Introduction
Breast cancer is a common malignant tumor with a
high mortality rate in females. According to World Health
Organization statistics, ~1,500,000 cases occur each year
worldwide, with ~200,000 new cases arising annually in China alone
(1). There has been major
development in the research of breast cancer pathogenesis,
particularly in the field of oncogene protein expression, with the
identification of cyclin D1, p53 and Rab (2–4). These
proteins have varying expression levels in tumor and normal breast
tissues, and are able to cause a variety of changes in the
biological behavior of cells (2–4).
WTH3 is a gene that was identified during early
studies of multiple drug resistance (5). In a previous study involving
drug-resistant MCF-7/AdrR cells, the promotor of the WTH3 gene was
hypermethylated and the expression of WTH3 was downregulated
(6). WTH3 belongs to the Rab6
superfamily, whose expression product can bind to GTP. In contrast
to Rab6s, which is located on the Golgi apparatus, WTH3 is
primarily distributed in the cytoplasm and is unable to bind to the
cytomembrane to perform its biological functions. According to the
results of previous studies, WTH3 has an effect on the drug
resistance of breast cancer cells and has a bidirectional
regulatory function on certain cellular behaviors (7,8).
In the present study, the expression of WTH3 in
breast cancer tissue was detected. In addition, an MCF-7 and
MDA-MB-231 in vitro cell model was used to investigate the
possible mechanisms underlying the effects of WTH3 on breast
cancer.
Materials and methods
Clinical specimens and cell lines
Breast cancer tissue specimens were collected from
five patients aged between 55 and 60 years old at the Chongqing
Hospital of Traditional Chinese Medicine (Chongqing, China).
Written informed patient consent was obtained from the patients and
the study was approved by the ethics committee of Chongqing
Hospital of Traditional Chinese Medicine. Human breast cancer cell
lines, MCF-7 and MDA-MB-231, were purchased from the Cell Resource
Center of the Shanghai Institutes for Biological Sciences at the
Chinese Academy of Sciences (Shanghai, China). MDA-MB-231-WTH3
(WTH3 overexpression) and MCF-7 WTH3-/- (WTH3 knockout) cell lines
were previously established by the current research group and
maintained in our laboratory. Cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; Gibco Life Technologies, Beijing,
China) supplemented with 10% (v/v) heat-inactivated fetal bovine
serum (FBS; Gibco) and penicillin-streptomycin (100 IU/ml-100
lg/ml; Gibco) at 37°C in a humid atmosphere (5% CO2 to
95% air). The cells were harvested by brief incubation with 0.25%
Tryptase (Sigma-Aldrich, St. Louis, MO, USA).
Reverse transcription quantitative
polymerase chain reaction (PCR)
Total RNA was extracted using an RNeasy kit (Sangon
Biotech, Shanghai, China). Reverse transcription was conducted with
0.5 µg extracted RNA using the First Strand cDNA Synthesis kit
(Beyotime Institute of Biotechnology, Haimen, China). PCR primer
pairs were as follows: WTH3 sense, 5′-GATGGAACAATCGGGCTTCG-3′ and
antisense, 5′-GCTGCTACACGTCGAAAGAGC-3′; β-actin sense,
5′-GACGACATGGAGAAGATCTGG-3′ and antisense,
5′-ATCGGGCAGCTCGTAGCTCTTC-3′), where β-actin was used as an
internal control. The reaction was performed at 95°C for 5 min,
followed by 35 cycles at 95°C for 15 sec, 60°C for 30 sec and 72°C
for 30 sec, and finally 72°C for 10 min. PCR products were run on
2.0% agarose gels containing 0.5 lg/ml ethidium bromide and
photographed under a UV transilluminator; relative light
intensities were analyzed using AlphaEaseFC software.
