compared with the scores on the date of the SCI; however,
the average BBB score was significantly higher in the group III
rats than that in the group II rats between days 5 and 28 post-SCI.
On day 28, the BBB score of the group III rats was 13.74±0.66
points, whereas the group II rats scored 10.22±0.74 points
(P<0.05). A BBB score of 14 is indicative of consistent
weight-supported plantar steps and front-hind limb coordination
(23); neither of the scores in
groups II or III exceeded the 14-point threshold (mean in group III
= 13.74)
 | Table I.BBB score of each group at different
time-points. |
Table I.
BBB score of each group at different
time-points.
| Days post-SCI | Group I
(score) | Group II
(score) | Group III
(score) |
|---|
| 0 | 21.00±0.00 | 21.00±0.00 | 21.00±0.00 |
| 1 | 21.00±0.00 | 0.69±0.24 | 0.79±0.23 |
| 3 | 21.00±0.00 | 2.36±0.30 | 2.53±0.38 |
| 5 | 21.00±0.00 | 4.82±0.31 |
5.03±0.33a |
| 7 | 21.00±0.00 | 6.62±0.40 |
7.55±0.37b |
| 14 | 21.00±0.00 | 7.54±0.42 |
9.65±0.44b |
| 28 | 21.00±0.00 | 10.22±0.74 |
13.74±0.66b |
Histological assessments: Visual
study
Following the initial injury, tissue edema appeared
immediately in the dorsal region of the spinal cord in groups II
and III, while no fresh bleeding spots were observed at day 3. Scar
formation was observed in the region of the lesion and
conglutination with the endorhachis was apparent at days 14 and 28
post-SCI. In addition, the spinal cord was atrophic with a
reduction in the diameter. In group I, areas of scar formation were
observed in the region of the lesion and conglutination with the
endorhachis was apparent at days 14 and 28 post-SCI. This may have
been a result of the trauma of the surgery; however, no obvious
edema in the spinal cord was observed and the posterior central
blood vessel and the structure of the spinal cord were clearly
visible.
H&E staining
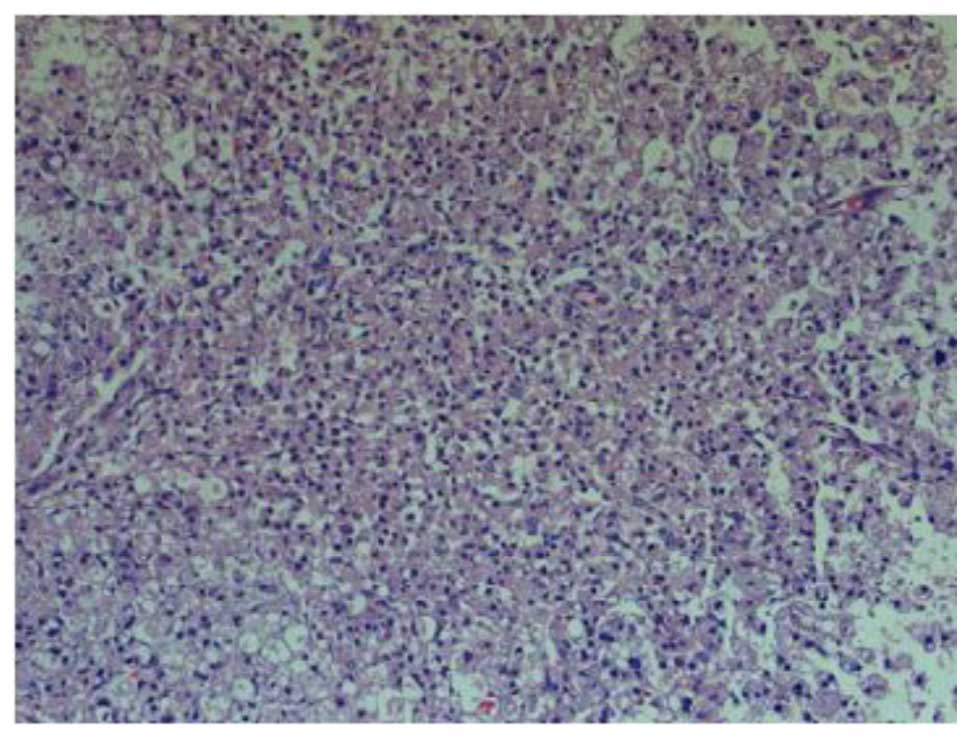
At 1 day post-SCI, H&E staining in the group II
rats showed a large area of structural damage, multifocal
hemorrhage and inflammatory cell infiltration. Notably, neuron
pyknosis and chromatin condensation could be observed, which
indicated cell apoptosis (Fig. 1).
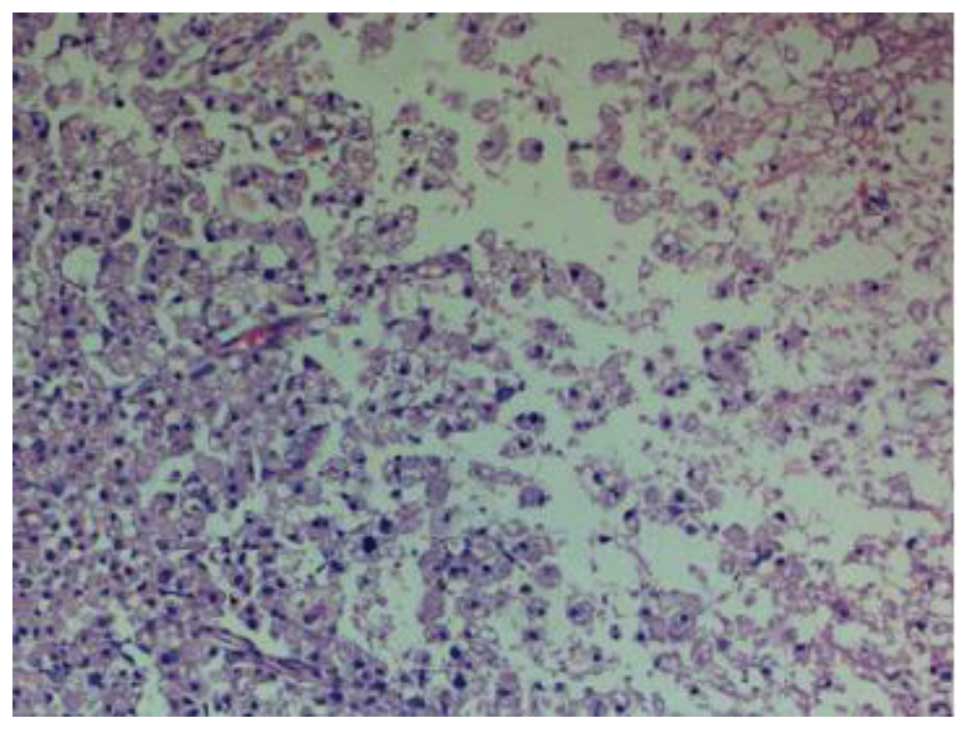
At 14 days post-SCI, H&E staining in the group II rats showed a
small hemorrhagic focus in the gray and white matter of the spinal
cord; evident destruction to the structure of the spinal cord was
observed, and neurons were found to be dissolved and liquefied in
the gray matter (Fig. 2). The
resulting large, liquefied and necrotic area formed a cystic space.
