Introduction
Lumbosacral radiculopathy is a relatively common
clinical condition that occurs as a result of mechanical
deformation and biochemical irritation caused by intervertebral
disc (IVD) herniation. Although mechanical nerve root compression
may contribute to the symptoms of radiculopathy, it is currently
believed that an inflammatory or immune component is the key
mediator of radicular pain (1,2). The
nucleus pulposus (NP) tissue may be autoantigenic; this theory is
supported by the accumulation of lymphocytes in regional lymph
nodes following exposure to autologous NP (3) and injury to the annulus fibrosus
(4). A number of animal studies
applied healthy autologous NP to spinal nerve roots, as a model of
non-compressive disc herniation, which induced an inflammatory and
immune response resulting in neuronoglial apoptosis, a decrease in
nerve conduction velocity, the onset of gait abnormality,
mechanical allodynia and thermal hyperalgesia (5–12).
Inflammatory cells (predominantly macrophages) in herniated disc
tissue that have been harvested during surgery synthesize
proinflammatory mediators and cytokines, such as phospholipase A2
(13,14), leukotrienes (14), immunoglobulins (14), fibroblast growth factor (15–17),
matrix metalloproteinases (18–21),
nitric oxide (18–20), interleukin (IL)-1 (14,22–24),
IL-4 (25), IL-6 (14,19,22,23,26,27),
IL-8 (27), IL-10 (26), IL-17 (12), IL-20 (28), IL-23 (12), prostaglandin E2 (18,19,22,27,29),
tumor necrosis factor-α (14,22–24,30–32),
interferon γ (25),
granulocyte-macrophage colony stimulating factor (22,26),
nerve growth factor (21), substance
P (21,33), cyclooxygenase-2 (31,32,34,35),
monocyte chemoattractant protein-1 (36) and vascular endothelial growth factor
(37).
Numerous studies have focused on a new subset of T
cells, known as T-helper type 17 (Th17) cells, which are associated
with autoimmune diseases. IL-17 is a cytokine associated with
inflammation, autoimmunity (38),
inflammatory bowel disease (39),
psoriasis (40), rheumatoid
arthritis (41) and the defense
against certain bacterial and viral infections (42). IL-17A and IL-17B, along with the
IL-17 receptor and IL-17 receptor-like protein, have been found to
localize in chondrocytes in the fracture callus and in the
epiphyseal growth plate during an endochondral differentiation
program (43).
Previous studies have examined the association
between IL-17 and disc diseases, particularly lumbar disc
herniation (LDH). According to the findings of those studies, IL-17
participates in the local inflammatory response in an autologous NP
model of radiculopathy (44). The
presence of IL-17 has been examined in herniated disc tissues of
patients with LDH, demonstrating the involvement of Th17
lymphocytes in disc degeneration (45,46).
IL-17 is currently being used as a marker of inflammation and
immune activation. There is, however, limited research on the
expression of IL-17 in different disc pathologies in LDH. The
present study investigated the differences in IL-17 expression
among patients with different types of LDH. Specimens were
collected from patients who had undergone surgery and were
investigated through pathological and immunological
observations.
Materials and methods
Patients
IVD specimens from 46 patients who had received
conservative therapy for ≥3 months between March and October 2009
were obtained through primary lumbar discectomy for radiculalgia.
The subjects comprised 24 men and 22 women aged 18–76 years (mean
age, 45.2±13.1 years) (Table I).
Patients showed either no improvement in radiculopathy or initial
improvement during conservative therapy, which was, however,
followed by relapse. Patients were assessed according to symptoms,
physical examination (sensation, muscle strength and straight leg
raising test) and image data. Certain patients also underwent the
femoral nerve stretch test if it was considered that the L3/4
herniated lumbar disc could affect the femoral nerve. The study
protocol was approved by the Ethics Committee of Tianjin Hospital
(Tianjin, China). Herniation was present at L3/4 in 3 discs, L4/5
in 32 discs and L5/S1 in 17 discs. The presence or absence of
perforation of the posterior longitudinal ligament could not be
conclusively confirmed by magnetic resonance imaging. The type of
herniation was, therefore, confirmed during the surgery, based on
whether or not the annulus fibrosus was broken. The patients were
divided into two groups: The contained disc herniation (CDH) and
the non-contained disc herniation (NCDH) groups. Specimens
collected during surgery were examined by immunopathology.
 | Table I.Clinical characteristics of the 46
patients with lumbar disc herniation. |
Table I.
Clinical characteristics of the 46
patients with lumbar disc herniation.
