Introduction
In the same way that benign prostatic hyperplasia
(BPH) is a widespread age-related pathological condition that
affects men worldwide, prostatism-like symptoms in women, referred
to as female lower urinary tract symptoms (LUTS), are also common,
mainly including voiding (‘obstructive’) and filling (‘irritative’)
symptoms, such as weak stream, hesitancy, intermittency, nocturia,
daytime frequency and urgency (1).
LUTS are highly prevalent in women, particularly perimenopausal
women, but they are rarely reported as they are considered to be
part of the aging process and it is assumed that no effective
treatment is available. LUTS increase and become more aggravating
with advancing age in the majority of individuals (2–6). Voiding
symptoms were observed to be more common than filling symptoms in a
study of female patients with LUTS who visited a urological clinic.
Furthermore, functional bladder outlet obstruction was more
prevalent than detrusor underactivity in these female patients
(7).
A similarly high prevalence of filling and voiding
LUTS in men and women suggests that certain aspects of the
underlying etiology may be identical. Adrenergic receptors
(adrenoceptors, ARs) were originally categorized into αAR and βAR
subgroups. However, the application of molecular biological methods
in the last few years has confirmed a total of nine AR subtypes:
α1A (formerly named α1c), α1B,
α1D, α2A, α2B, α2C,
β1, β2 and β3 (8). The α1DAR predominates in the
female detrusor and spinal cord (9,10). The
α1AAR is expressed at significantly higher levels than
other α1AR subtypes are in the female urethra (11,12).
These findings suggest that voiding and filling symptoms have a
correlation with the expression of these α1AR subtypes,
and indicates that α1-adrenoceptor antagonists
(α1ARAs) may be used as a potentially novel treatment
for female patients with LUTS.
In clinical practice, however, the use of
α1ARAs to treat LUTS in women has been adopted based on
limited studies, anecdotal case reports and local experience. The
specific mechanism by which α1ARAs act in the treatment
of female LUTS has not been established; however, some small
sample-size clinical trials have confirmed that α1ARAs
are able to significantly improve voiding and filling symptoms in
female patients with LUTS (13–16).
A meta-analysis was, therefore, carried out to
determine the effectiveness of α1ARAs versus placebo for
female patients with LUTS.
Materials and methods
Search strategy
Pubmed (1966–2013), Embase (1974–2013), the Cochrane
Library (issue 4, 2013), the Chinese Biomedical Literature database
(1978–2013), the Chinese Sci-Tech Periodical Full-Text database
(1989–2013) and the Chinese Periodical Full-Text database
(1994–2013) were searched for randomized controlled trials in which
α1ARAs, terazosin, tamsulosin, doxazosin or alfuzosin
were compared with placebo in female patients with LUTS. Related
references and included studies on the Internet, according to
search engines such as Google™, were also searched, and a manual
search was used to find key Chinese publications in associated
fields. The reference lists of included studies and reviews were
searched by hand and experts in the field were contacted;
unpublished studies were not sought. No limits based on language
were imposed.
This search strategy was used to obtain the titles
and abstracts from randomized controlled trials associated with the
subject matter of this review. The titles and abstracts were
screened independently by two reviewers (Long Cheng and Hao-Han
Wang), who discarded studies that were inapplicable. Two reviewers
independently assessed the retrieved titles and abstracts of all
the identified trials to confirm fulfillment of the inclusion
criteria. Disagreements were resolved in consultation with Xiao-Kan
Xiong. Data extraction was carried out independently by the same
authors using standard data extraction forms.
Inclusion criteria
The patients were women with LUTS, aged 20 to 70
years, with a total I-PSS ≥8. Written informed consent was
provided. Excluded cases were pregnancy, breastfeeding, stress
incontinence, urinary tract infection, neurological diseases
including diabetes mellitus with neuropathy, previous pelvic
surgery or radiation, medical conditions mimicking LUTS and
concomitant medications affecting the lower urinary tract.
Types of outcome measures
The main outcome measures were total I-PSS and
maximum urinary flow rate (MUFR). The I-PSS quality of life (QOL)
and average urinary flow rate (AFR) were also observed and
analyzed.
Types of intervention
The types of intervention were α1ARAs
versus placebo.