Numerous swollen axons and neovascularization were additionally
observed in the white matter, and nerve fiber disorganization was
apparent. Twenty-eight days after SCI, the hemorrhagic focus in the
gray and white matter was almost entirely absorbed, and further
destruction of the spinal cord was observed; the neurons that were
dissolved and liquefied in the gray matter formed numerous vacuolar
structures. Furthermore, a reduction in the inflammatory cell
infiltration, and newborn disordered blood-vessels were observed
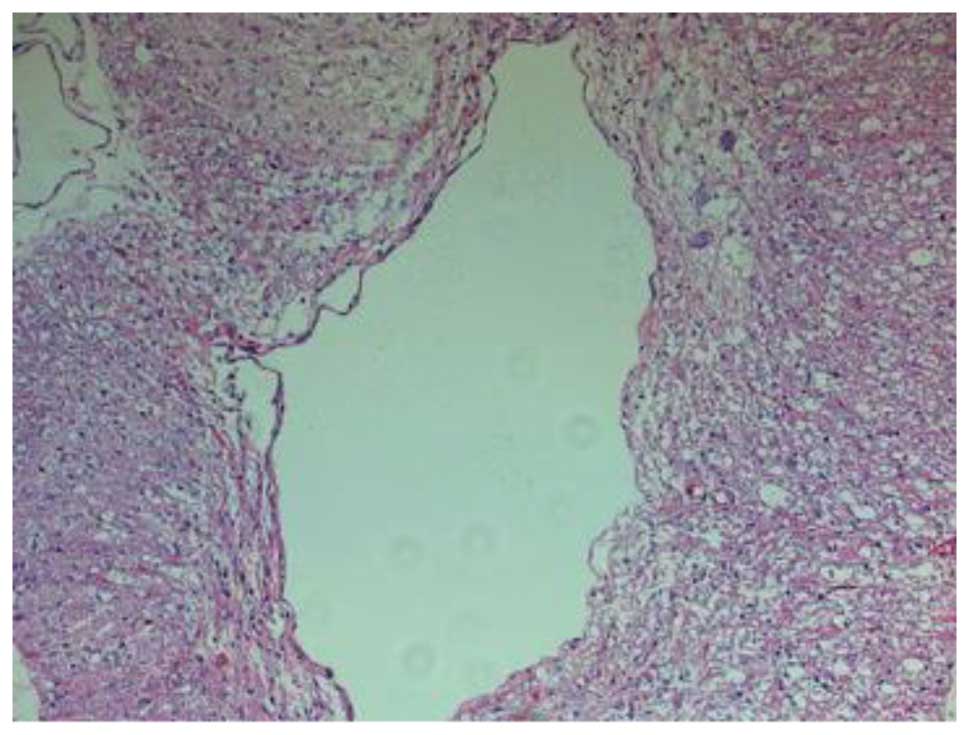
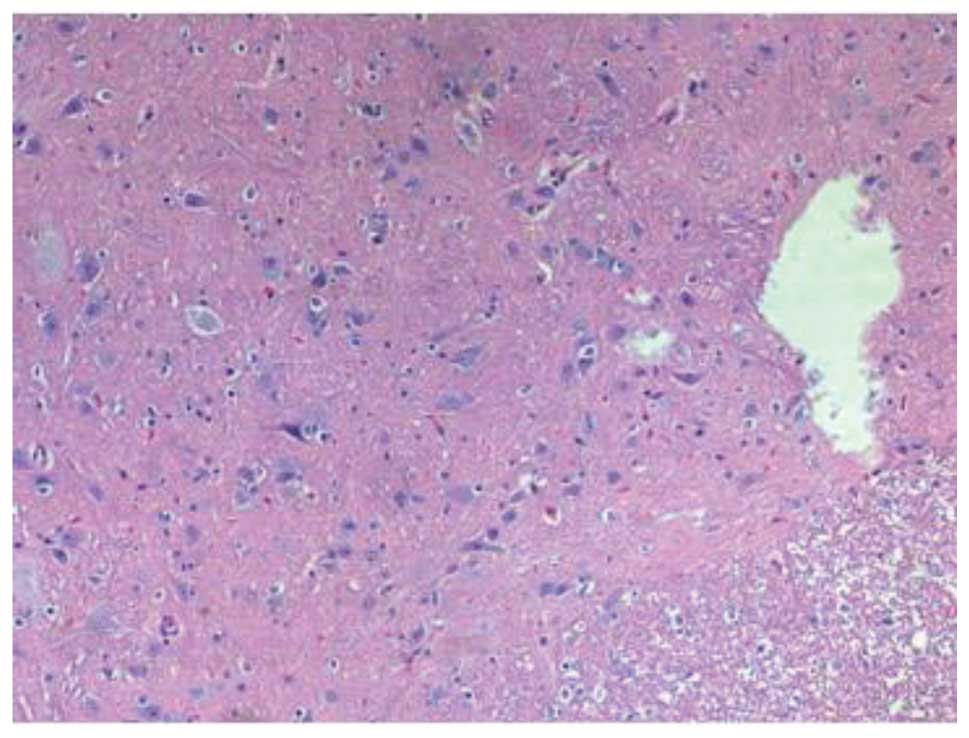
(Fig. 3). Fourteen days after the
SCI, H&E staining in group III revealed damage to the structure
of the spinal cord, in addition to inflammatory cell infiltration,
neuron dehydration and disintegration, hyperplastic and
hypertrophic gliocytes and the formation of cystic spaces; however,
the damage was less severe and widespread compared with that in
group II. Furthermore, fewer apoptotic cells were observed in group
III than in group II. At 28 days post-SCI, it was observed that the
inflammatory cell infiltration in the group III rats was reduced,
and fewer apoptotic cells were present compared with the group II
rats (Fig. 4). In summary, the
spinal cord pathological changes that occurred following injury
were significantly attenuated by batroxobin on the 5th, 7th, 14th
and 28th days postoperatively.