| Case no. | Gender | Age, years | Level (side) | Duration of
sciatica, months | SLR test,
degrees | Sensory disturbance
of lower leg | Muscle weakness of
lower leg |
|---|
| 1 | F | 22 | L5/S1 (R) | 6 | 40 | + | + |
| 2 | M | 23 | L4/5 (L) | 48 | 50 | – | – |
| 3 | M | 28 | L4/5 (R) | 24 | 40 | + | – |
|
|
|
| L5/S1 (R) |
|
|
|
|
| 4 | F | 55 | L4/5 (L) | 2 | 45 | + | + |
| 5 | F | 53 | L4/5 (L) | 1 | 30 | + | + |
| 6 | M | 53 | L4/5 (R) | 2 | 70 | + | – |
|
|
|
| L5/S1 (R) |
|
|
|
|
| 7 | M | 46 | L5/S1 (R) |
0.2 | 50 | + | – |
| 8 | M | 44 | L4/5 (R) | 2 | 50 | – | + |
| 9 | F | 21 | L5/S1 (L) | 4 | 30 | + | – |
| 10 | F | 46 | L5/S1 (L) | 5 | 40 | – | – |
| 11 | F | 46 | L4/5 (R) | 5 | 30 | + | + |
| 12 | F | 41 | L5/S1 (L) | 3 | 50 | + | + |
| 13 | F | 36 | L4/5 (R) | 1 | 40 | + | + |
|
|
|
| L5/S1 (R) |
|
|
|
|
| 14 | M | 52 | L4/5 (R) |
1.2 | 50 | + | + |
| 15 | M | 18 | L4/5 (R) | 1 | 10 | + | + |
| 16 | M | 42 | L5/S1 (R) | 10 | 50 | + |
|
| 17 | M | 56 | L4/5 (L) | 1 | 45 | + | + |
| 18 | M | 56 | L4/5 (L) | 2 | 70 | + | + |
| 19 | F | 44 | L4/5 (L) | 1 | 30 | + | + |
|
|
|
| L5/S1 (R) |
|
|
|
|
| 20 | F | 52 | L3/4 (R) |
0.3 |
FNST+ | + | + |
| 21 | F | 47 | L5/S1 (R) | 4 | 50 | + | + |
| 22 | M | 20 | L4/5 (R) | 6 | 20 | + | – |
| 23 | M | 39 | L4/5 (L) | 30 | 30 | + | – |
| 24 | M | 54 | L4/5 (R) |
0.3 | 40 | – | – |
| 25 | M | 34 | L4/5 (R) | 4 | 20 | + | + |
| 26 | M | 54 | L4/5 (R) | 2 | 30 | + | + |
| 27 | F | 44 | L4/5 (L) | 2 | 30 | + | + |
| 28 | M | 42 | L3/4 (R) | 60 |
FNST+ | + | + |
| 29 | M | 58 | L4/5 (R) | 2 | 40 | + | + |
| 30 | M | 47 | L3/4 (L) | 48 |
FNST− | + | + |
| 31 | M | 52 | L4/5 (L) | 3 | 40 | + | + |
|
|
|
| L5/S1 (R) |
|
|
|
|
| 32 | F | 40 | L5/S1 (R) |
0.2 | 50 | + | + |
| 33 | F | 39 | L4/5 (L) | 12 | 30 | + | + |
|
|
|
| L5/S1 (R) |
|
|
|
|
| 34 | F | 56 | L4/5 (L) | 3 | 40 | + | + |
| 35 | M | 76 | L4/5 (R) | 6 | 30 | + | + |
| 36 | F | 67 | L4/5 (R) | 2 | 70 | + | + |
| 37 | F | 68 | L4/5 (R) | 8 | 20 | + | + |
| 38 | F | 63 | L4/5 (L) | 2 | 0 | + | + |
| 39 | F | 40 | L4/5 (L) | 36 | 30 | + | + |
| 40 | M | 48 | L4/5 (L) | 36 | 70 | + | – |
| 41 | M | 21 | L5/S1 (L) | 9 | 20 | + | – |
| 42 | F | 43 | L4/5 (L) |
0.5 | 80 | + | – |
| 43 | M | 52 | L4/5 (L) |
0.7 | 30 | + | + |
| 44 | F | 52 | L5/S1 (R) |