Western blot analysis
Western blot analysis was used to evaluate the
protein expression levels of WTH3 and matrix metalloproteinase
(MMP)-2 in breast tumor tissue and cancer cell lines in
vitro. The tumor tissue was dispersed mechanically with
phosphate-buffered saline (PBS) and the supernatant was collected,
from which the protein concentration was determined using a
bicinchoninic acid protein assay kit (Beyotime). The cancer cells
were harvested and the cell lysates (30 µg protein/lane) were
fractionated by 10% SDS-PAGE. The protein was electrotransferred
onto polyvinylidene fluoride membranes (EMD Millipore, Billerica,
CA, USA), and the protein levels were determined using the
dilutions of the primary antibodies overnight at 4°C, including a
mouse polyclonal anti-WTH-3 (1:2,500, #ab168296; Abcam, Cambridge,
MA, USA) and monoclonal anti-MMP-2 (1:800, #sc-13595; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) antibodies. The primary
antibodies were washed in 0.05% Tween-20/PBS, then incubated with a
goat anti-mouse horseradish peroxidase (HRP)-conjugated IgG
secondary antibody for 40 min at room temperature (1:800, ZDR-5307;
ZSGB-BIO, Bejing, China). The bound antibodies were visualized
using an enhanced chemiluminescence reagent (Amersham Pharmacia
Biotech, Piscataway, NJ, USA) and quantified using a LAS 4000 mini
chemiluminescence imaging system (GE Healthcare, Little Chalfont,
UK).
Cell Counting Kit-8 (CCK-8) assay for
cell proliferation activity
Breast cancer cells at the logarithmic growth phase
were seeded into 96-well plates (2×103 cells/well). The
cells were incubated at 37°C for 18–24 h for adherence, after which
incubation continued for 1–7 days to form a sigmoid growth curve.
Cell proliferation activity was detected by adding 10 µl CCK-8