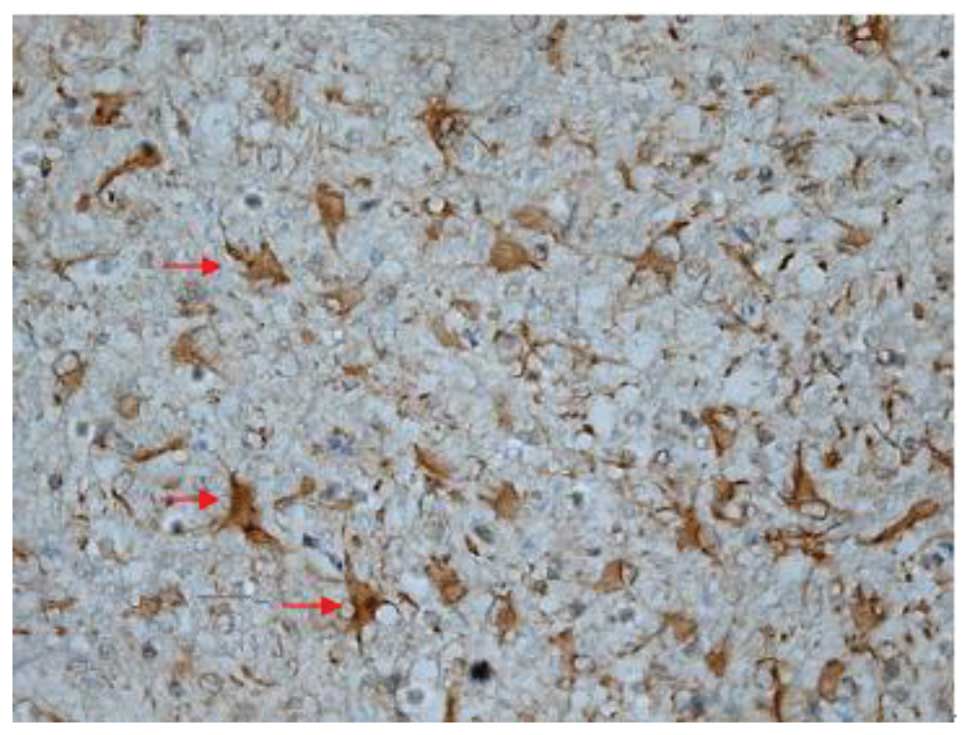
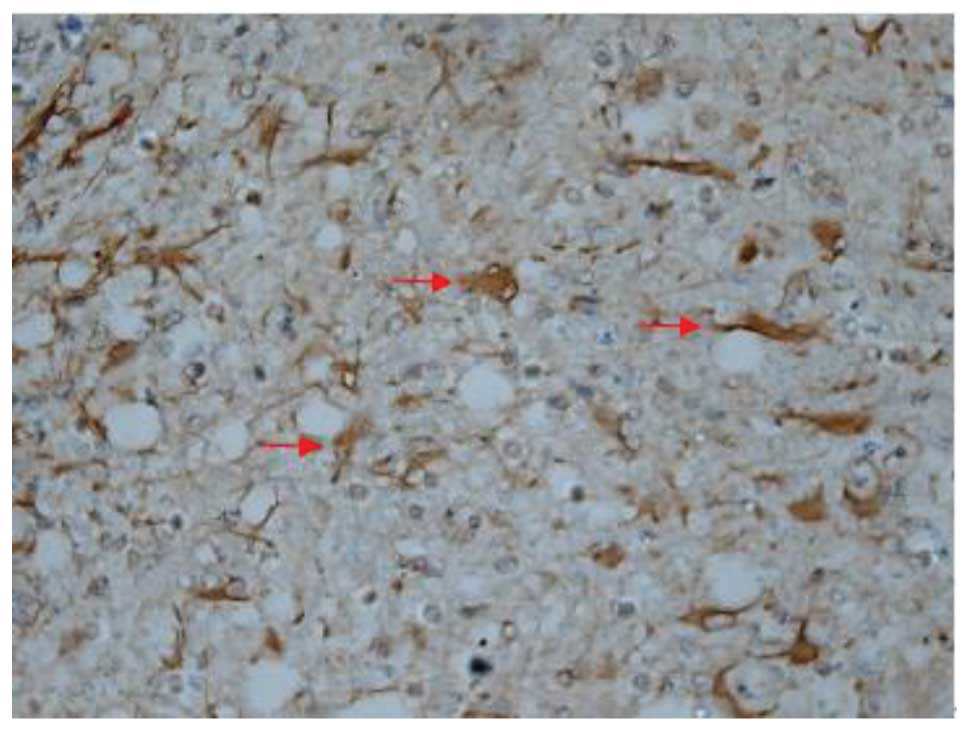
Effect of batroxobin on cellular
apoptosis in the spinal cord
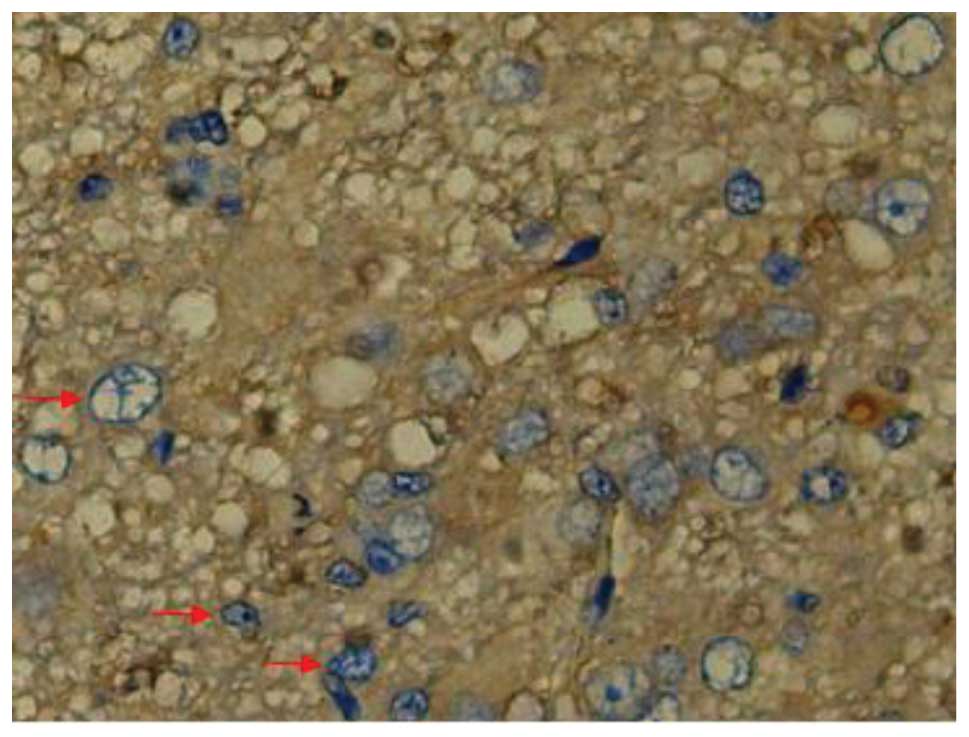
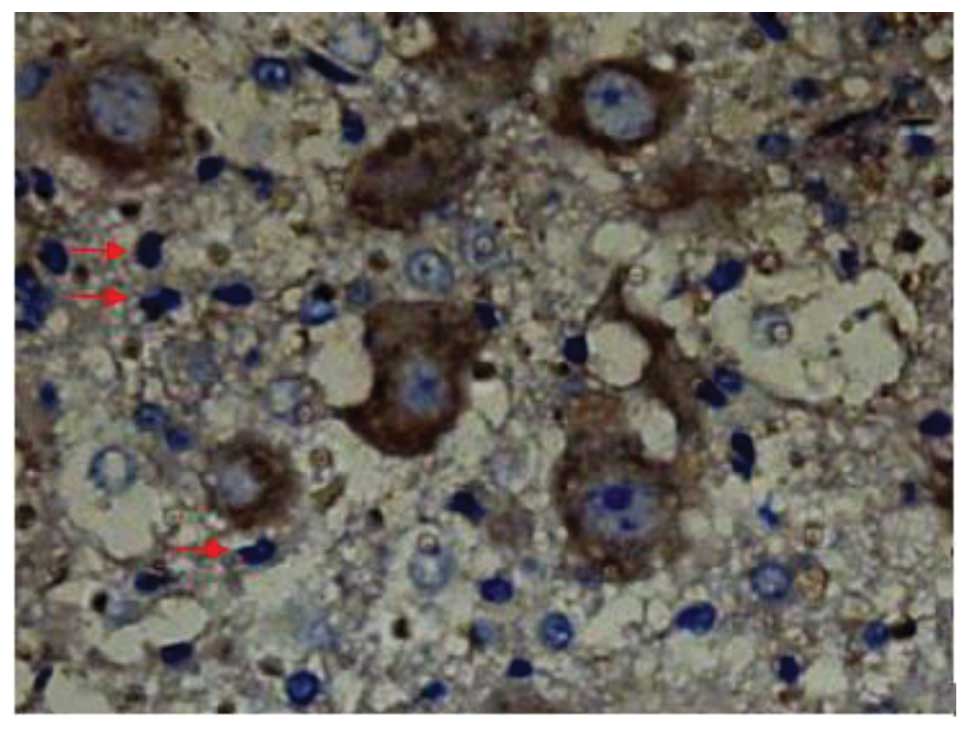
SCI-induced cellular apoptosis could be detected
using the TUNEL test. As shown in Fig.
5, apoptotic cells were barely detectable in group I at 1 day
after SCI, as little apoptosis occurred in the absence of injury.
In group II, an increased number of apoptotic cell bodies
(indicated by arrows) were found at 1 day after SCI (Fig. 6), and this number continued to remain
high from day 3 to day 5 (Fig. 7),
prior to tapering until day 28. Compared with group II, however, a
significant reduction in the number of apoptotic cell bodies
(indicated by arrows) was detected in group III at 1 day after SCI
(Fig. 8), and this number remained
at a lower level from day 3 to day 5 (Fig. 9). Following treatment with
batroxobin, the number of apoptotic cells was found to decrease
significantly. This indicated that batroxobin inhibited cellular
apoptosis subsequent to injury. The number of apoptotic cells in
the field of view on the slides in each group was counted under a
microscope and analyzed. The result of the TUNEL test indicated
that the severity of tissue damage and neuronal loss was
considerably milder in group III than that in group II (Table II).
 | Table II.TUNEL test for apoptosis
detection. |
Table II.
TUNEL test for apoptosis
detection.