0.3 | 30 | + | + |
| 45 | F | 39 | L4/5 (L) | 12 | 30 | + | + |
| 46 | M | 52 | L5/S1 (L) |
0.5 | 30 | + | + |
Preparation of pathological
specimens
IVDs were harvested during discectomy and specimens
underwent light microscopic examination. The specimens for light
microscopy were fixed in 10% formalin and embedded in paraffin.
Paraffin sections (5-µm-thick) were cut and stained with
hematoxylin and eosin and toluidine blue. Surgical specimens were
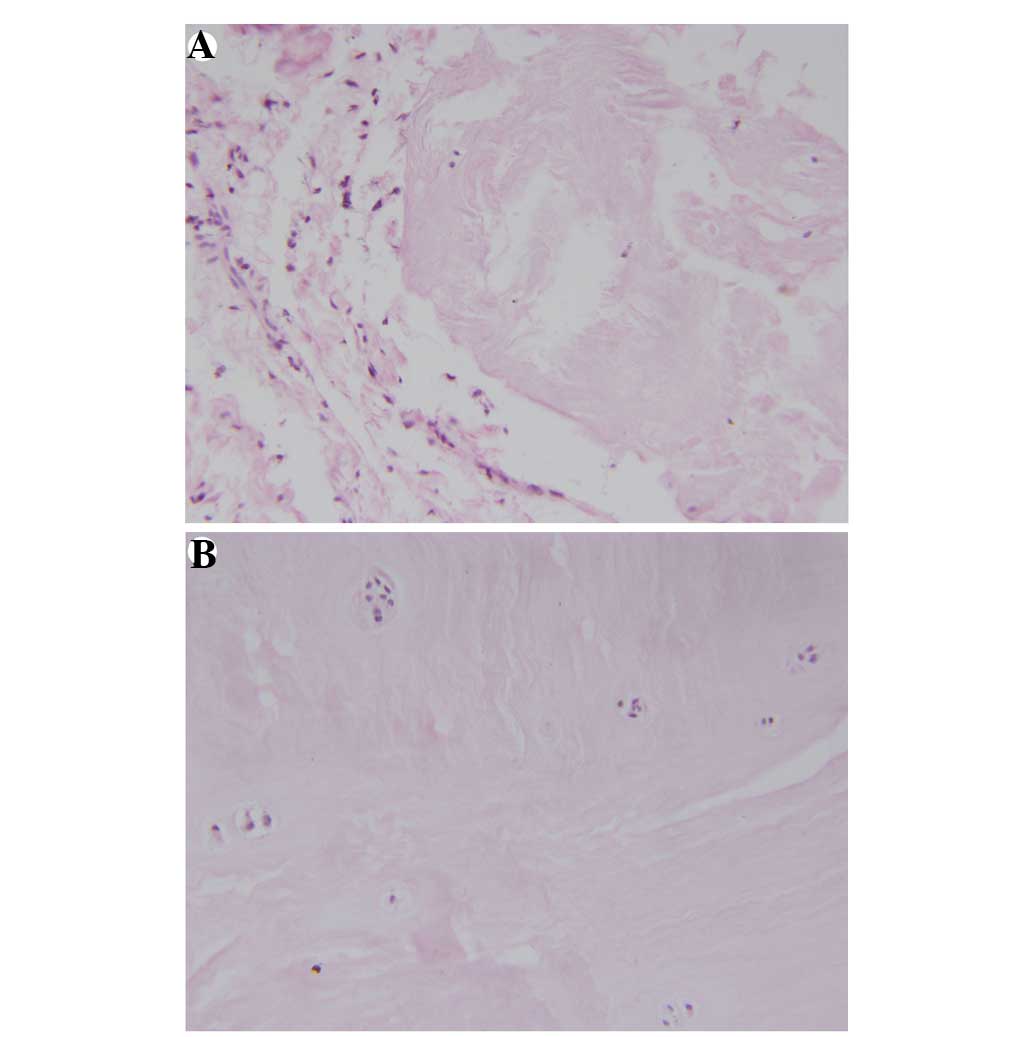
qualitatively graded according to whether or not inflammatory
changes existed in the herniation (47) (Fig. 1)
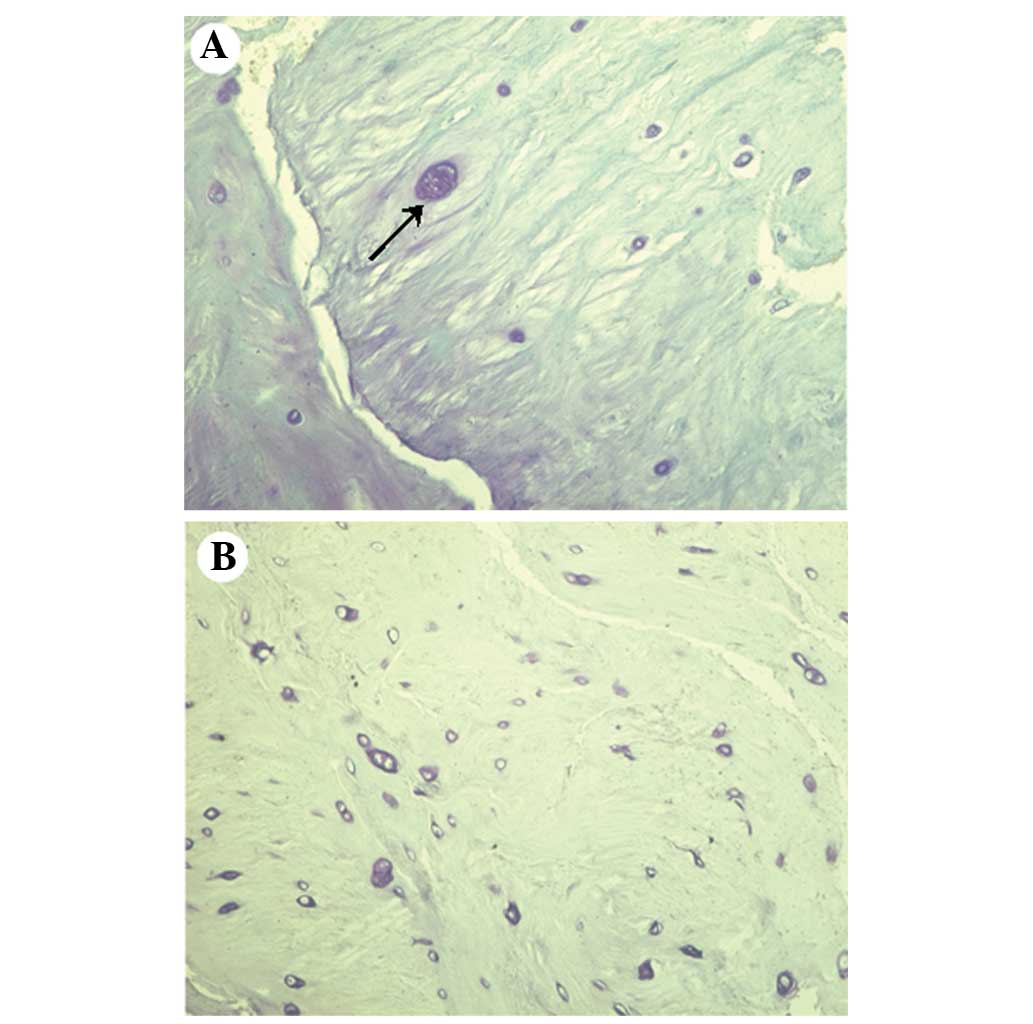
and hypertrophic chondrocytes were present in the specimens
(Fig. 2), as shown by the amount of
toluidine blue staining.
Immunohistochemistry of IL-17
Immunohistochemistry of IL-17 was performed
according to the streptavidin-peroxidase (SP) method, using an
SP-9001 Rabbit SP kit (Tianjin Haoyang Biological Manufacture Co.,
Ltd., Tianjin, China). The sections were baked at 60°C for 1 h,
deparaffinized, rehydrated, immersed in 3%
H2O2 in phosphate-buffered saline (PBS) for
10 min, heated in a sodium citrate bath in a microwave oven, washed
in PBS and blocked in 10% sheep serum for 15 min at room
temperature. The sections were then incubated with primary rabbit
polyclonal anti-human IL-17 antibodies (cat. no. sc-7927; Santa
Cruz Biotechnology Inc., Santa Cruz, CA, USA), and diluted 1:50 in
a blocking solution at 4°C in a humidified chamber overnight.
Incubation with biotinylated secondary antibody (Tianjin Hao Yang
Biological Manufacture Co. Ltd., Tianjin, China) was performed for
15 min at room temperature, and an avidin-biotin alkaline
phosphatase complex reaction was performed for 15 min, also at room
temperature. Color was developed with diaminobenzidine, and the
sections were counterstained with hematoxylin for 1 min.
Tris-buffered saline was used as a negative control instead of the
primary antibodies, and the conditions were selected so that there
was no signal in the negative control samples. Five slices of IVDs
were used to determine the number of IL-17- immunoreactive cells
per 0.015625 mm2 of histological section for each
evaluation. The evaluation was blinded with respect to the grouping
of the specimens.