Methodological quality assessment and
level of evidence
The Cochrane collaboration tool in Review Manager,
version 5.2 (Cochrane Collaboration, Copenhagen,. Denmark) was used
for assessing the risk of bias in order to evaluate the
methodological quality of each randomized controlled trial. The
Grades of Recommendation, Assessment, Development and Evaluation
(GRADE) approach was applied to assess the level of evidence, and
GRADEprofiler software, version 3.6 (Cochrane Collaboration) was
used to create the evidence profile.
Statistical analysis
The data were analyzed using Stata (version 12.0;
Stata Corporation, College. Station, TX, USA) and data were
extracted and pooled for summary estimates. Results are expressed
for continuous outcomes as weighted mean difference or standardized
mean difference, and dichotomous outcomes as relative risk with 95﹪
confidence intervals (CI). The χ2 statistical test was
used to assess heterogeneity between trials and the Ι2
statistical test was used to assess the extent of inconsistency. A
fixed effect model was used for calculations of summary estimates
and their 95﹪ CI, unless there was significant heterogeneity, in
which case results were confirmed using a random effects
statistical model. Subgroup analyses were designed to explore
important clinical differences that may be expected to alter the
magnitude of treatment effects.
Results
Search results
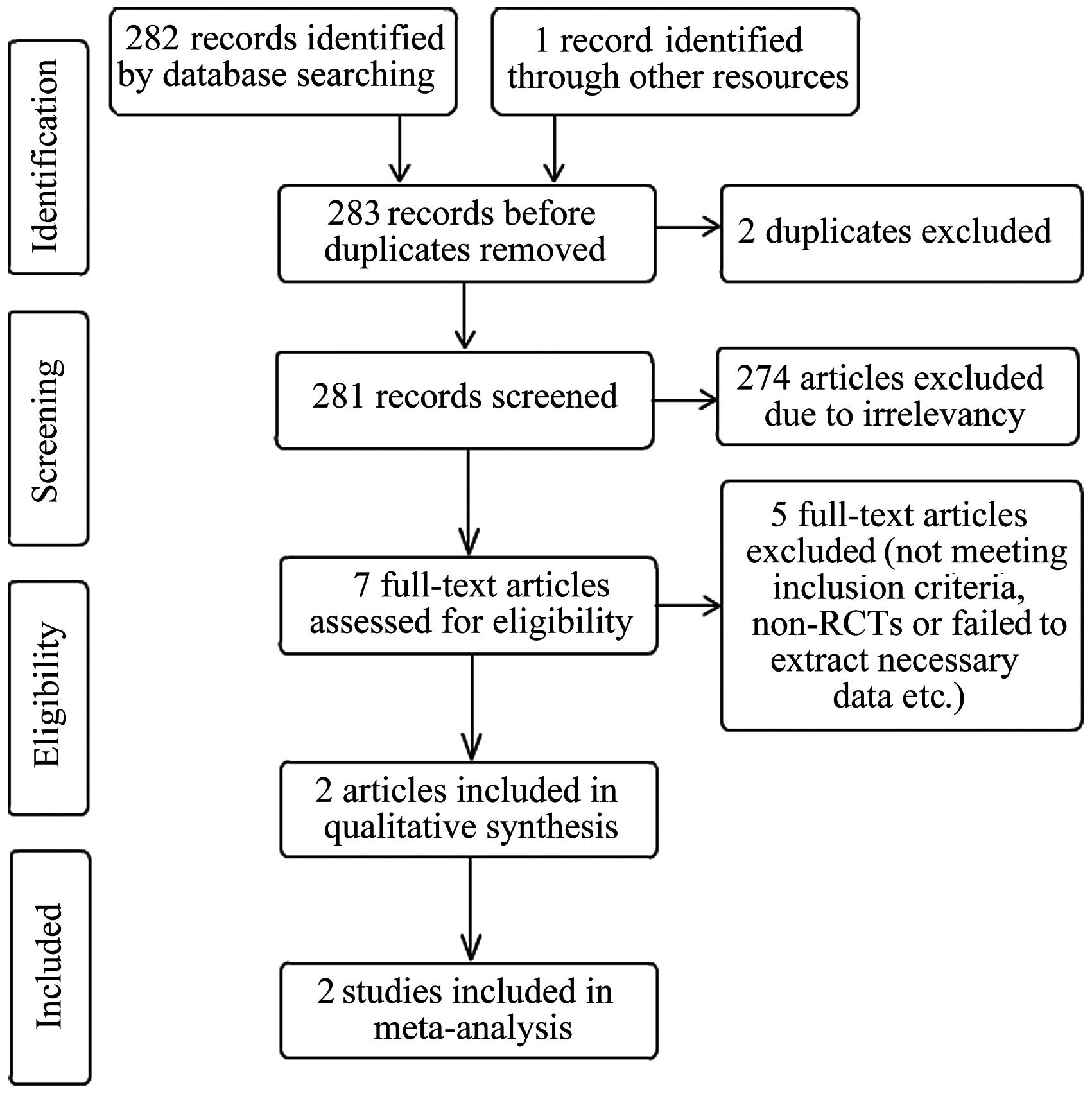
The selection process is depicted in Fig. 1. Seven potentially eligible trials
were identified and five trials were subsequently excluded for the
following reasons: Two were not controlled; one failed to extract
necessary data such as the mean and standard deviations of the
outcome index; one did not include a placebo group; and one was an
unpublished conference article that contained no detailed data. Two
randomized controlled trials totaling 213 patients [133 in the
study by Pummangura et al (16) and 80 in the study by Low et al
(15)] were included. The two trials
reported two types of outcome including total I-PSS and MUFR.