| Days post-SCI | Group I | Group II | Group III |
|---|
| 1 | 15.30±0.32 | 65.48±4.03 |
63.65±1.58a |
| 3 | 15.08±0.63 | 49.14±2.86 |
39.64±1.33b |
| 5 | 15.03±0.40 | 32.80±2.34 |
28.53±1.29b |
| 7 | 15.11±0.42 | 25.04±0.83 |
23.05±0.62b |
| 14 | 15.08±0.42 | 21.92±0.65 |
21.53±0.47a |
| 28 | 15.13±0.59 | 21.39±0.59 |
20.98±0.35a |
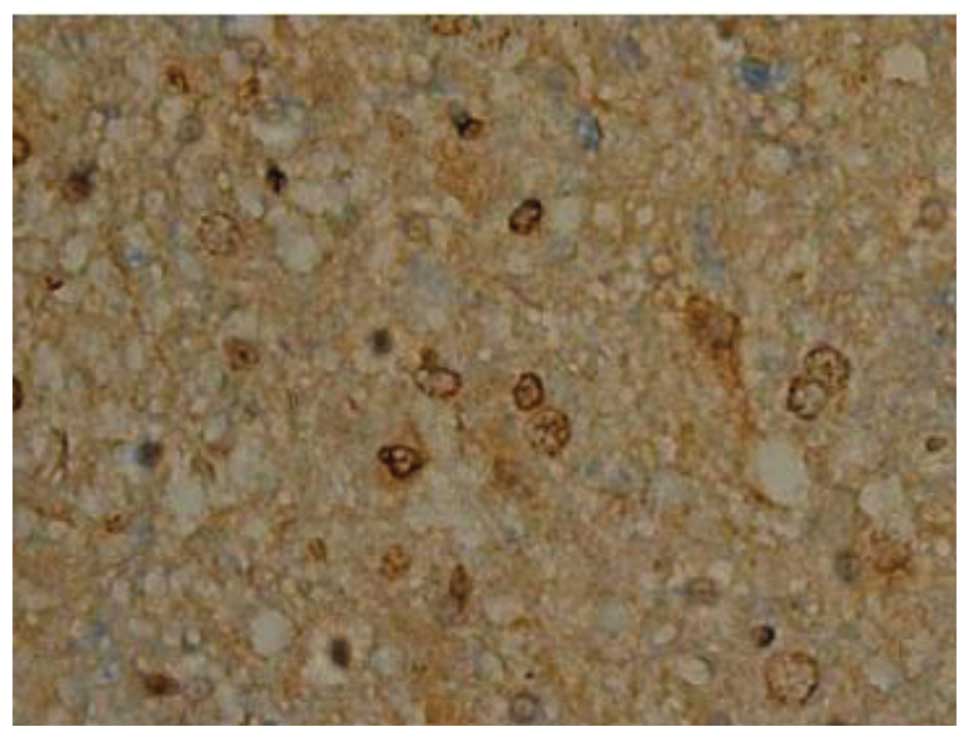
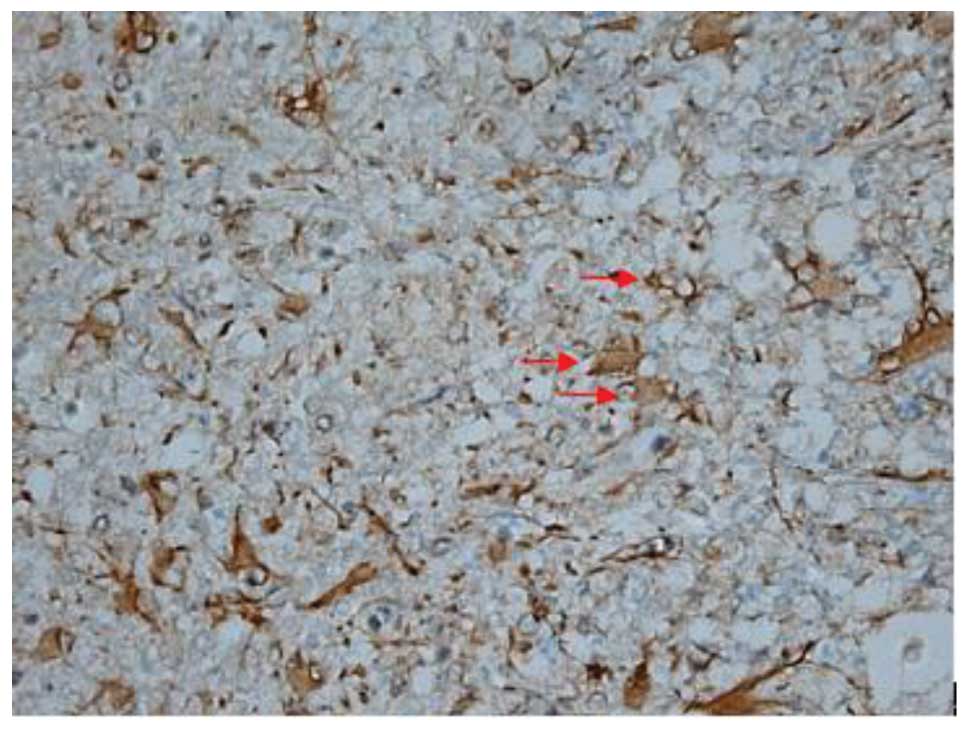
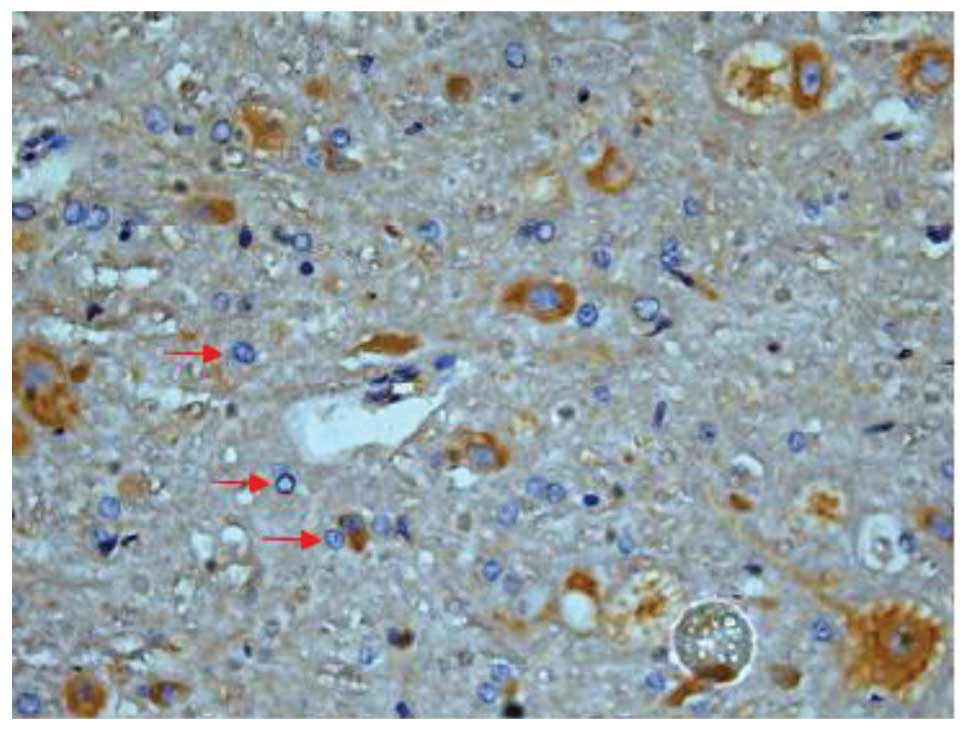
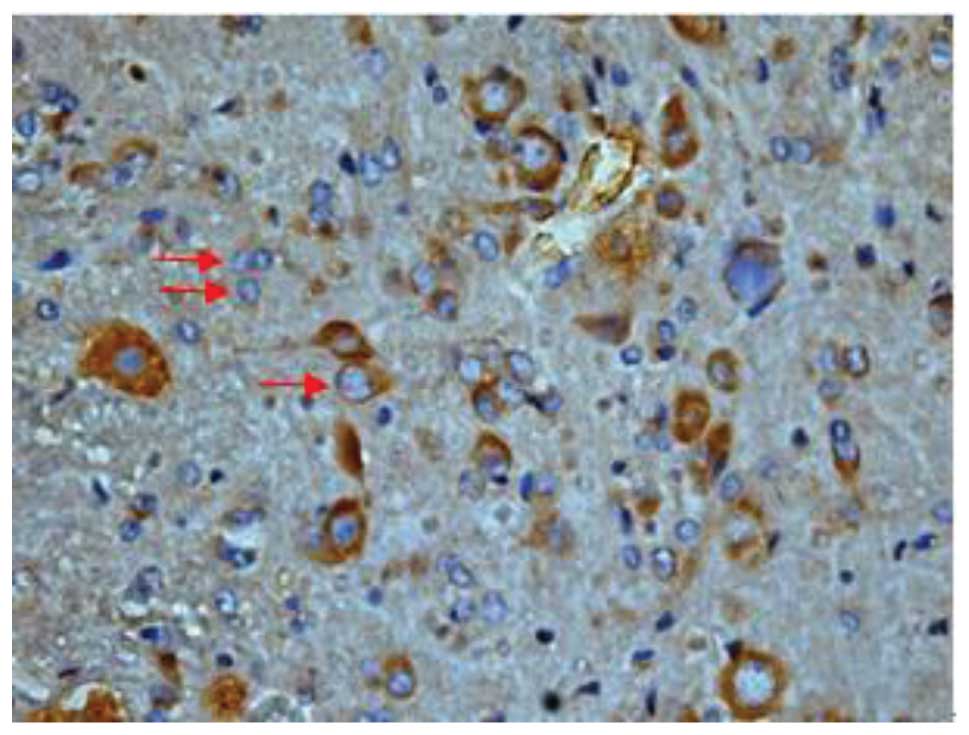
Effect of batroxobin on VEGF
expression in the spinal cord
High-level constitutive expression of VEGF was
observed in groups II and III; however, immunohistochemical study
of VEGF in the spinal cord sections showed significant differences
between the two groups. Compared with group II, batroxobin promoted
the expression of VEGF between days 1 (Figs. 10 and 11) and 14 after injury. It was noted that
the mean number of VEGF-positive cells per section was maximized at
day 3 in groups II and III (Figs.