solution to each well, and the light absorbance of the solution
[optical density (OD)] was measured at 450 nm using a microplate
reader (Molecular Devices LLC, Sunnyvale, CA, USA). A cell growth
curve was generated with time as the abscissa and OD450
as ordinates.
Invasion and migration assay
A 24-well Transwell chamber (Corning, Inc., Corning,
NY, USA) was used to evaluate the motility and invasive ability of
the carcinoma cells. The upper surfaces of the polycarbonate
filters with 8 µm pores were coated with 100 µg Matrigel (Corning,
Inc.). The lower chambers were filled with 800 µl DMEM with 10%
FBS. A cell suspension (2.5×104 cells/100 µl) was placed
in the upper chambers and incubated at 37°C in a CO2
incubator. Following incubation, any cancer cells remaining on the
upper surface of the filters were removed by wiping with cotton
swabs. The cells that had migrated to the lower surface were
stained with Giemsa stain (Sigma-Aldrich). Five fields of vision
were randomly selected and the number of cells on the lower surface
of the filters were counted under an Olympus microscope (Olympus
Corporation, Tokyo, Japan) at a magnification of x400.
Statistical analysis
Data are presented as the mean ± standard deviation
and were analyzed using SPSS 13.0 software (SPSS, Inc., Chicago,
IL, USA). Statistical differences between groups were analyzed by
one-way analysis of variance and Fisher's least significant
difference t-test, where P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of WTH3 in breast cancer
tissue
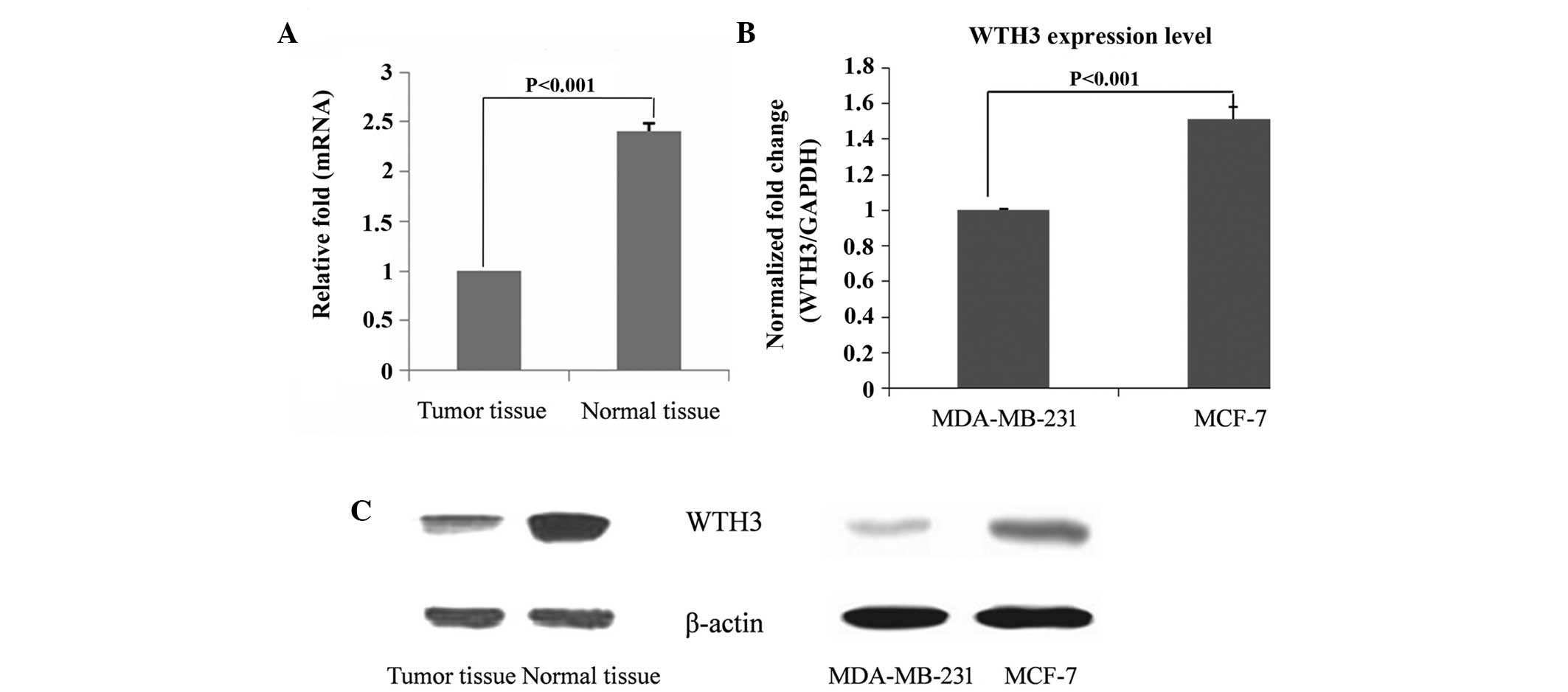
Compared with the normal breast tissue, the
expression of WTH3 in breast tumor tissue was downregulated at an
mRNA and protein level (Fig. 1A;
P<0.001). The metastatic ability of MDA-MB-231 cells is higher