Statistical analysis
The statistical significance of the differences in
the neovascularization, granulation tissue and hypertrophic
chondrocytes between the two groups was assessed using the
χ2 test. The number of IL-17-immunoreactive cells in the
herniated IVDs was compared by an independent-samples t-test.
P<0.05 indicated a statistically significant difference.
Results
Baseline characteristics
In Table I the
clinical conditions of the patients have been introduced. Six
patients suffered from two segments of lumbar disc herniation so
the age and male/female ratio could be compared in all
patients.
Differences in the pathological
results from the specimens collected from patients with LDH
Thirty-one discs were classified as NCDH and 21 as
CDH, based on the position of the IVD that was observed during the
surgery (Table II).
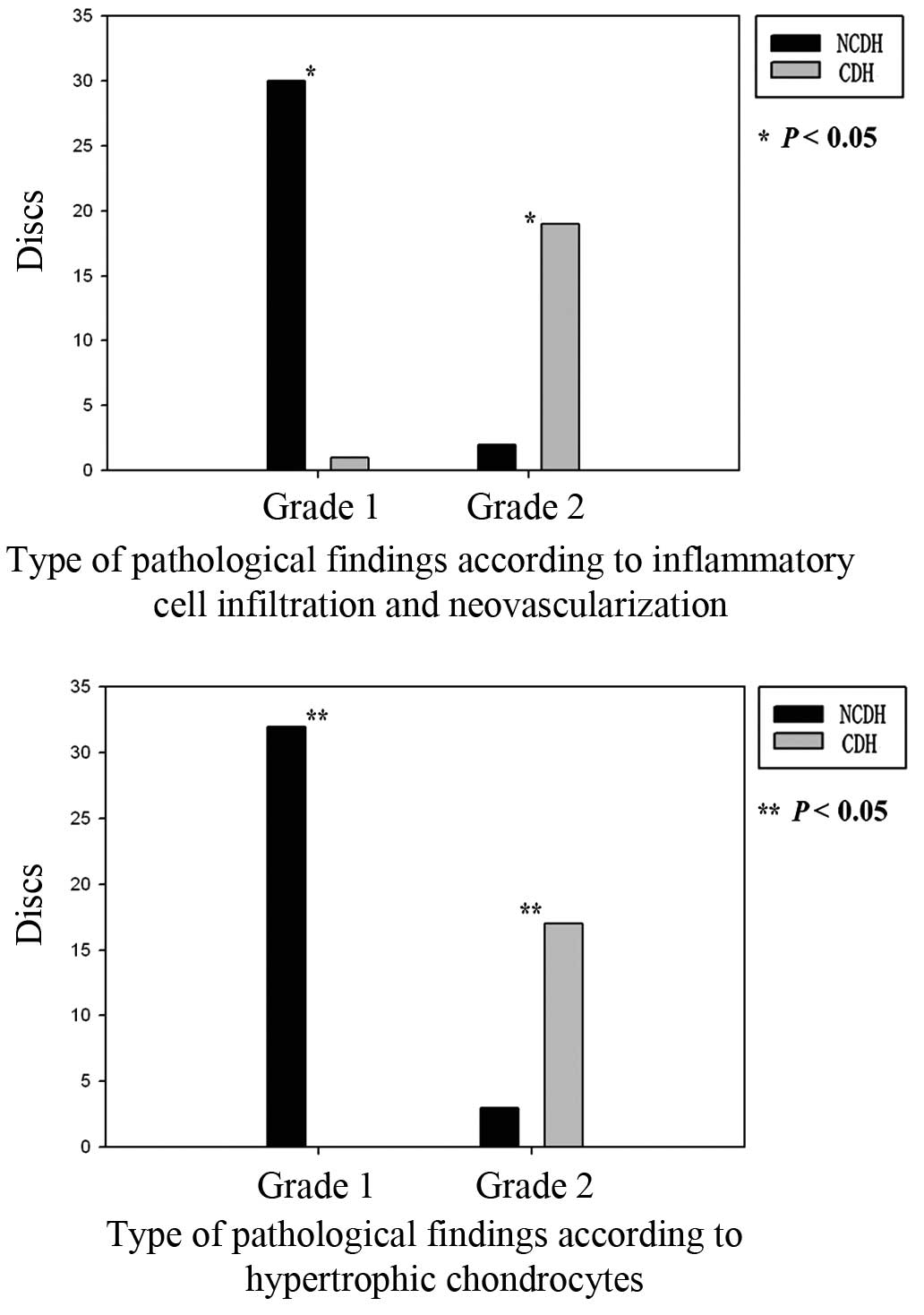
Neovascularization and granulation tissues were observed in 30 out
of 31 (97%) herniated tissues in the NCDH group. Hypertrophic
chondrocytes were detected in the IVD specimens from the entire
NCDH group (Fig. 3).
 | Table II.Summary of surgical and pathological
findings in the 46 patients with lumbar disc herniation. |
Table II.
Summary of surgical and pathological
findings in the 46 patients with lumbar disc herniation.