Patients were matched for age and the severity of urinary symptoms.
Co-morbidities that clinically indicated symptoms similar to LUTS
were not sought.
Assessing the risk of bias, and
characteristics of included studies
The main characteristics of the two included studies
are shown in Table I. The new ‘Risk
of bias’ tool in Review Manager 5.2 was used to assess risk of bias
(Table II).
 | Table I.Characteristics of the two
studies. |
Table I.
Characteristics of the two
studies.
|
|
|
| No. | Intervention |
| TI-PSSa | MUFRa | I-PSS
QOLa | AFRa |
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Study (ref.) | Gender | Age(years) | T | C | T | C |
Pharmacologyb | T | C | P-value | T | C | P-value | T | C | P-value | T | C | P-value |
|---|
| 15 | Female | 20–70 | 40 | 40 | Terazosin | Placebo |
α1A=α1B=α1D | 15.6 | 14.38 | >0.05 | 21.73 | 20.13 | >0.05 | 4.78 | 5.03 | >0.05 |
| – |
|
| 16 | Female | 27–69 | 65 | 68 | Tamsulosin | Placebo |
α1A=α1D>α1B | 18.2 | 22.50 | 0.001c | 18.00 | 18.80 | >0.05 |
| – |
| 7.00 | 7.70 | >0.05 |
 | Table II.Assessment of the risk of bias in the
studies. |
Table II.
Assessment of the risk of bias in the
studies.
| Study (ref.) | Random sequence
generation | Allocation
concealment | Blinding of
participants and personnel | Blinding of outcome
assesment | Incomplete outcome
data | Selective
reporting | Other bias |
|---|
| 15 | Low riska | Unclear
riska | Low
riskb | Low
riskc | Low
riskd | Low
riske | Low
riskf |
| 16 | Low
riskg | Low
riskg | Low
riskh | Low
riskc | Low
riski | Low