compared with MCF-7 cells, and the mRNA expression of WTH3 in the
MDA-MB-231 cells was lower compared with the MCF-7 cells (Fig. 1B; P<0.001). As shown in Fig. 1C, the expression of WTH3 in normal
tissue was higher compared with tumor tissue, while in the same
time in MDA-MB-31 cells with high metastatic ability the expression
of WTH3 was reduced compared with MCF-7 cells (Fig. 1B; P<0.001).
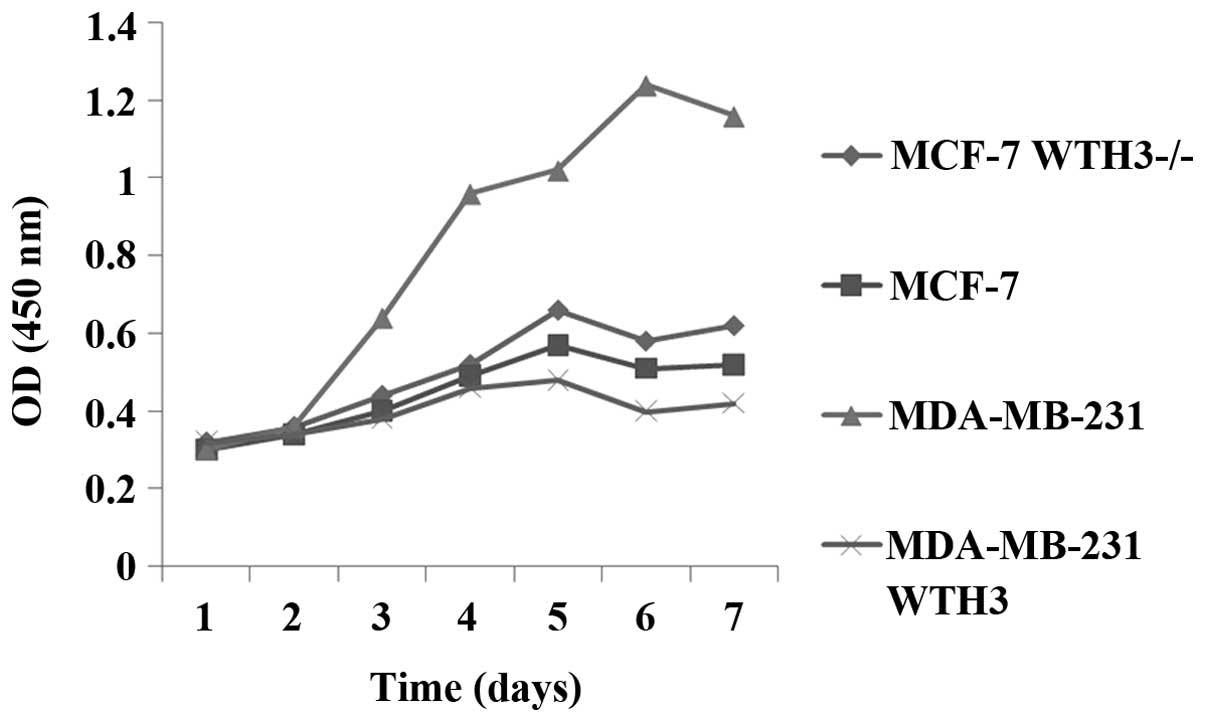
Measurement of cell proliferation
capacity
As shown in Fig. 2,
the proliferation ability of MDA-MB-231 cells was significantly
stronger compared with that of MCF-7 cells. From day 2, the
proliferation activity of MDA-MB-231 WTH3 cells with overexpression
of WTH3 was markedly decreased (P<0.05). Compared with the
original MCF-7 cells, the WTH3 knockout cell line, MCF-7 WTH3-/-,
showed improved proliferation activity (P<0.05)
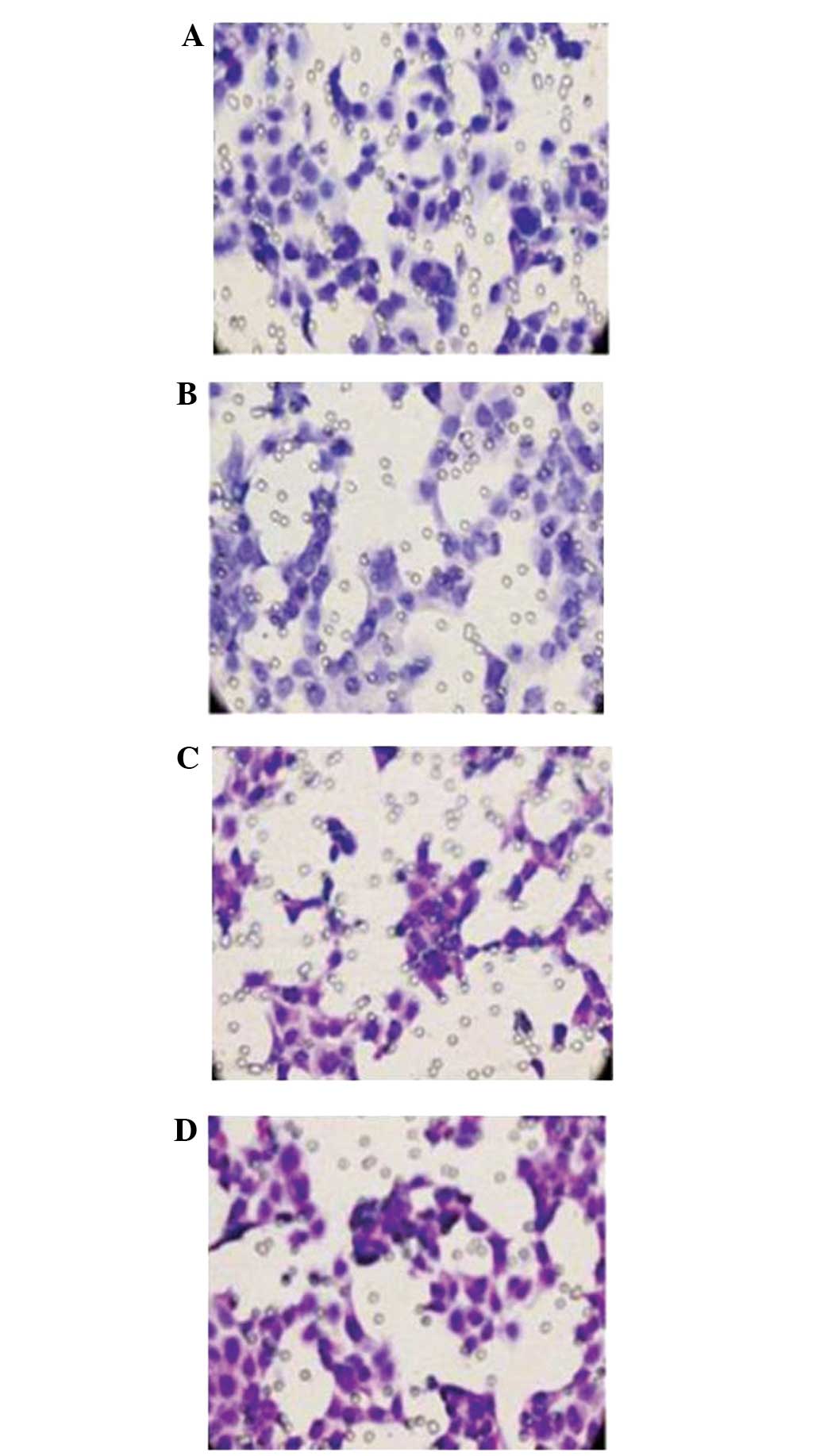
Inhibition of breast cancer cell
invasion and migration
The inhibitory effect of WTH3 on the invasion and
migration of breast carcinoma cells was examined using an invasion
assay with Matrigel-coated filters. The results showed that
MDA-MB-231 cells had a higher invasive ability than MCF-7 cells.
Furthermore, the invasive ability of the WTH3 knockout (MCF-7
WTH3-/-) cell line was increased and WTH3 overexpression cell line
(MDA-MB-231 WTH3) were decreased (P<0.01). As shown in Fig. 3, WTH3 inhibited the invasion and
migration of breast cancer cells.
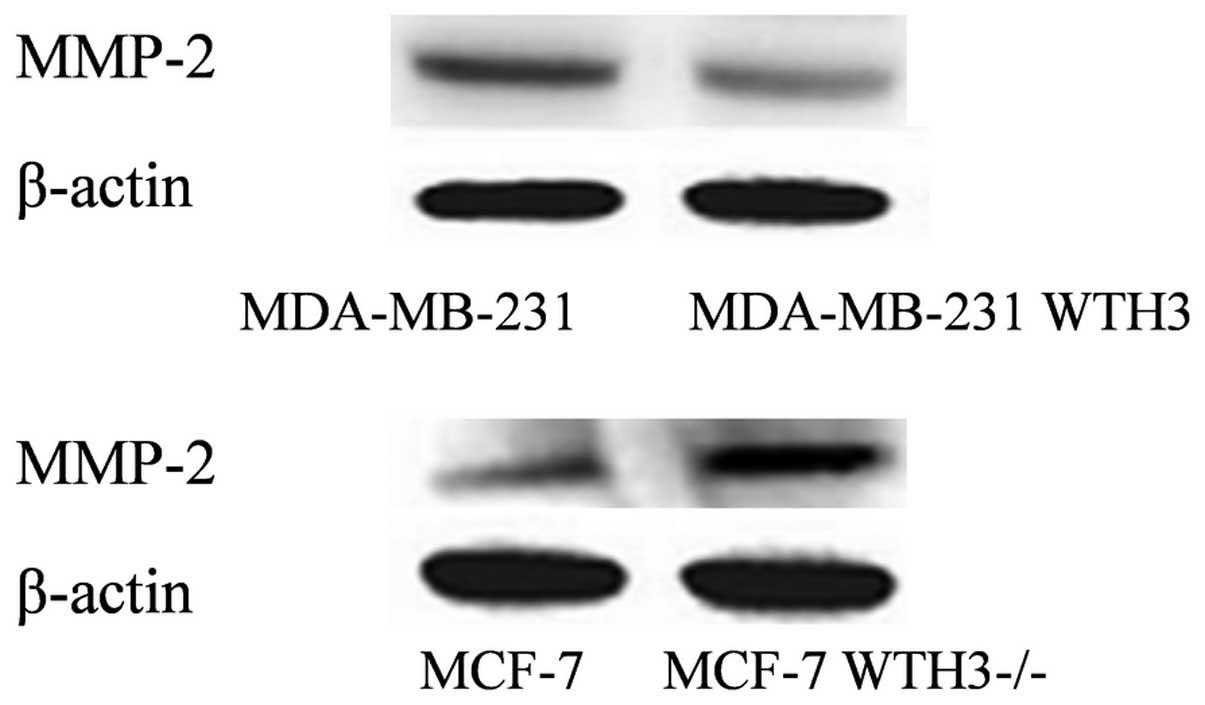
Effect of WTH3 on the expression of
MMP-2 in breast cancer cells
Compared with the original MDA-MB-231 cell line, the
expression of MMP-2 in the MDA-MB-231 WTH3 cell line was decreased
(P<0.01). MMP-2 expression in the MCF-7 WTH3-/- cells was higher
compared with the MCF-7 cell line (P<0.001). The expression