| Case no. | Level (side) | Type of hernia | Blood vessels
around hernia | Hypertrophic
chondrocytes |
|---|
| 1 | L5/S1 (R) | NC | 1 | I |
| 2 | L4/5 (L) | C | 2 | II |
| 3 | L4/5 (R) | C | 2 | I |
|
| L5/S1 (R) | C | 2 | II |
| 4 | L4/5 (L) | C | 2 | II |
| 5 | L4/5 (L) | NC | 1 | I |
| 6 | L4/5 (R) | C | 2 | II |
|
| L5/S1 (R) | C | 2 | II |
| 7 | L5/S1 (R) | NC | 1 | II |
| 8 | L4/5 (R) | C | 1 | II |
| 9 | L5/S1 (L) | C | 2 | II |
| 10 | L5/S1 (L) | C | 2 | II |
| 11 | L4/5 (R) | NC | 1 | I |
| 12 | L5/S1 (L) | NC | 1 | I |
| 13 | L4/5 (R) | C | 2 | I |
|
| L5/S1 (R) | C | 2 | I |
| 14 | L4/5 (R) | NC | 1 | I |
| 15 | L4/5 (R) | C | 2 | II |
| 16 | L5/S1 (R) | C | 2 | II |
| 17 | L4/5 (L) | C | 2 | II |
| 18 | L4/5 (L) | NC | 1 | II |
| 19 | L4/5 (L) | C | 2 | II |
|
| L5/S1 (R) | NC | 2 | I |
| 20 | L3/4 (R) | NC | 1 | I |
| 21 | L5/S1 (R) | NC | 1 | I |
| 22 | L4/5 (R) | NC | 1 | I |
| 23 | L4/5 (L) | NC | 2 | II |
| 24 | L4/5 (R) | NC | 1 | I |
| 25 | L4/5 (R) | NC | 1 | I |
| 26 | L4/5 (R) | NC | 1 | I |
| 27 | L4/5 (L) | C | 2 | II |
| 28 | L3/4 (R) | NC | 1 | I |
| 29 | L4/5 (R) | NC | 1 | I |
| 30 | L3/4 (L) | NC | 1 | I |
| 31 | L4/5(L) | C | 2 | II |
|
| L5/S1 (R) | C | 2 | II |
| 32 | L5/S1 (R) | NC | 1 | I |
| 33 | L4/5(L) | NC | 1 | I |
|
| L5/S1 (L) |
| 1 | I |
| 34 | L4/5 (L) | NC | 1 | I |
| 35 | L4/5 (R) | NC | 1 | I |
| 36 | L4/5 (R) | NC | 1 | I |
| 37 | L4/5 (R) | C | 2 | II |
| 38 | L4/5 (L) | NC | 1 | I |
| 39 | L4/5 (L) | NC | 1 | I |
| 40 | L4/5 (L) | C | 2 | II |
| 41 | L5/S1 (L) | NC | 1 | I |
| 42 | L4/5 (L) | NC | 1 | I |
| 43 | L4/5 (L) | NC | 1 | I |
| 44 | L5/S1 (R) | NC | 1 | I |
| 45 | L4/5 (L) | NC | 1 | I |
| 46 | L5/S1 (L) | NC | 1 | I |
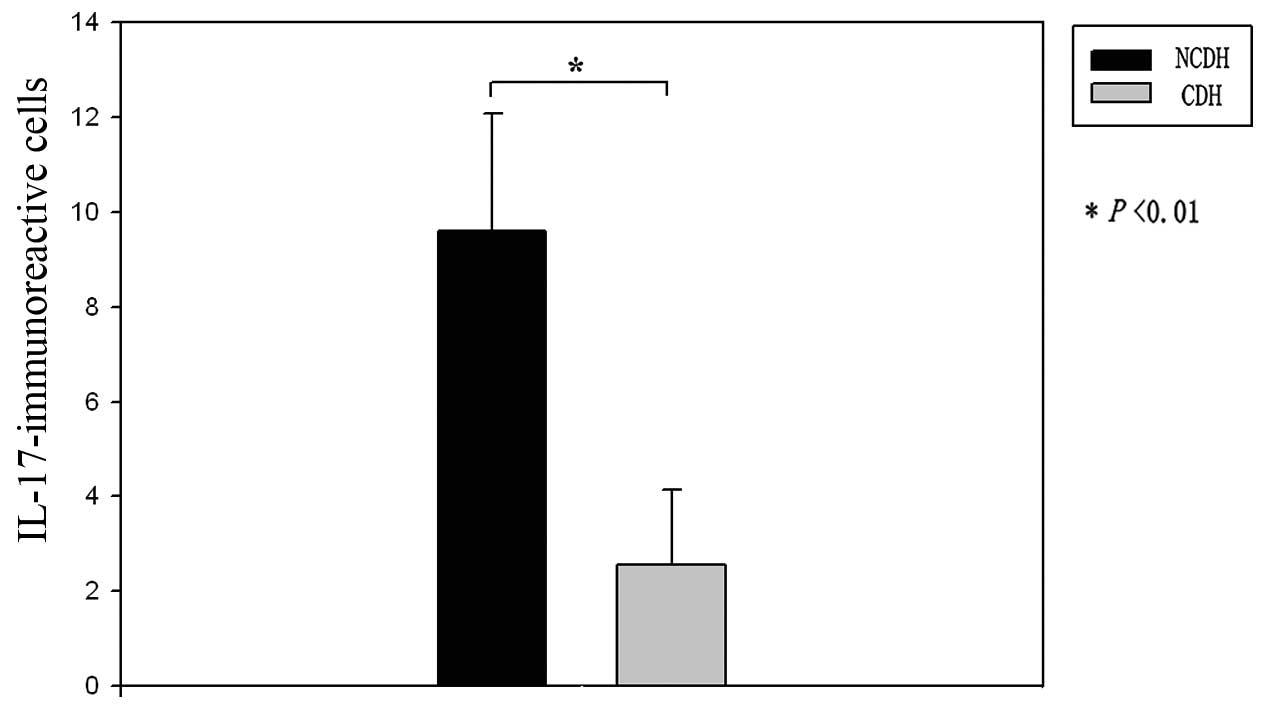
Pattern of distribution of
IL-17-immunoreactive cells in the IVDs
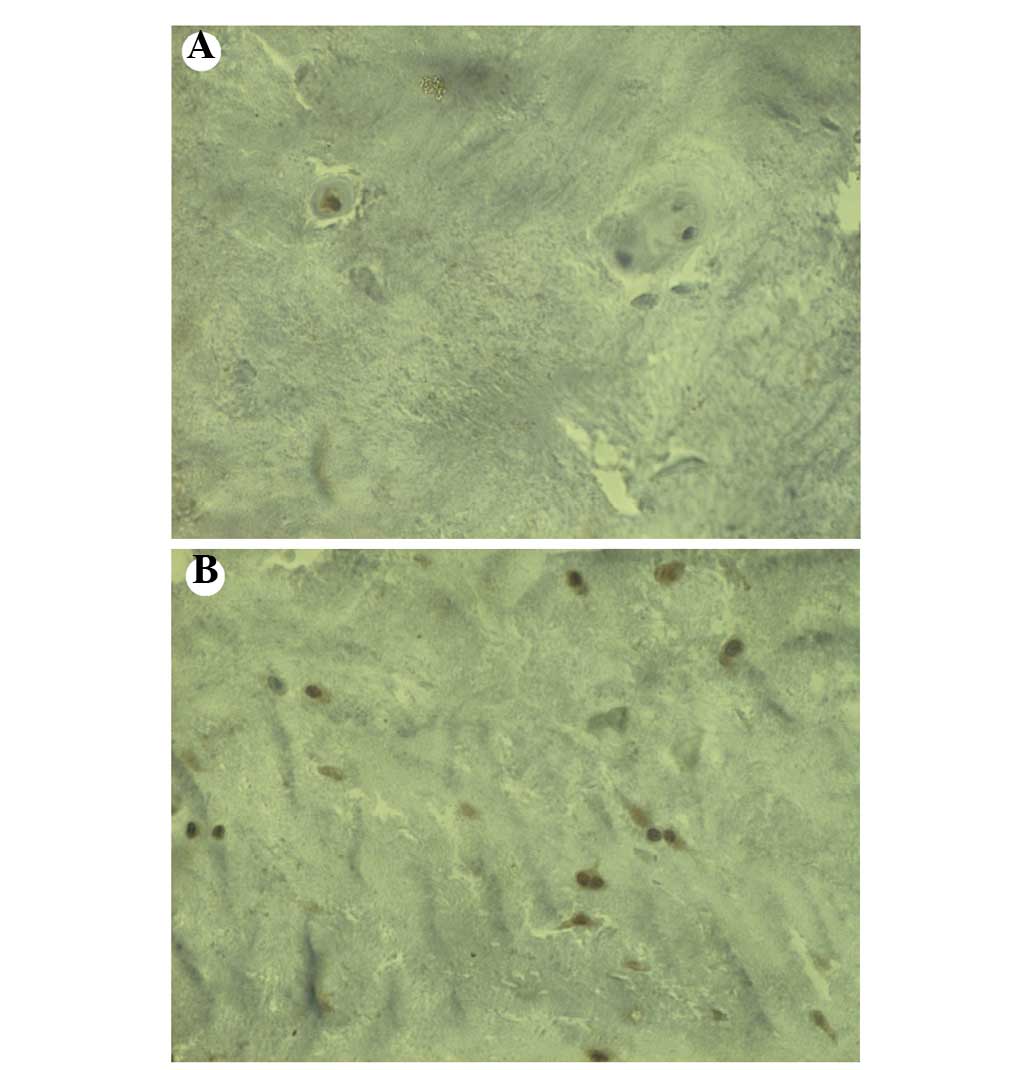
IL-17 immunoreactivity was observed in the IVD
specimens from both the NCDH and CDH groups. The number of
IL-17-immunoreactive cells in the IVD specimens from patients in
the NCDH group was significantly greater than that in the IVD
specimens from patients in the CDH group (Figs. 4 and 5).
Discussion
LDH is the most common cause of sciatica, a
condition that severely limits the ability to perform daily
activities. Although there is a high incidence of LDH in society,
controversy still surrounds the pathogenesis and treatment of the
disease. In 1962, Naylor proposed that chronic lower back pain
could be due to an autoimmune mechanism (48). In the last few decades, numerous
studies have proposed a possible immunological mechanism for nerve
injury in LDH. By acting as a biochemical or immunological
irritant, herniated disc material could contribute to a patient's
clinical signs and symptoms (13–15).
Previous studies have provided evidence for the
neovascularization and infiltration of macrophages and other
inflammatory cells into herniated disc tissues (12,49). In