riskj | Low
riskf |
Meta-analysis results
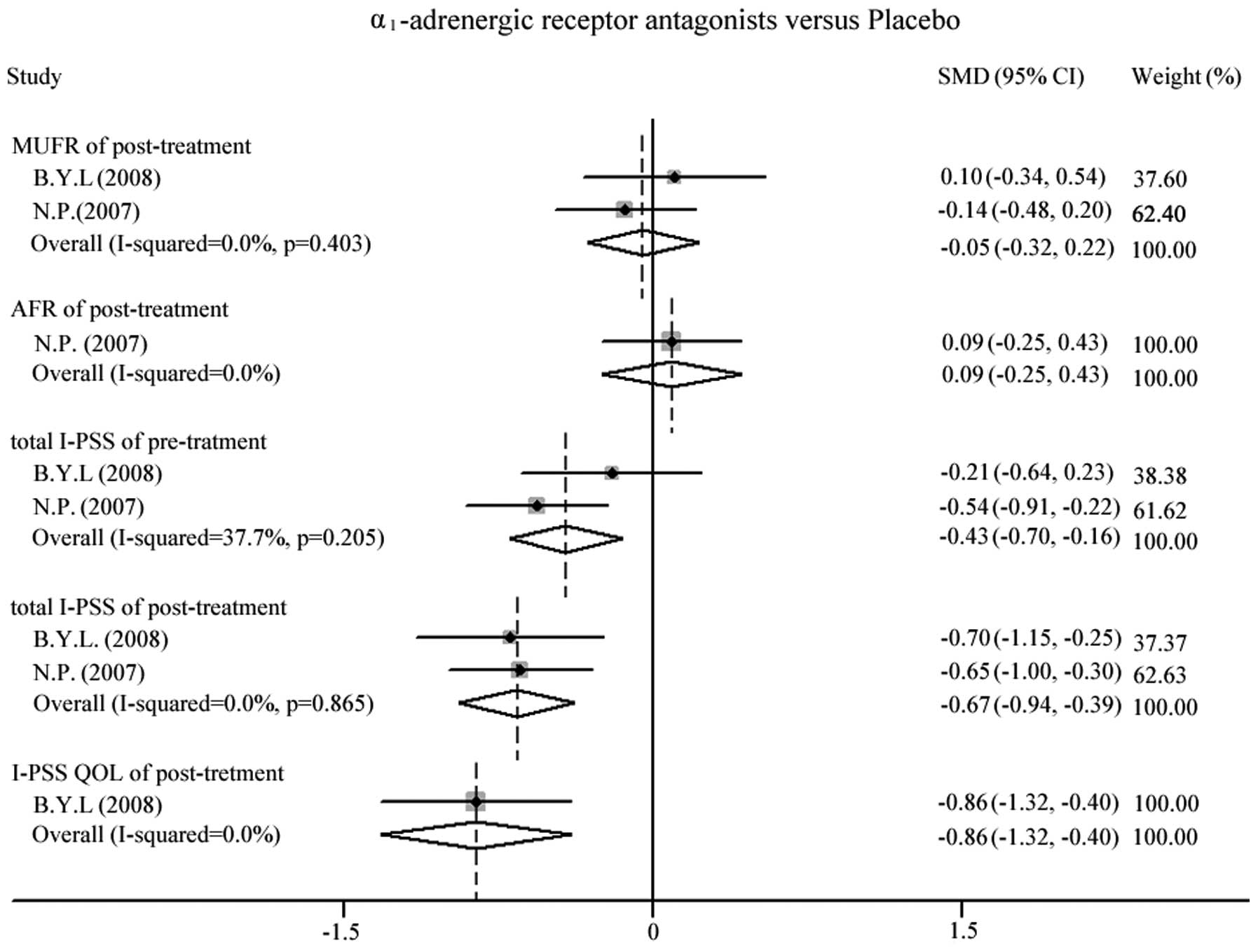
Results of the analysis are depicted in Fig. 2. Following almost 4 weeks of
treatment, the MUFR post-treatment [standardized mean difference
(SMD, −0.05; 95% CI, −0.32 to 0.22] between the experimental and
control groups was found not to differ. The improvement of AFR was
not significant (SMD, 0.09; 95% CI, −0.25 to 0.43) in the study
conducted by Pummangura et al (16). The meta-analysis indicates that the
two groups are inhomogeneous, due to a statistically significant
difference (P=0.001) in the baseline I-PSS between experimental and
control groups (16). In general,
the total I-PSS was decreased in both groups following treatment
compared with the baseline. In patients receiving α1ARAs
and placebo, the total I-PSS following treatment was lower than
that prior to treatment, but the total I-PSS was significantly
lower in females treated with α1ARAs than in females
treated with placebo (SMD, −0.67; 95% CI, −0.94 to −0.39). In
addition, the I-PSS QOL post-treatment was lower in the
α1ARA group compared with that in the placebo-treated
group (SMD, −0.86; 95% CI, −1.32 to −0.40) (15).
GRADE profile of evidence
The quality of evidence in the included studies, as
determined by the GRADE approach, is shown in Table III. Two critical outcome measures:
Total I-PSS and I-PSS QOL, and two important outcomes: MUFR and
AFR, were judged to indicate high-quality evidence.
 | Table III.GRADE profile of evidence of the
included studies. |
Table III.