12 and 13). This indicated that
batroxobin could promote the expression of VEGF, which played a
central role in inducing angiogenesis (Table III).
 | Table III.Number of VEGF-positive cells per
section. |
Table III.
Number of VEGF-positive cells per
section.
| Days post-SCI | Group I | Group II | Group III |
|---|
| 1 | 3.66±0.03 | 53.89±1.87 |
55.02±1.44a |
| 3 | 3.66±0.01 | 61.35±1.89 |
69.63±4.69b |
| 5 | 3.66±0.04 | 67.11±3.03 |
79.10±4.61b |
| 7 | 3.66±0.08 | 59.75±1.30 |
61.37±2.90b |
| 14 | 3.66±0.01 | 30.51±0.85 |
40.50±1.97b |
| 28 | 3.66±0.02 | 20.08±0.35 | 20.41±0.72 |
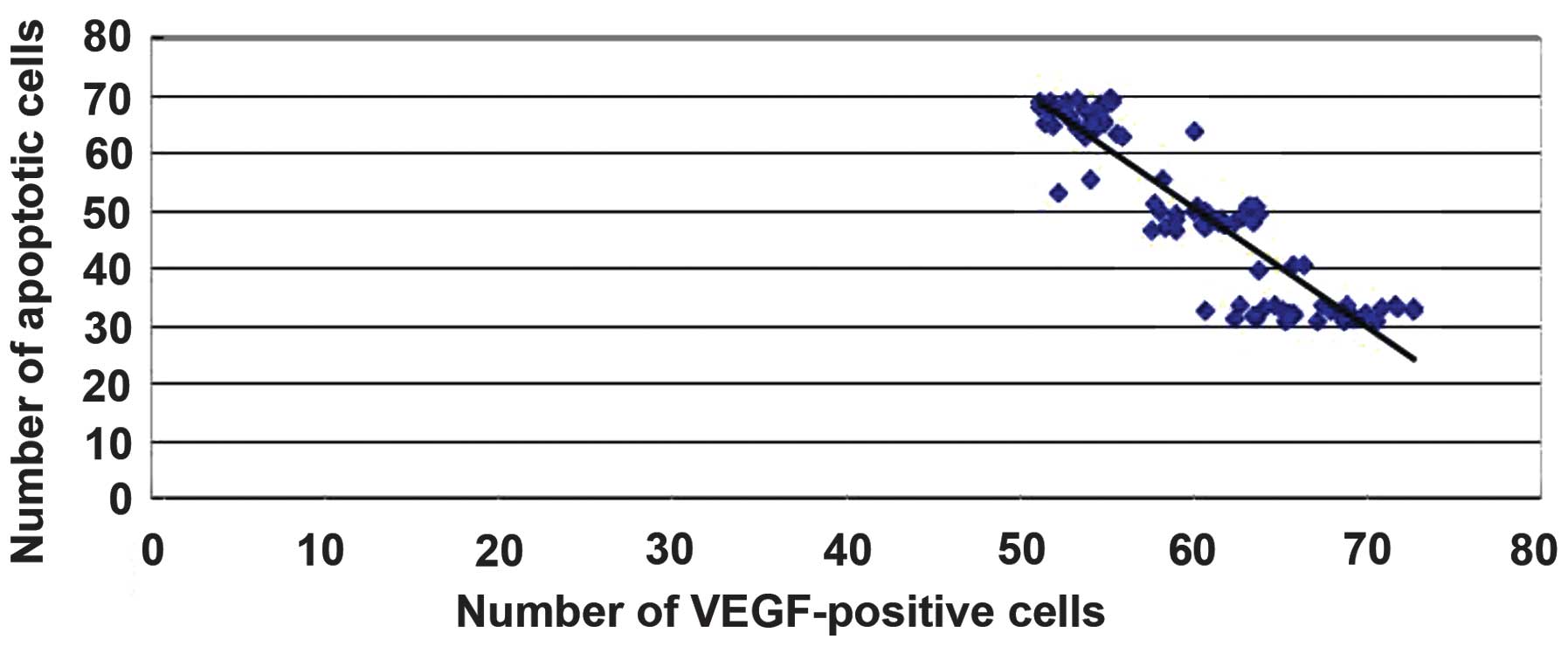
Correlation of VEGF expression with
the number of apoptotic cells
A significant correlation was found between the
degree of VEGF expression and the number of apoptotic cells
following injury (r=-0.90052, P<0.05). These data suggest that
batroxobin may exert protective effects by promoting the expression
of VEGF in order to reduce apoptosis in SCI in rats (Fig. 14).