the current study, IVDs from patients with NCDH had significantly
more neovascularization and granulation tissue than patients with
CDH. This is consistent with results from previous studies
(37,47), which showed that annulus fibrosus
disruption could induce changes in the microenvironment surrounding
herniated disc tissues. The present study evaluated the differences
in neovascularization between the CDH and NCDH groups; however, no
post-treatment follow-up evaluations were performed. A recent study
has shown that severe histodegeneration in LDH is associated with
enhanced neovascularization and a potentially spontaneous
regression of the herniated tissue (50). The process of angiogenesis in the
degenerated IVD affects the pre- and postoperative quality of life
of the patients and may also serve as a predictive factor for
postoperative results (51).
Through histological observations of intact rabbit
IVDs, Kim et al (52)
confirmed that chondrocytes in the NP originate from the cartilage
endplate. Following the identification of chondrocytes in IVDs, an
increasing number of studies have focused on the effects of
chondrocyte apoptosis on physiological changes in IVDs (52,53). The
frequency of chondrocyte apoptosis in the sequestrated NP (SNP) and
in the remaining NP (RNP) was the same (53). The pathways involved in chondrocyte
apoptosis in the SNP and the RNP differed among individuals and
included intrinsic and/or extrinsic pathways (53). In the current study, hypertrophic
chondrocytes were more prevalent in specimens from the NCDH group
than in specimens from the CDH group.
Immunohistochemical analysis showed that IL-17 was
expressed in human herniated IVD tissues. IL-17 is a cytokine
associated with inflammation and autoimmunity. The number of
IL-17-immunoreactive cells found in the specimens from patients
with NCDH was significantly higher than that found in the specimens
from patients with CDH.
The present study had a few limitations. Each group
contained only a small number of cases, including patients of
different ages; however, the intensity of pain or IL-17 expression
in each patient was not compared. The results of this study suggest
that the expression of IL-17 in herniated disc tissue may be a
cause of lower back pain in LDH; however, further studies should be
conducted in order to investigate the pathophysiological mechanisms
of herniated disc tissue-induced pain.
In conclusion, the present results demonstrate that
neovascularization, granulation tissue and hypertrophic
chondrocytes are found in herniated disc tissues. IL-17 is
expressed in human IVDs. Following its expression and secretion in
inflamed human herniated disc tissues, IL-17 acts in an autoimmune
manner to regulate inflammation and angiogenesis during the healing
process. The present study demonstrates that IL-17 contributes to
the pathogenesis of human IVD herniation by promoting autoimmune
inflammation, chemotaxis and angiogenesis.