GRADE profile of evidence of the
included studies.
|
|
|
| Quality
assessment | No. of
patients |
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Outcome | No. of study | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other
considerations | α1−AR
antagonists | Placebo | Absolute effect,
SMD (95% CI) | Quality | Importance |
|---|
| TI-PSS | 2 | RCT | Not serious | Not serious | Not serious | Not serious | None | 105 | 108 | −0.67 (−0.94 to
−0.39) | High | Critical |
| MUFR | 2 | RCT | Not serious | Not serious | Not serious | Not serious | None | 105 | 108 | −0.05 (−0.32 to
0.22) | High | Important |
| I-PSS QOL | 1 | RCT | Not serious | Not serious | Not serious | Not serious | None | 40 | 40 | −0.86 (−1.32 to
−0.4) | High | Critical |
| AFR | 1 | RCT | Not serious | Not serious | Not serious | Not serious | None | 65 | 68 | 0.09 (−0.25 to
0.43) | High | Important |
Discussion
A randomized double-blind study of 29 women in New
York reported by Lepor and Theune (17) in 1995 indicated that terazosin was
not effective for relieving prostatism-like symptoms. Another open
non-randomized trial that used doxazosin, demonstrated that
α1ARAs were at least as effective as the anticholinergic
drug hyoscyamine in reducing the total I-PSS (13). These studies were excluded due to
non-conformity of the inclusion criteria. In the study by Chang
et al (14), which comprised
97 female patients and was not placebo controlled, the outcome
suggested that tamsulosin improved voiding symptoms and urodynamic
parameters in nearly one-third of women with voiding difficulty,
and comparable good therapeutic response rates were observed
between patients with bladder outlet obstruction and detrusor
underactivity (14). This
disagreement among studies of whether α1ARAs are more
effective than placebo in the treatment of female LUTS was
perplexing; hence, the collection and evaluation of studies
concerning the efficacy of α1ARAs in the treatment of
female LUTS in the present meta-analysis was important and it may
provide clinicians with a temporary guideline for selecting
α1ARA treatments.
Although the number of studies included in the
present meta-analysis was small, the quality of the two studies and
the reliability of the outcome measures were high. Due to the low
number of participants it was not possible to reach a reliable
conclusion; however, a low risk of bias and a high GRADE quality of
evidence suggested that the outcome measures were reliable, at
least in terms of the two included studies. The two studies
mentioned randomized, double-blind, allocation concealment, and one
of them included intent-analysis. The majority of participants were
Asian, so any differences due to ethnicity could not be observed.
In clinical trials conducted in the future, it may be appropriate
to expand the sample size and select participants of different
nationalities.
The bladder, bladder neck and urethra are
responsible for urine storage and voiding in females. During the
storage phase of the micturition cycle, the bladder relaxes to
accommodate increasing volumes of urine at acceptable pressures,
and the bladder neck and urethra contract to provide resistance to
prevent involuntary leakage. During the micturition phase, the
bladder neck and urethral muscle relax to allow the detrusor to
contract and expel urine without major resistance. The
α1DAR predominates in the female detrusor and the
α1AAR is expressed at higher levels than other
α1AR subtypes in the female urethra (including the
bladder neck) (9–12). Therefore, the efficacy of
α1ARAs for the treatment of female LUTS may be explained
by the targeting of two possible mechanisms. The first is
dysfunction of the bladder neck and urethra, causing functional
outlet obstruction and secondary detrusor overactivity, which is
similar to bladder outlet obstruction in men with BPH. The second
possibility is increased α1AR activity in the detrusor,
causing frequency and urgency (18);
however, from the meta-analysis results, only total I-PSS and I-PSS
QOL improved following α1ARA treatment with no
alteration of the MUFR and AFR compared with those of the placebo
groups. If α1ARAs relax the bladder neck and urethral
muscle, it is possible that MUFR and AFR could improve. More
studies are required to confirm whether the difference of total
I-PSS and I-PSS QOL is associated with MUFR and AFR in the control
group. In terms of terazosin and tamsulosin blocking
α1A- and α1DARs (Table I), the bladder neck, urethral muscle
and detrusor would be relaxed under the effect of these drugs. It
is hypothesized that the fact that there was no clear alteration of
the MUFR and AFR was as a result of the functional bladder outlet
obstruction remitting and the detrusor pressure reducing over time.
Highly selective α1AARAs may improve the MUFR and AFR
for women with LUTS including functional bladder outlet obstruction
along with possible detrusor overactivity.
In the present analysis, α1ARAs were
indicated to be more effective than placebo in reducing total I-PSS
and improving QOL in females with LUTS; however, MUFR and AFR did
not increase significantly and it was not clear whether the
alteration of total I-PSS and IPSS QOL was associated with changes
of MUFR and AFR for females with LUTS under α1ARA
treatment. As the sample size was small and the patients were from
a limited geographical area, further clinical trials are required
to expand the sample size and select participants of different
ethnicities. More high-quality, multi-center, randomized controlled
trials are required.