Discussion
SCI is a serious and common CNS trauma, leading to
irreversible damage to the sensory and motor functions. At present,
treatment strategies for patients with SCI have been focusing
increasingly on the surgical stabilization of the initial injury to
prevent further loss of neurological function, without much
attention being paid to nerve cell protection and a reduction of
cell death, as has been a focus for the treatment of stroke
(24). In a previous study, the
potential of the nervous system to adapt to SCI from a functional
(neuronal plasticity) and a structural (neuronal remodeling)
perspective was demonstrated (25).
However, following the primary SCI, a secondary injury expands
continuously for ∼4 weeks; understanding the mechanism and finding
measures to control this secondary injury are of great importance.
Following SCI, the response of the host can generate an ischemic
environment that can lead to cell death. Furthermore, this ischemic
environment limits cell transplantation approaches that could be
used to promote spinal cord regeneration (26). It has been widely accepted that
apoptosis is the most common form of cell death following SCI. The
number of apoptotic cells is dependent on a number of factors,
including external stimulation, injury severity, secondary edema
and ischemia (27). By focusing on
the regulation of apoptosis subsequent to SCI it has been found
that the inhibition of this apoptosis could effectively protect the
nerve cells (28).
A previous study has shown that enhancing spinal
cord blood circulation reduces the secondary injury (29). The delivery of angiogenic factors,
such as VEGF, from poly(lactide-co-glycolide) scaffolds formed by
the gas foaming process can induce a local increase in blood vessel
formation (30,31). VEGF signals are considered to act as
neurotrophic factors (10,13,32).
Previous studies have demonstrated the direct neurotrophic effects
of VEGF on peripheral nerves (33)
and reported increased neuron density and viability in
mesencephalic explant cultures treated with VEGF (34). Successful neurotrophic or
neuroprotective and tissue-sparing effects have also been observed
following VEGF treatment in traumatic SCI (35,36).
Tissue edema is one of the main causes of secondary damage
subsequent to SCI (37,38). The application of VEGF following SCI
can decrease vascular permeability and tissue edema in the spinal
cord, and alleviate the deterioration of functional recovery
(39,40).
The aim of the present study was to examine the
effect of batroxobin, a drug widely used in various ischemic
disorders (19,20), in reducing the secondary damage
following SCI. One of the concerns for batroxobin administration in
SCI is the possibility of inducing bleeding in the injured cord. It
has been reported that a downstream product of batroxobin,
fibrinopeptide-A, forms an unstable clot and even shortens the
bleeding time in vivo (18,41). In
the present study, batroxobin effectively increased the expression
of VEGF and reduced the number of apoptotic cells, which suggests
that the batroxobin has a positive effect on the secondary damage
following SCI, promoting neuronal survival and improving locomotor
recovery.
In conclusion, this study has underlined the
potential of batroxobin for improving the functional outcome
following SCI. Since batroxobin is clinically widely used, its
beneficial effect in reducing SCI can be utilized in therapeutic
strategies; however, future studies are required to detail the
mechanisms underlying the batroxobin-induced decrease in apoptosis
and the promotion of functional recovery following SCI.
References
|
1
|
Cao HQ and Dong ED: An update on spinal
cord injury research. Neurosci Bull. 29:94–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McEwen ML, Sullivan PG, Rabchevsky AG and
Springer JE: Targeting mitochondrial function for the treatment of
acute spinal cord injury. Neurotherapeutics. 8:168–179. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Inman DM and Steward O: Physical size does
not determine the unique histopathological response seen in the
injured mouse spinal cord. J Neurotrauma. 20:33–42. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mautes AE, Weinzierl MR, Donovan F and
Noble LJ: Vascular events after spinal cord injury: Contribution to
secondary pathogenesis. Phys Ther. 80:673–687. 2000.PubMed/NCBI
|
|
5
|
Kwon BK, Tetzlaff W, Grauer JN, Beiner J
and Vaccaro AR: Pathophysiology and pharmacologic treatment of
acute spinal cord injury. Spine J. 4:451–464. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Byrnes KR, Stoica BA, Fricke S, Di
Giovanni S and Faden AI: Cell cycle activation contributes to
post-mitotic cell death and secondary damage after spinal cord
injury. Brain. 130:2977–2992. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mattson MP: Apoptosis in neurodegenerative
disorders. Nat Rev Mol Cell Biol. 1:120–129. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mizuno Y, Mochizuki H, Sugita Y and Goto
K: Apoptosis in neurodegenerative disorders. Intern Med.
37:192–193. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lewis KM, Turner RJ and Vink R: Blocking
neurogenic inflammation for the treatment of acute disorders of the
central nervous system. Int J Inflamm. 2013:5784802013.
|
|
10
|
Sondell M, Lundborg G and Kanje M:
Vascular endothelial growth factor has neurotrophic activity and
stimulates axonal outgrowth, enhancing cell survival and Schwann
cell proliferation in the peripheral nervous system. J Neurosci.
19:5731–5740. 1999.PubMed/NCBI
|
|
11
|
Schoch HJ, Fischer S and Marti HH:
Hypoxia-induced vascular endothelial growth factor expression
causes vascular leakage in the brain. Brain. 125:2549–2557. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pereira Lopes FR, Lisboa BC, Frattini F,
et al: Enhancement of sciatic nerve regeneration after vascular
endothelial growth factor (VEGF) gene therapy. Neuropathol Appl
Neurobiol. 37:600–612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jin K, Zhu Y, Sun Y, Mao XO, Xie L and
Greenberg DA: Vascular endothelial growth factor (VEGF) stimulates
neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA.