Acknowledgements
This study was supported by a grant from the
Scientific Research Fund of Tianjin Municipal Administration of
Traditional Chinese Medicine (grant no. 13123), National Natural
Science Foundation of China (grant no. 81401792) and Project of
Natural Science Foundation of Tianjin of China (grant no.
14JCQNJC11700).
References
|
1
|
McLain RF, Kapural L and Mekhail NA:
Epidural steroids for back and leg pain: Mechanism of action and
efficacy. Cleve Clin J Med. 71:961–970. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Geiss A, Larsson K, Rydevik B, Takahashi I
and Olmarker K: Autoimmune properties of nucleus pulposus: An
experimental study in pigs. Spine (Phila Pa 1976). 32:168–173.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirsch C and Schajowicz F: Studies on
structural changes in the lumbar annulus fibrosus. Acta Orthop
Scand. 22:184–231. 1952. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kanerva A, Kommonen B, Grönblad M, et al:
Inflammatory cells in experimental intervertebral disc injury.
Spine (Phila Pa 1976). 22:2711–2715. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Olmarker K, Nordborg C, Larsson K and
Rydevik B: Ultrastructural changes in spinal nerve roots induced by
autologous nucleus pulposus. Spine (Phila Pa 1976). 21:411–414.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Olmarker K, Iwabuchi M, Larsson K and
Rydevik B: Walking analysis of rats subjected to experimental disc
herniation. Eur Spine J. 7:394–399. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Otani K, Arai I, Mao GP, et al: Nucleus
pulposus-induced nerve root injury: Relationship between blood flow
and motor nerve conduction velocity. Neurosurgery. 45:614–620.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Igarashi T, Kikuchi S, Shubayev V and
Myers RR: 2000 Volvo Award winner in basic science studies:
Exogenous tumor necrosis factor-alpha mimics nucleus
pulposus-induced neuropathology. Molecular, histologic, and
behavioral comparisons in rats. Spine (Phila Pa 1976).
25:2975–2980. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Olmarker K, Størkson R and Berge OG:
Pathogenesis of sciatic pain: A study of spontaneous behavior in
rats exposed to experimental disc herniation. Spine (Phila Pa
1976). 27:1312–1317. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kallakuri S, Takebayashi T, Ozaktay AC, et
al: The effects of epidural application of allografted nucleus
pulposus in rats on cytokine expression, limb withdrawal and nerve
root discharge. Eur Spine J. 14:956–964. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Murata Y, Nannmark U, Rydevik B, et al:
Nucleus pulposus-induced apoptosis in dorsal root ganglion
following experimental disc herniation in rats. Spine (Phila Pa
1976). 31:382–390. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shamji MF, Allen KD, So S, et al: Gait
abnormalities and inflammatory cytokines in an autologous nucleus
pulposus model of radiculopathy. Spine (Phila Pa 1976). 34:648–654.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saal JS, Franson RC, Dobrow R, et al: High
levels of inflammatory phospholipase A2 activity in lumbar disc
herniations. Spine (Phila Pa 1976). 15:674–678. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goupille P, Jayson MI, Valat JP and
Freemont AJ: The role of inflammation in disk herniation-associated
radiculopathy. Semin Arthritis Rheum. 28:60–71. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tolonen J, Grönblad M, Vanharanta H, et
al: Growth factor expression in degenerated intervertebral disc
tissue. An immunohistochemical analysis of transforming growth
factor beta, fibroblast growth factor and platelet-derived growth
factor. Eur Spine J. 15:588–596. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nagano T, Yonenobu K, Miyamoto S, et al:
Distribution of the basic fibroblast growth factor and its receptor
gene expression in normal and degenerated rat intervertebral discs.
Spine (Phila Pa 1976). 20:1972–1978. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tolonen J, Grönblad M, Virri J, et al:
Basic fibroblast growth factor immunoreactivity in blood vessels
and cells of disc herniations. Spine (Phila Pa 1976). 20:271–276.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kang JD, Stefanovic-Racic M, McIntyre LA,
et al: Toward a biochemical understanding of human intervertebral
disc degeneration and herniation. Contributions of nitric oxide,
interleukins, prostaglandin E2, and matrix metalloproteinases.
Spine (Phila Pa 1976). 22:1065–1073. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kang JD, Georgescu HI, McIntyre-Larkin L,
et al: Herniated lumbar intervertebral discs spontaneously produce
matrix metalloproteinases, nitric oxide, interleukin-6, and
prostaglandin E2. Spine (Phila Pa 1976). 21:271–277. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brisby H, Byröd G, Olmarker K, et al:
Nitric oxide as a mediator of nucleus pulposus-induced effects on
spinal nerve roots. J Orthop Res. 18:815–820. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Richardson SM, Doyle P, Minogue BM, et al:
Increased expression of matrix metalloproteinase-10, nerve growth
factor and substance P in the painful degenerate intervertebral
disc. Arthritis Res Ther. 11:R1262009. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takahashi H, Suguro T, Okazima Y, et al:
Inflammatory cytokines in the herniated disc of the lumbar spine.
Spine (Phila Pa 1976). 21:218–224. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ozaktay AC, Cavanaugh JM, Asik I, et al:
Dorsal root sensitivity to interleukin-1 beta, interleukin-6 and
tumor necrosis factor in rats. Eur Spine J. 11:467–475. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hoyland JA, Le Maitre C and Freemont AJ:
Investigation of the role of IL-1 and TNF in matrix degradation in
the intervertebral disc. Rheumatology (Oxford). 47:809–814. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Geiss A, Larsson K, Junevik K, et al:
Autologous nucleus pulposus primes T cells to develop into
interleukin-4-producing effector cells: An experimental study on
the autoimmune properties of nucleus pulposus. J Orthop Res.