levels of WTH3 and MMP-2 were shown to have a negative association
(Fig. 4).
Discussion
Previous studies have demonstrated that mutations in
the BRCA1, BRCA2 and P53 genes can partially explain the
pathogenesis of breast cancer (8,9).
However, the incidence of breast cancer may also be associated with
other unknown genes, and studies have suggested that the
tumorigenesis and metastasis of certain cancer types is strongly
associated with the loss of Rab gene function (10–12). The
Rab family is the largest subfamily of the small GTP-binding
protein Ras superfamily, which plays a regulatory role as a
GTP-dependent molecular switch at different stages of vesicular
transport (13). The life processes
of eukaryotic cells are accompanied by protein transport between
organelles; this intracellular protein transportation is dependent
on vesicular transport. Rab proteins are key regulatory factors in
the process of intracellular vesicle transport (14,15).
Mutations in the Rab gene or abnormal expression may cause disorder
in the process of vesicle transport. If proteins are unable to be
accurately transported to their destination, a variety of diseases
may arise, including tumors (3).
WTH3 is a newly identified member of the Ras family
homologous to RAB6/RAB6C encoding small G proteins consisting of
254 amino acids, which protein is highly homologous to the protein
of 208 amino acids encoded by RAB6/RAB6C (16,17).
Differences between the proteins include substitutions at amino
acids 19 and 22 in WTH3, and an extended sequence of 46 amino acids
without cysteine in the carboxy-terminal of WTH3. Therefore, WTH3
does not undergo modification following translation and is unable
to bind to the membrane to exert a biological function (18–20).