99:11946–11950. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Herrera JJ, Nesic O and Narayana PA:
Reduced vascular endothelial growth factor expression in contusive
spinal cord injury. J Neurotrauma. 26:995–1003. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sakowski SA, Heavener SB, Lunn JS, Fung K,
Oh SS, Spratt SK, Hogikyan ND and Feldman EL: Neuroprotection using
gene therapy to induce vascular endothelial growth factor-A
expression. Gene Ther. 16:1292–1299. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma Y, Liu W, Wang Y, Chao X, Qu Y, Wang K
and Fei Z: VEGF protects rat cortical neurons from mechanical
trauma injury induced apoptosis via the MEK/ERK pathway. Brain Res
Bull. 86:441–446. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Storkebaum E, Lambrechts D and Carmeliet
P: VEGF: Once regarded as a specific angiogenic factor, now
implicated in neuroprotection. BioEssays. 26:943–954. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
You WK, Choi WS, Koh YS, Shin HC, Jang Y
and Chung KH: Functional characterization of recombinant
batroxobin, a snake venom thrombin-like enzyme, expressed from
Pichia pastoris. FEBS Lett. 571:67–73. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bell WR Jr: Defibrinogenating enzymes.
Drugs. 54:(Suppl 3). 18–31. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gusev EI, Skvortsova VI, Suslina ZA,
Avakian GN, Martynov MIu, Temirbaeva SL, Tanashian MA, Kamchtnov
PR, Stakhovskaia LV and Efremova NM: Batroxobin in patients with
ischemic stroke in the carotid system (the multicenter study). Zh
Nevrol Psikhiatr Im S S Korsakova. 106:31–34. 2006.[(In Russian)].
PubMed/NCBI
|
|
21
|
Shiraishi T, Kubo T and Matsunaga T:
Chronological study of recovery of sudden deafness treated with
defibrinogenation and steroid therapies. Acta Otolaryngol.
111:867–871. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Black P, Markowitz RS, Damjanov I,
Finkelstein SD, Kushner H, Gillespie J and Feldman M: Models of
spinal cord injury: Part 3. Dynamic load technique. Neurosurgery.
22:51–60. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Basso DM, Beattie MS and Bresnahan JC:
Graded histological and locomotor outcomes after spinal cord
contusion using the NYU weight-drop device versus transection. Exp
Neurol. 139:244–256. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Goldsmith HS: The evolution of omentum
transposition: From lymphedema to spinal cord, stroke and
Alzheimer's disease. Neurol Res. 26:586–593. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yiu G and He Z: Glial inhibition of CNS
axon regeneration. Nat Rev Neurosci. 7:617–627. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Y, Gu J, Feng X, Wang H, Tao Y and
Wang J: Effects of Nogo-A receptor antagonist on the regulation of
the Wnt signaling pathway and neural cell proliferation in newborn
rats with hypoxic ischemic encephalopathy. Mol Med Rep. 8:883–886.
2013.PubMed/NCBI
|
|
27
|
Beattie MS, Hermann GE, Rogers RC and
Bresnahan JC: Cell death in models of spinal cord injury. Prog
Brain Res. 137:37–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, Gu J, Wang J, et al: BDNF and NT-3
expression by using glucocorticoid-induced bicistronic expression
vector pGC-BDNF-IRES-NT3 protects apoptotic cells in a cellular
injury model. Brain Res. 1448:137–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jia LY, Yao AH, Kuang F, Zhang YK, Shen XF
and Ju G: Beneficial effect of the traditional chinese drug
shu-xue-tong on recovery of spinal cord injury in the rat. Evid
Based Complement Alternat Med. 2011:8621972011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ennett AB, Kaigler D and Mooney DJ:
Temporally regulated delivery of VEGF in vitro and in vivo. J
Biomed Mater Res A. 79:176–184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Peters MC, Polverini PJ and Mooney DJ:
Engineering vascular networks in porous polymer matrices. J Biomed
Mater Res. 60:668–678. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun Y, Jin K, Xie L, Childs J, Mao XO,
Logvinova A and Greenberg DA: VEGF-induced neuroprotection,
neurogenesis, and angiogenesis after focal cerebral ischemia. J
Clin Invest. 111:1843–1851. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sondell M, Sundler F and Kanje M: Vascular
endothelial growth factor is a neurotrophic factor which stimulates
axonal outgrowth through the flk-1 receptor. Eur J Neurosci.
12:4243–4254. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Silverman WF, Krum JM, Mani N and
Rosenstein JM: Vascular, glial and neuronal effects of vascular
endothelial growth factor in mesencephalic explant cultures.
Neuroscience. 90:1529–1541. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Facchiano F, Fernandez E, Mancarella S,
Maira G, Miscusi M, D'Arcangelo D, Cimino-Reale G, Falchetti ML,
Capogrossi MC and Pallini R: Promotion of regeneration of
corticospinal tract axons in rats with recombinant vascular
endothelial growth factor alone and combined with adenovirus coding
for this factor. J Neurosurg. 97:161–168. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Widenfalk J, Lipson A, Jubran M,
Hofstetter C, Ebendal T, Cao Y and Olson L: Vascular endothelial
growth factor improves functional outcome and decreases secondary
degeneration in experimental spinal cord contusion injury.
Neuroscience. 120:951–960. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Narayana PA, Grill RJ, Chacko T and Vang
R: Endogenous recovery of injured spinal cord: Longitudinal in vivo
magnetic resonance imaging. J Neurosci Res. 78:749–759. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Maikos JT and Shreiber DI: Immediate
damage to the blood-spinal cord barrier due to mechanical trauma. J
Neurotrauma. 24:492–507. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Patel CB, Cohen DM, Ahobila-Vajjula P,
Sundberg LM, Chacko T and Narayana PA: Effect of VEGF treatment on
the blood-spinal cord barrier permeability in experimental spinal
cord injury: Dynamic contrast-enhanced magnetic resonance imaging.
J Neurotrauma. 26:1005–1016. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sundberg LM, Herrera JJ and Narayana PA:
In vivo longitudinal MRI and behavioral studies in experimental
spinal cord injury. J Neurotrauma. 27:1753–1767. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Adams RA, Passino M, Sachs BD, Nuriel T
and Akassoglou K: Fibrin mechanisms and functions in nervous system
pathology. Mol Interv. 4:163–176. 2004.PubMed/NCBI
|