27:97–103. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rand N, Reichert F, Floman Y and
Rotshenker S: Murine nucleus pulposus-derived cells secrete
interleukins-1-beta, −6, and −10 and granulocyte-macrophage
colony-stimulating factor in cell culture. Spine (Phila Pa 1976).
22:2598–2602. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Burke JG, Watson RW, McCormack D, et al:
Intervertebral discs which cause low back pain secrete high levels
of proinflammatory mediators. J Bone Joint Surg Br. 84:196–201.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang K-Y, Lin R-M, Chen W-Y, et al: IL-20
may contribute to the pathogenesis of human intervertebral disc
herniation. Spine (Phila Pa 1976). 33:2034–2040. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
O'Donnell JL and O'Donnell AL:
Prostaglandin E2 content in herniated lumbar disc disease. Spine
(Phila Pa 1976). 21:1653–1656. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Olmarker K and Larsson K: Tumor necrosis
factor alpha and nucleus-pulposus-induced nerve root injury. Spine
(Phila Pa 1976). 23:2538–2544. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoshida T, Park JS, Yokosuka K, et al:
Effect of a nonprotein bioactive agent on the reduction of
cyclooxygenase-2 and tumor necrosis factor-alpha in human
intervertebral disc cells in vitro. J Neurosurg Spine. 9:411–418.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Iwabuchi S, Ito M, Chikanishi T, et al:
Role of the tumor necrosis factor-alpha, cyclooxygenase-2,
prostaglandin E2, and effect of low-intensity pulsed ultrasound in
an in vitro herniated disc resorption model. J Orthop Res.
26:1274–1278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ohtori S, Inoue G, Koshi T, et al:
Substance P-saporin down-regulates substance P receptor
immunoreactive sensory dorsal root ganglion neurons innervating the
lumbar intervertebral discs in rats. Spine (Phila Pa 1976).
31:2987–2991. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Miyamoto H, Saura R, Harada T, et al: The
role of cyclooxygenase-2 and inflammatory cytokines in pain
induction of herniated lumbar intervertebral disc. Kobe J Med Sci.
46:13–28. 2000.PubMed/NCBI
|
|
35
|
Miyamoto H, Saura R, Doita M, et al: The
role of cyclooxygenase-2 in lumbar disc herniation. Spine (Phila Pa
1976). 27:2477–2483. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yoshida M, Nakamura T, Kikuchi T, et al:
Expression of monocyte chemoattractant protein-1 in primary
cultures of rabbit intervertebral disc cells. J Orthop Res.
20:1298–1304. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Haro H, Kato T, Komori H, et al: Vascular
endothelial growth factor (VEGF)-induced angiogenesis in herniated
disc resorption. J Orthop Res. 20:409–415. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Caruso R, Pallone F and Monteleone G:
Emerging role of IL-23/IL-17 axis in H pylori-associated pathology.
World J Gastroenterol. 13:5547–5551. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fujino S, Andoh A, Bamba S, et al:
Increased expression of interleukin 17 in inflammatory bowel
disease. Gut. 52:65–70. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Loffredo S, Ayala F, Marone G, et al:
Immunopathogenesis of psoriasis and pharmacological perspectives. J
Rheumatol Suppl. 83:9–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hwang SY and Kim HY: Expression of IL-17
homologs and their receptors in the synovial cells of rheumatoid
arthritis patients. Mol Cells. 19:180–184. 2005.PubMed/NCBI
|
|
42
|
Hou W, Kang HS and Kim BS: Th17 cells
enhance viral persistence and inhibit T cell cytotoxicity in a
model of chronic virus infection. J Exp Med. 206:313–328. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kokubu T, Haudenschild DR, Moseley TA, et
al: Immunolocalization of IL-17A, IL-17B, and their receptors in
chondrocytes during fracture healing. J Histochem Cytochem.
56:89–95. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shamji MF, Allen KD, So S, et al: Gait
abnormalities and inflammatory cytokines in an autologous nucleus
pulposus model of radiculopathy. Spine (Phila Pa 1976). 34:648–654.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shamji MF, Setton LA, Jarvis W, et al:
Proinflammatory cytokine expression profile in degenerated and
herniated human intervertebral disc tissues. Arthritis Rheum.
62:1974–1982. 2010.PubMed/NCBI
|
|
46
|
Cheng L, Fan W, Liu B, et al: Th17
lymphocyte levels are higher in patients with ruptured than
non-ruptured lumbar discs, and are correlated with pain intensity.
Injury. 44:1805–1810. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kobayashi S, Meir A, Kokubo Y, et al:
Ultrastructural analysis on lumbar disc herniation using surgical
specimens: Role of neovascularization and macrophages in hernias.
Spine (Phila Pa 1976). 34:655–662. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Naylor A: The biophysical and biochemical
aspects of intervertebral disc herniation and degeneration. Ann R
Coll Surg Engl. 31:91–114. 1962.PubMed/NCBI
|
|
49
|
Doita M, Kanatani T, Ozaki T, et al:
Influence of macrophage infiltration of herniated disc tissue on
the production of matrix metalloproteinases leading to disc
resorption. Spine (Phila Pa 1976). 26:1522–1527. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rätsep T, Minajeva A and Asser T:
Relationship between neovascularization and degenerative changes in
herniated lumbar intervertebral discs. Eur Spine J. 22:2474–2480.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
David G, Ciurea AV, Iencean SM and Mohan
A: Angiogenesis in the degeneration of the lumbar intervertebral
disc. J Med Life. 3:154–161. 2010.PubMed/NCBI
|
|
52
|
Kim KW, Lim TH, Kim JG, et al: The origin
of chondrocytes in the nucleus pulposus and histologic findings
associated with the transition of a notochordal nucleus pulposus to
a fibrocartilaginous nucleus pulposus in intact rabbit
intervertebral discs. Spine (Phila Pa 1976). 28:982–990. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ha KY, Kim BG, Kim KW, et al: Apoptosis in
the sequestrated nucleus pulposus compared to the remaining nucleus
pulposus in the same patient. Spine (Phila Pa 1976). 36:683–689.
2011. View Article : Google Scholar : PubMed/NCBI
|