Since WTH3 was identified during the study of tumor drug
resistance, a number of studies have investigated the methylation
and transcriptional regulation of the gene (5,21). In
the present study, the expression level of WTH3 in human breast
cancer tissue and breast cancer cell lines was investigated and an
abnormal expression was observed. Using the previously constructed
cell lines, the results of the present study indicated that WTH3
was able to inhibit certain biological behaviors of the breast
cancer cells, including cell proliferation, migration and invasion.
WTH3 may inhibit cell proliferation through the activation of one
or more tumor suppressor genes, including the P53 transcription
factor, or negative regulation of the WT transcription factor and
DNA repair factors BRCA1 and BRCA2, all of which can be effective
in promoting apoptosis and the inhibition of tumor cell growth
(21,22). However, the exact mechanism
underlying the effects of WTH3 requires further study.
Tumor invasion and metastasis is a multi-step
cascade amplification process. The tumor cells leave the original
lesions, pass through the extracellular matrix and basement
membrane, migrate to the site of vascular invasion, adhere to the
vascular endothelium, enter the circulatory system and migrate
through blood vessels into the extracellular matrix to form
metastases (23). During the entire
process of tumor invasion and metastasis, the degradation of the
basement membrane and extracellular matrix is important. MMPs are
enzymes that are essential for this process. MMPs play a role in
the degradation of the extracellular matrix by promoting
epithelial-mesenchymal transition, promoting the activation of
growth factors, their receptors and angiogenesis, and facilitating
tumor invasion and metastasis (24–27).