References
|
1
|
Fitzpatrick JM: Facts and future lines of
research in lower urinary tract symptoms in men and women: An
overview of the role of α1-adrenoreceptor antagonist. BJU Int.
85:(Suppl 2). 1–5. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Møller LA, Lose G and Jørgensen T: The
prevalence and bothersomeness of lower urinary tract symptoms in
women 40–60 years of age. Acta Obstet Gynecol Scand. 79:298–305.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wennberg AL, Molander U, Fall M, Edlund C,
Peeker R and Milsom I: A longitudinal population-based survey of
urinary incontinence, overactive bladder and other lower urinary
tract symptoms in women. Eur Urol. 55:783–791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wehrberger C, Madersbacher S, Jungwirth S,
Fischer P and Tragl KH: Lower urinary tract symptoms and urinary
incontinence in a geriatric cohort - a population-based analysis.
BJU Int. 110:1516–1521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coyne KS, Sexton CC, Thompson CL, Milsom
I, Irwin D, Kopp ZS, Chapple CR, Kaplan S, Tubaro A, Aiyer LP and
Wein AJ: The prevalence of lower urinary tract symptoms (LUTS) in
the USA, the UK and Sweden: Results from the Epidemiology of LUTS
(EpiLUTS) study. BJU Int. 104:352–360. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coyne KS, Sexton CC, Bell JA, Thompson CL,
Dmochowski R, Bavendam T, Chen CI and Quentin Clemens J: The
prevalence of lower urinary tract symptoms (LUTS) and overactive
bladder (OAB) by racial/ethnic group and age: Results from
OAB-POLL. Neurourol Urodyn. 32:230–237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi YS, Kim JC, Lee KS, Seo JT, Kim HJ,
Yoo TK, Lee JB, Choo MS, Lee JG and Lee JY: Analysis of female
voiding dysfunction: A prospective, multi-center study. Int Urol
Nephrol. 45:989–994. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schwinn DA and Roehrborn CG:
Alpha1-adrenoceptor subtypes and lower urinary tract symptoms. Int
J Urol. 15:193–199. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Malloy BJ, Price DT, Price RR, Bienstock
AM, Dole MK, Funk BL, Rudner XL, Richardson CD, Donatucci CF and
Schwinn DA: Alpha1-adrenergic receptor subtypes in human detrusor.
J Urol. 160:937–943. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Andersson KE: Mode of action of
alpha1-adrenoreceptor antagonists in the treatment of lower urinary
tract symptoms. BJU Int. 85:(Suppl 2). 12–18. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Michel MC and Vrydag W: Alpha1-, alpha2-
and beta-adrenoceptors in the urinary bladder, urethra and
prostate. Br J Pharmacol. 147:(Suppl 2). S88–S119. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nasu K, Moriyama N, Fukasawa R, Tsujimoto
G, Tanaka T, Yano J and Kawabe K: Quantification and distribution
of α1-adrenoreceptor subtype mRNAs in human proximal urethra. Br J
Pharmacol. 123:1289–1293. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Serels S and Stein M: Prospective study
comparing hyoscyamine, doxazosin, and combination therapy for the
treatment of urgency and frequency in women. Neurourol Urodyn.
17:31–36. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang SJ, Chiang IN and Yu HJ: The
effectiveness of tamsulosin in treating women with voiding
difficulty. Int J Urol. 15:981–985. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Low BY, Liong ML, Yuen KH, Chee C, Leong
WS, Chong WL, Khan NA, Cheah PY and Liong KK: Terazosin therapy for
patients with female lower urinary tract symptoms: a randomized,
double-blind, placebo controlled trial. J Urol. 179:1461–1469.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pummangura N and Kochakarn W: Efficacy of
tamsulosin in the treatment of lower urinary tract symptoms (LUTS)
in women. Asian J Surg. 30:131–137. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lepor H and Theune C: Randomized
double-blind study comparing the efficacy of terazosin versus
placebo in women with prostatism-like symptoms. J Urol.
154:116–118. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kakizaki H and Koyanagi T: Current view
and status of the treatment of lower urinary tract symptoms and
neurogenic lower urinary tract dysfunction. BJU Int. 85:(Suppl 2).
25–30. 2000. View Article : Google Scholar : PubMed/NCBI
|