Previous studies revealed that the expression of MMP-2 in breast
cancer tissue is higher compared with normal breast tissue. Such
abnormal expression can promote tumor growth, invasion and
metastasis by increasing the vascular permeability of cancer cells
and the degradation of the extracellular matrix (28,29).
The results of the current study revealed that the
breast cancer cell line with high invasive capacity, MDA-MB-231
WTH3, had a lower invasive ability following elevation of WTH3
expression. In addition, the number of invasive and migratory cells
was significantly decreased, which consequently inhibited the
malignancy of the cancer cells. However, in the MCF-7 WTH3-/- cell
line, the invasion and metastatic ability of the cells was markedly
increased as a result of WTH3 gene knockout. Further investigation
using the four cell lines demonstrated a negative association
between MMP-2 and WTH3 expression, indicating that the inhibitory
metastatic mechanism of WTH3 may be associated with its inhibitory
effects on MMP-2 expression.
In summary, the expression of WTH3 differs between
human breast cancer tissue and normal breast tissue. Furthermore,
WTH3 can inhibit tumor proliferation, invasion and metastasis;
thus, WTH3 is an important and promising therapeutic target for
breast cancer therapy.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 81271598).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saitoh M, Ohmichi M, Takahashi K, Kawagoe
J, Ohta T, Doshida M, Takahashi T, Igarashi H, Mori-Abe A, Du B,
Tsutsumi S and Kurachi H: Medroxyprogesterone acetate induces cell
proliferation through up-regulation of cyclin D1 expression via
phosphatidylinositol 3-kinase/Akt/nuclear factor-kappaB cascade in
human breast cancer cells. Endocrinology. 146:4917–4925. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheng KW, Lahad JP, Kuo WL, Lapuk A,
Yamada K, Auersperg N, Liu J, Smith-McCune K, Lu KH, Fishman D,
Gray JW and Mills GB: The RAB25 small GTPase determines
aggressiveness of ovarian and breast cancers. Nat Med.
10:1251–1256. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dookeran KA, Dignam JJ, Ferrer K, Sekosan
M, McCaskill-Stevens W and Gehlert S: p53 as a marker of prognosis
in African-American women with breast cancer. Ann Surg Oncol.
17:1398–1405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tian K, Wang Y, Huang Y, Sun B, Li Y and
Haopeng Xu: Methylation of WTH3, a possible drug resistant gene,
inhibits p53 regulated expression. BMC Cancer. 8:3272008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shan J, Mason JM, Yuan L, Barcia M, Porti
D, Calabro A, Budman D, Vinciguerra V and Xu H: Rab6c, a new member
of the rab gene family, is involved in drug resistance in MCF7/AdrR
cells. Gene. 257:67–75. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shan J and Yuan L: WTH3, a new member of
the Rab6 gene family, and multidrug resistance. Biochim Biophys
Acta. 1589:112–123. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abdel-Fatah TM, Powe DG, Agboola J, et al:
The biological, clinical and prognostic implications of p53
transcriptional pathways in breast cancers. J Pathol. 220:419–434.
2010.PubMed/NCBI
|
|
9
|
van Beers EH, van Welsem T, Wessels LF, et
al: Comparative genomic hybridization profiles in human BRCA1 and
BRCA2 breast tumors highlight differential sets of genomic
aberrations. Cancer Res. 65:822–827. 2005.PubMed/NCBI
|
|
10
|
Culine S, Honoré N, Closson V, Lang P,
Bertoglio J, Tavitian A and Olofsson B: A possible role for the
Ras-related Rab2 protein in the immunological events associated
with hematological malignancies. Nouv Rev Fr Hematol. 35:41–44.
1993.PubMed/NCBI
|
|
11
|
He H, Dai F, Yu L, She X, Zhao Y, Jiang J,
Chen X and Zhao S: Identification and characterization of nine
novel human small GTPases showing variable expressions in liver
cancer tissues. Gene Expr. 10:231–242. 2002.PubMed/NCBI
|
|
12
|
Amillet JM, Ferbus D, Real FX, Antony C,
Muleris M, Gress TM and Goubin G: Characterization of human Rab20
overexpressed in exocrine pancreatic carcinoma. Hum Pathol.
37:256–263. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pereira-Leal JB and Seabra MC: The
mammalian Rab family of small GTPases: definition of family and
subfamily sequence motifs suggests a mechanism for functional
specificity in the Ras superfamily. J Mol Biol. 301:1077–1087.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tuvim MJ, Adachi R, Hoffenberg S and
Dickey BF: Traffic control: Rab GTPases and the regulation of
interorganellar transport. News Physiol Sci. 16:56–61.
2001.PubMed/NCBI
|
|
15
|
Calero M, Chen CZ, Zhu W, et al: Dual
prenylation is required for Rab protein localization and function.
Mol Biol Cell. 14:1852–1867. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goud B and Zahraoui A: Small GTP-binding
protein associated with Golgi cisternae. Nature. 345:553–556. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Echard A, Opdam FJ, de Leeuw HJ, et al:
Alternative splicing of the human Rab6A gene generates two close
but functionally different isoforms. Mol Biol Cell. 11:3819–3833.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morikawa RK, Aoki J, Kano F, et al:
Intracellular phospholipase A1gamma (iPLA1gamma) is a novel factor
involved in coat protein complex I- and Rab6-independent retrograde
transport between the endoplasmic reticulum and the Golgi complex.
J Biol Chem. 284:26620–26630. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Martinez O, Antony C, Pehau-Arnaudet G,
Berger EG, Salamero J and Goud B: GTP-bound forms of rab6 induce
the redistribution of Golgi proteins into the endoplasmic
reticulum. Proc Natl Acad Sci USA. 94:1828–1833. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martinez O, Schmidt A, Salaméro J, Hoflack
B, Roa M and Goud B: The small GTP-binding protein rab6 functions
in intra-Golgi transport. J Cell Biol. 127:1575–1588. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tian K, Wang Y and Xu H: WTH3 is a direct
target of the p53 protein. Br J Cancer. 96:1579–1586. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tian K, Jurukovski V, Yuan L, Shan J and
Xu H: WTH3, which encodes a small G protein, is differentially
regulated in multidrug-resistant and sensitive MCF7 cells. Cancer
Res. 65:7421–7428. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Perlikos F, Harrington KJ and Syrigos KN:
Key molecular mechanisms in lung cancer invasion and metastasis: a
comprehensive review. Crit Rev Oncol Hematol. 87:1–11. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Patruno A, Pesce M, Marrone A, Speranza L,
Grilli A, De Lutiis MA, Felaco M and Reale M: Activity of matrix
metallo proteinases (MMPs) and the tissue inhibitor of MMP (TIMP)-1
in electromagnetic field-exposed THP-1 cells. J Cell Physiol.
227:2767–2774. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang K, Palm J, König J, Seeland U,
Rosenkranz S, Feiden W, Rübe C and Rübe CE:
Matrix-Metallo-Proteinases and their tissue inhibitors in
radiation-induced lung injury. Int J Radiat Biol. 83:665–676. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iwata H, Yamamoto M, Nemori R, Mizutani M,
Iwase T, Miura S, Obata Y, Hara Y, Omoto Y, Toyama T, Yamashita H,
Iwase H and Kobayashi S: Localization of gelatinolytic activity can
be detected in breast cancer tissues by film in situ zymography.
Breast Cancer. 8:111–115. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kurizaki T, Toi M and Tominaga T:
Relationship between matrix metalloproteinase expression and tumor
angiogenesis in human breast carcinoma. Oncol Rep. 5:673–677.
1998.PubMed/NCBI
|
|
28
|
Jezierska A and Motyl T: Matrix
metalloproteinase-2 involvement in breast cancer progression: a
mini-review. Med Sci Monit. 15:RA32–RA40. 2009.PubMed/NCBI
|
|
29
|
Brummer O, Athar S, Riethdorf L, Löning T
and Herbst H: Matrix-metalloproteinases 1, 2, and 3 and their
tissue inhibitors 1 and 2 in benign and malignant breast lesions:
an in situ hybridization study. Virchows Arch. 435:566–73. 1999.
View Article : Google Scholar : PubMed/NCBI
|